Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory
Abstract
1. Introduction
2. Results
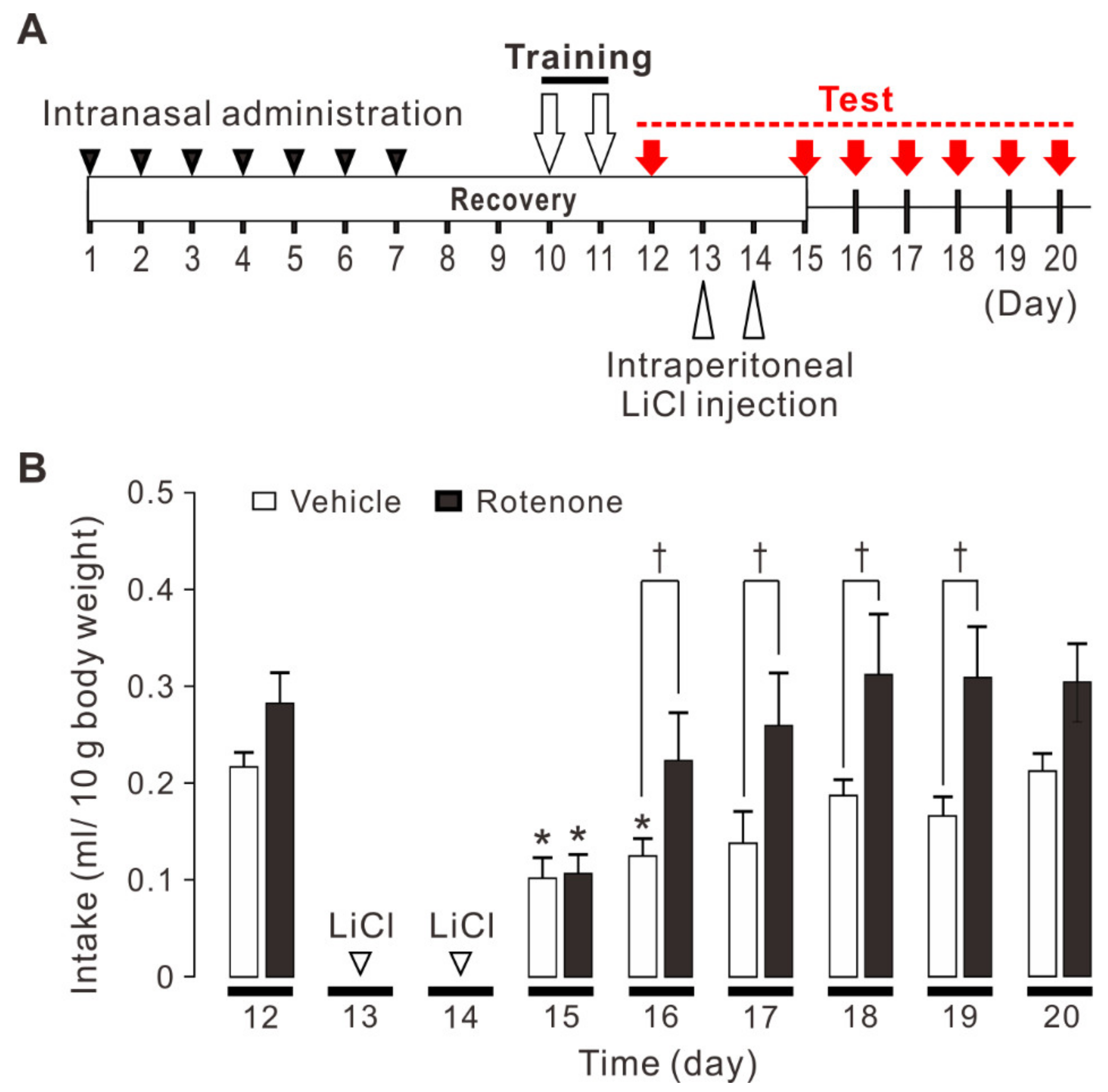
2.1. Conditioned Taste Aversion Memory is Impaired in Rotenone-Treated Mice
2.2. Intranasal Administration of Rotenone Impairs LTD but Not LTP in Layer V Pyramidal Neurons of the Mouse Insular Cortex
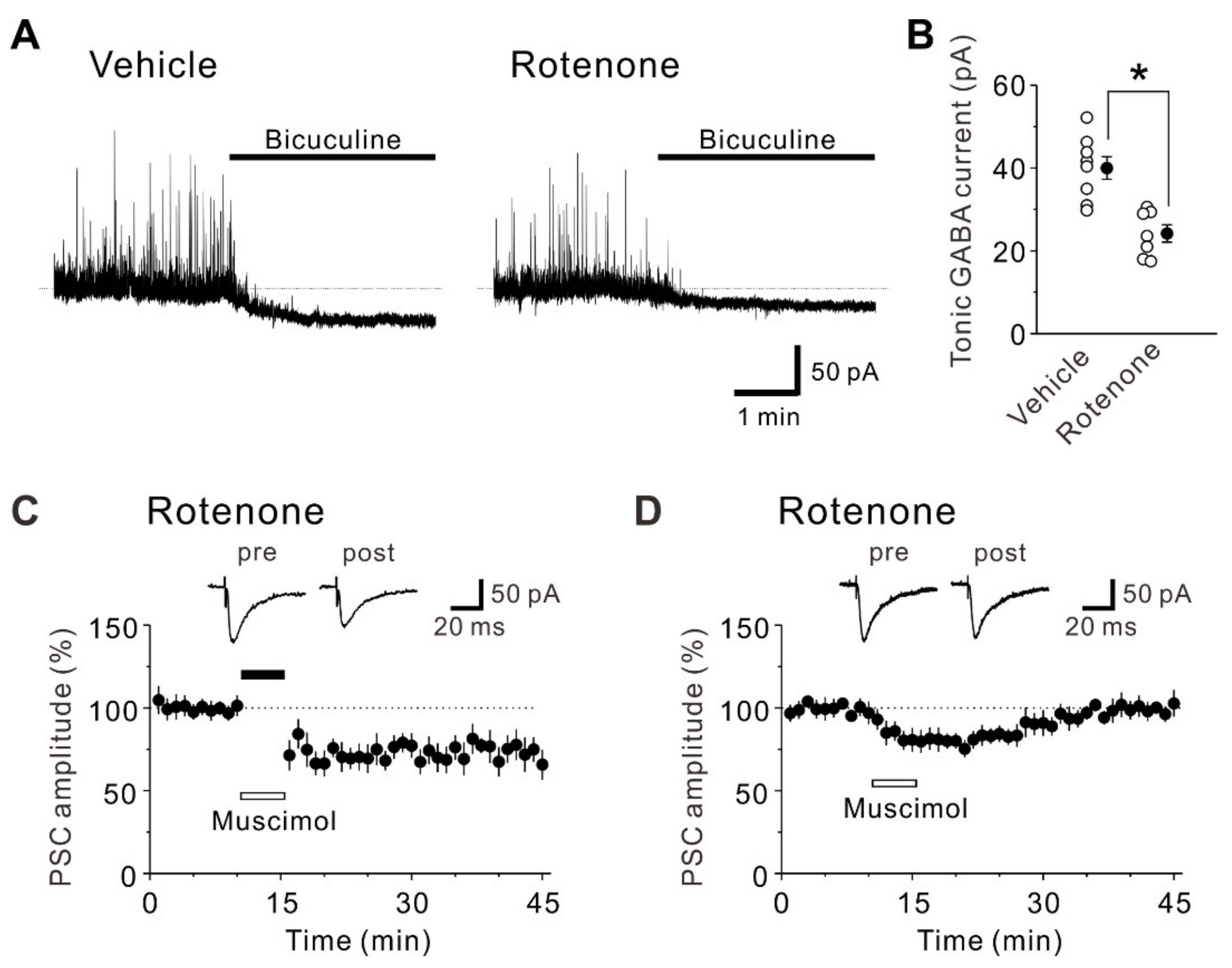
2.3. Intranasal Administration of Rotenone Affects Inhibitory Synaptic Transmission
2.4. The Intranasal Administration of Rotenone Reduces Tonic GABA Currents and Activation of GABAA Receptors Restores LTD in Rotenone-Treated Mice
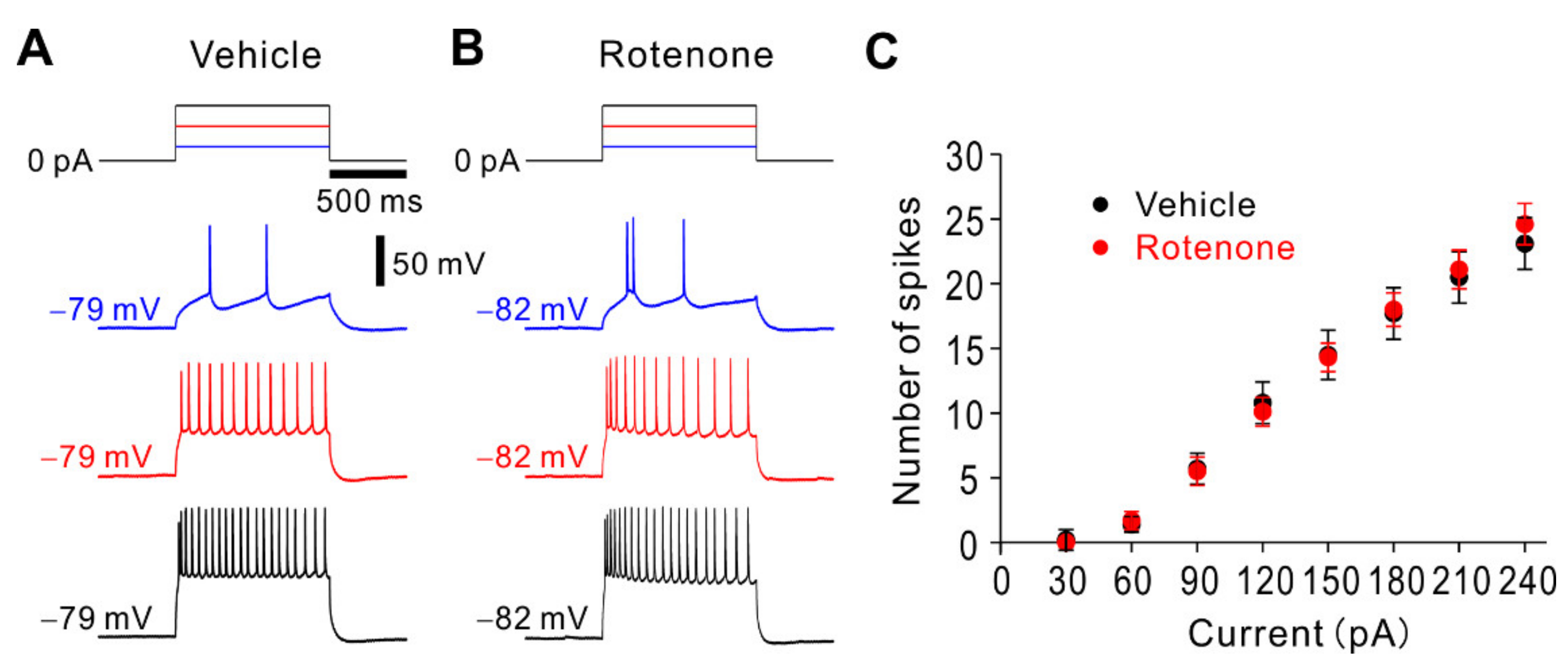
2.5. Intranasal Administration of Rotenone Has no Effect on Spike Firing Properties
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Conditioned Taste Aversion Test
4.3. Slice Preparation
4.4. Whole-Cell Patch-Clamp Recordings
4.5. Induction Protocol for Synaptic Plasticity
4.6. Drug Application for Whole-Cell Patch-Clamp Recordings
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCSF | Artificial cerebrospinal fluid |
| CS | Conditioned stimulus |
| DMSO | Dimethyl sulfoxide |
| EPSC | Excitatory postsynaptic current |
| GABAAR | GABAA receptor |
