Calcium Pyrophosphate Dihydrate Crystals Increase the Granulocyte/Monocyte Progenitor (GMP) and Enhance Granulocyte and Monocyte Differentiation In Vivo
Abstract
1. Introduction
2. Results
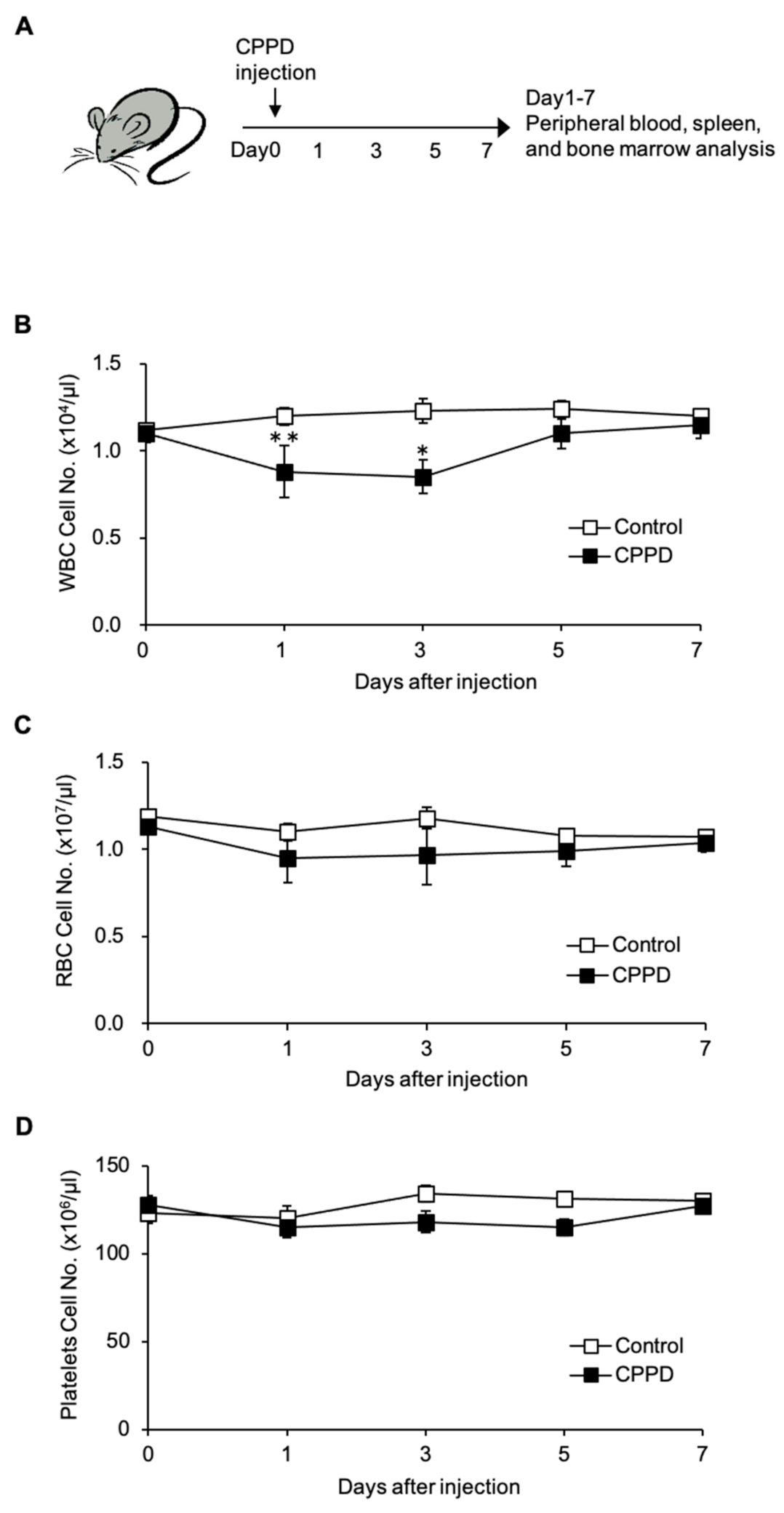
2.1. Calcium Pyrophosphate Dihydrate (CPPD) Crystal Administration Temporarily Reduces White Blood Cell Count
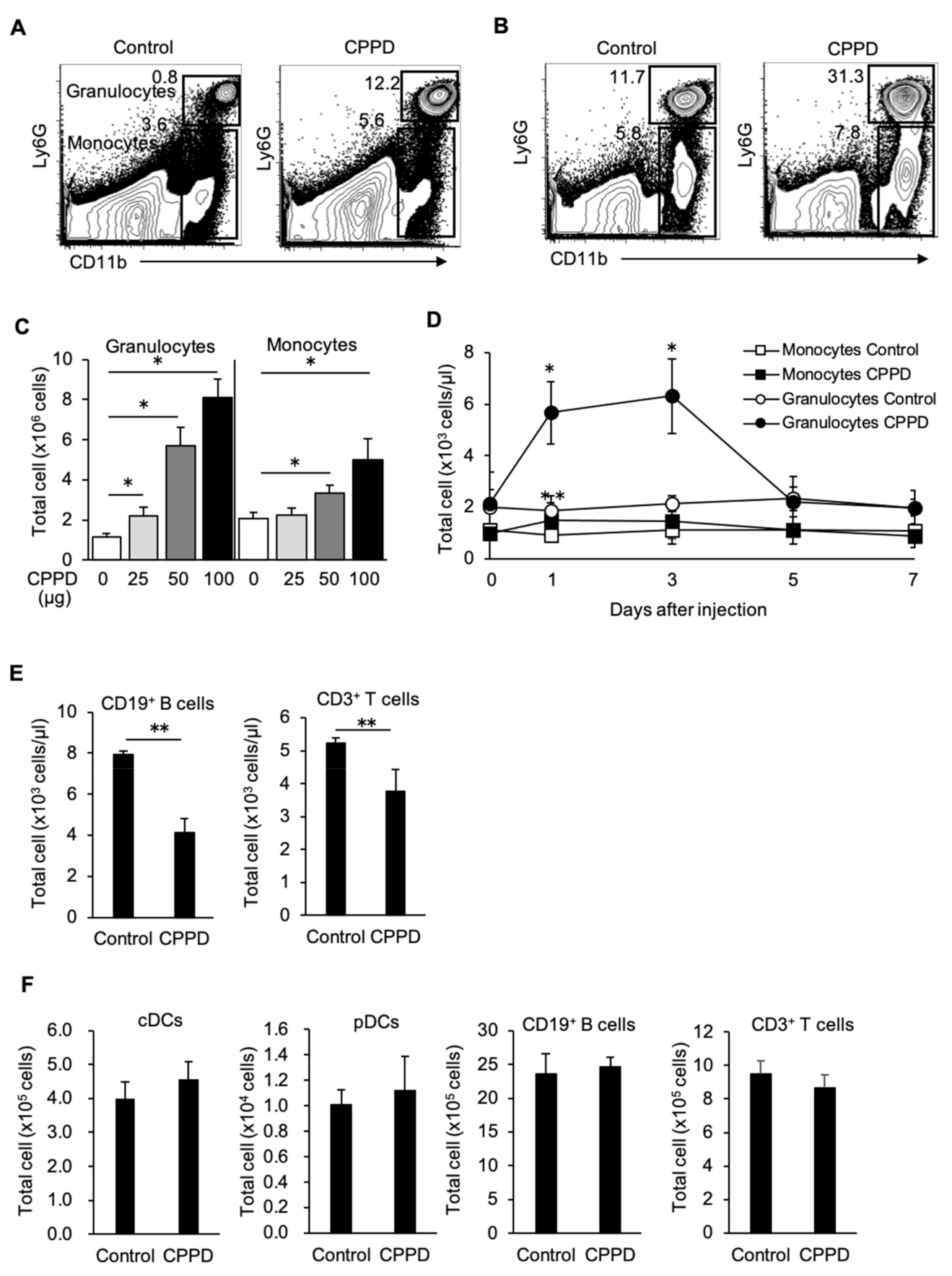
2.2. CPPD Crystal Administration Increases Granulocytes and Monocytes
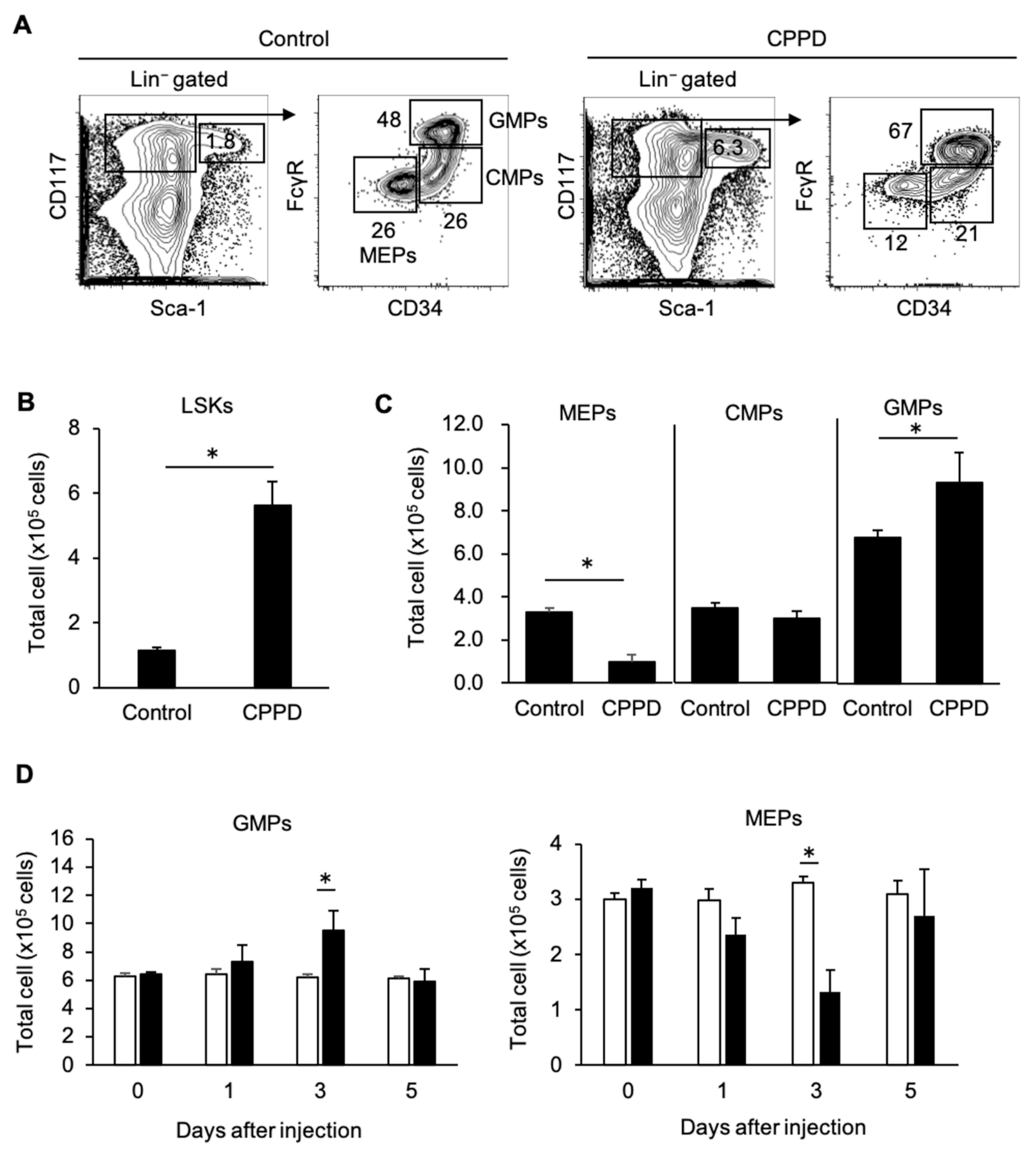
2.3. CPPD Administration Increases Granulocyte/monocyte Progenitor Cell Populations in the Bone Marrow
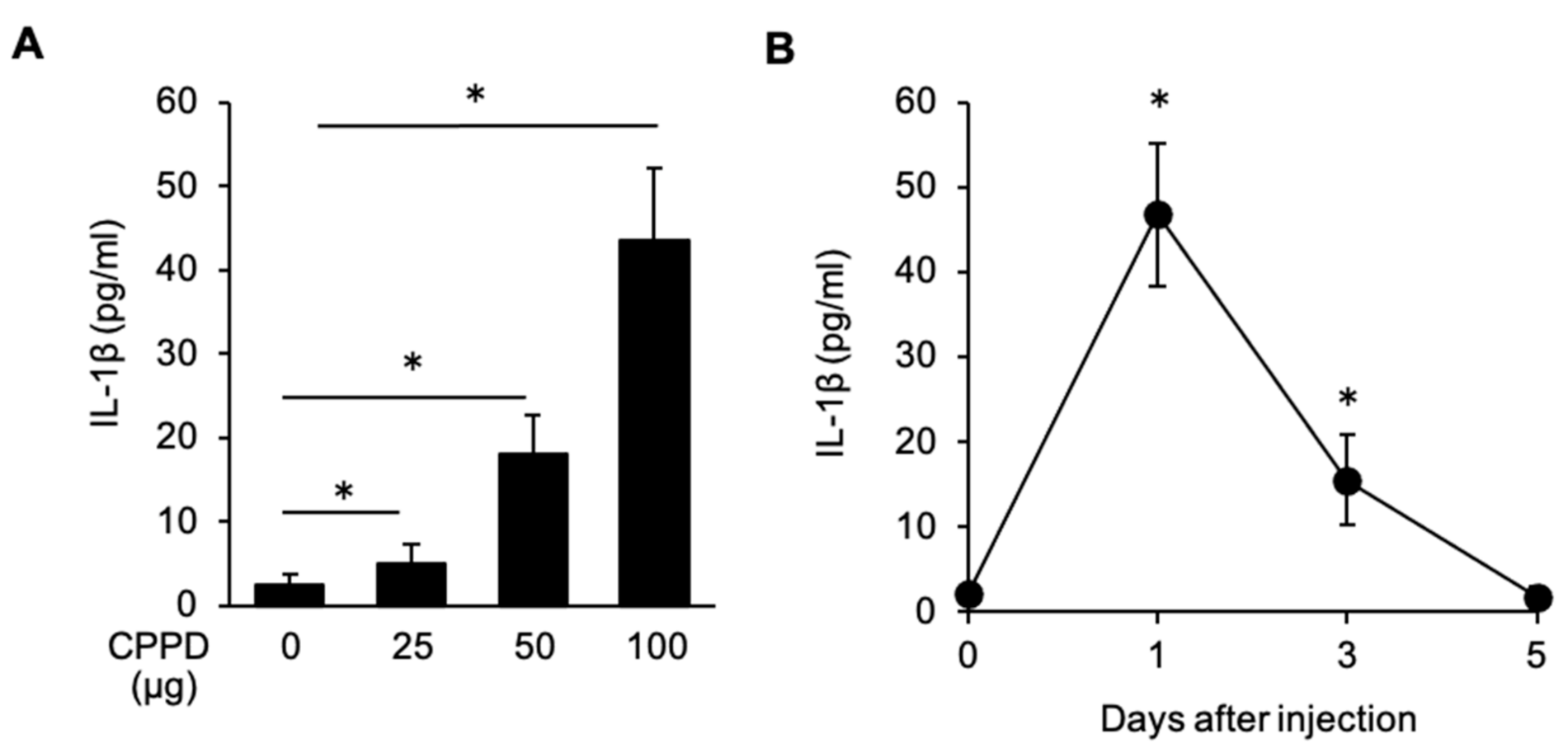
2.4. CPPD Administration Induces IL-1β Production in the Bone Marrow
3. Discussion
4. Material and Method
4.1. Mice
4.2. Reagent
4.3. Peripheral Blood Cell Count
4.4. FCM Analysis
4.5. ELISA