| IPSC | Inhibitory postsynaptic current |
| IPSP | Inhibitory postsynaptic potential |
| LSD | Least significant difference |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| NMDA | N-methyl-D-aspartate |
| nNOS | Neuronal nitric oxidase synthase |
| PD | Parkinson’s disease |
| PCS | Post synaptic currents |
| QHCl | Quinine hydrochloride |
| US | Unconditioned stimulus |
References
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Talpade, D.J.; Greene, J.G.; Higgins, D.S.; Greenamyre, J.T. In Vivo Labeling of Mitochondrial Complex I (NADH:UbiquinoneOxidoreductase) in Rat Brain Using [3H]Dihydrorotenone. J. Neurochem. 2008, 75, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Schuler, F.; Casida, J.E. Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim. Biophys. Acta Bioenerg. 2001, 1506, 79–87. [Google Scholar] [CrossRef]
- Alam, M.; Schmidt, W. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002, 136, 317–324. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Bobrovskaya, L. An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 2015, 46, 101–116. [Google Scholar] [CrossRef]
- Radad, K.; Al-Shraim, M.; Al-Emam, A.; Wang, F.; Kranner, B.; Rausch, W.-D.; Moldzio, R. Rotenone: From modelling to implication in Parkinson’s disease. Folia Neuropathol. 2018, 57, 317–326. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Féger, J.; Prigent, A.; Michel, P.P.; Parain, K.; Champy, P.; Ruberg, M.; Oertel, W.; Hirsch, E.C. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 2003, 84, 491–502. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Genter, M.B.; Kendig, E.L.; Knutson, M.D. Uptake of materials from the nasal cavity into the blood and brain: Are we finally beginning to understand these processes at the molecular level? Ann. N. Y. Acad. Sci. 2009, 1170, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, H.; Miyazono, S.; Noguchi, T.; Kashiwayanagi, M. Intranasal administration of rotenone in mice attenuated olfactory functions through the lesion of dopaminergic neurons in the olfactory bulb. NeuroToxicology 2015, 51, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, H.; Miyazono, S.; Noguchi, T.; Kashiwayanagi, M. Intranasal Administration of Rotenone to Mice Induces Dopaminergic Neurite Degeneration of Dopaminergic Neurons in the Substantia Nigra. Biol. Pharm. Bull. 2017, 40, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R. The insular cortex: A review. Prog. Brain Res. 2012, 195, 123–163. [Google Scholar]