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Annu. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Gaide, O.; Pétrilli, V.; Mayor, A.; Tschopp, J. NALP inflammasomes: A central role in innate immunity. Semin. Immunopathol. 2007, 29, 213–229. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Zamora, E.A.; Naik, R. Calcium Pyrophosphate Deposition Disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar]
- Dalbeth, N.; Choi, H.K.; Joosten, L.A.B.; Khanna, P.P.; Matsuo, H.; Perez-Ruiz, F.; Stamp, L.K. Gout. Nat. Rev. Dis. Primers 2019, 5, 69. [Google Scholar] [CrossRef]
- Pang, L.; Hayes, C.P.; Buac, K.; Yoo, D.G.; Rada, B. Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J. Immunol. 2013, 190, 6488–6500. [Google Scholar] [CrossRef]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Tschopp, J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004, 117, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Lioté, F. Monosodium urate and calcium pyrophosphate dihydrate (CPPD) crystals, inflammation, and cellular signaling. Jt. Bone Spine 2005, 72, 295–302. [Google Scholar] [CrossRef]
- Ikuta, K.; Weissman, I.L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. USA 1992, 89, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Seita, J.; Weissman, I.L. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef]
- Pietras, E.M.; Mirantes-Barbeito, C.; Fong, S.; Loeffler, D.; Kovtonyuk, L.V.; Zhang, S.; Lakshminarasimhan, R.; Chin, C.P.; Techner, J.M.; Will, B.; et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell. Biol. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- Orelio, C.; Peeters, M.; Haak, E.; van der Horn, K.; Dzierzak, E. Interleukin-1 regulates hematopoietic progenitor and stem cells in the midgestation mouse fetal liver. Haematologica 2009, 94, 462–469. [Google Scholar] [CrossRef][Green Version]
- Hestdal, K.; Ruscetti, F.W.; Chizzonite, R.; Ortiz, M.; Gooya, J.M.; Longo, D.L.; Keller, J.R. Interleukin-1 (IL-1) directly and indirectly promotes hematopoietic cell growth through type I IL-1 receptor. Blood 1994, 84, 125–132. [Google Scholar] [CrossRef]
- Dorshkind, K. IL-1 inhibits B cell differentiation in long term bone marrow cultures. J. Immunol. 1998, 141, 531–538. [Google Scholar]
- King, K.Y.; Goodell, M.A. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 2011, 11, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, T.; Mitroulis, I.; Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 2019, 20, 802–811. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onai, N.; Ogasawara, C. Calcium Pyrophosphate Dihydrate Crystals Increase the Granulocyte/Monocyte Progenitor (GMP) and Enhance Granulocyte and Monocyte Differentiation In Vivo. Int. J. Mol. Sci. 2021, 22, 262. https://doi.org/10.3390/ijms22010262
Onai N, Ogasawara C. Calcium Pyrophosphate Dihydrate Crystals Increase the Granulocyte/Monocyte Progenitor (GMP) and Enhance Granulocyte and Monocyte Differentiation In Vivo. International Journal of Molecular Sciences. 2021; 22(1):262. https://doi.org/10.3390/ijms22010262
Chicago/Turabian StyleOnai, Nobuyuki, and Chie Ogasawara. 2021. "Calcium Pyrophosphate Dihydrate Crystals Increase the Granulocyte/Monocyte Progenitor (GMP) and Enhance Granulocyte and Monocyte Differentiation In Vivo" International Journal of Molecular Sciences 22, no. 1: 262. https://doi.org/10.3390/ijms22010262
APA StyleOnai, N., & Ogasawara, C. (2021). Calcium Pyrophosphate Dihydrate Crystals Increase the Granulocyte/Monocyte Progenitor (GMP) and Enhance Granulocyte and Monocyte Differentiation In Vivo. International Journal of Molecular Sciences, 22(1), 262. https://doi.org/10.3390/ijms22010262