- Yamamoto, T. Cortical Organization in Gustatory Perception. Ann. N. Y. Acad. Sci. 1987, 510, 49–54. [Google Scholar] [CrossRef]
- Zhuo, M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience 2016, 338, 220–229. [Google Scholar] [CrossRef]
- Bermúdez-Rattoni, F.; Ramírez-Lugo, L.; Gutierrez, R.; Miranda, M.-I. Molecular signals into the insular cortex and amygdala during aversive gustatory memory formation. Cell. Mol. Neurobiol. 2004, 24, 25–36. [Google Scholar] [CrossRef]
- Elkobi, A.; Ehrlich, I.; Belelovsky, K.; Barki-Harrington, L.; Rosenblum, K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat. Neurosci. 2008, 11, 1149–1151. [Google Scholar] [CrossRef]
- Escobar, M.L.; Alcocer, I.; Chao, V. The NMDA receptor antagonist CPP impairs conditioned taste aversion and insular cortex long-term potentiation in vivo. Brain Res. 1998, 812, 246–251. [Google Scholar] [CrossRef]
- Kiefer, S.W.; Leach, L.R.; Braun, J.J. Taste agnosia following gustatory neocortex ablation: Dissociation from odor and generality across taste qualities. Behav. Neurosci. 1984, 98, 590–608. [Google Scholar] [CrossRef]
- Yiannakas, A.; Rosenblum, K. The Insula and Taste Learning. Front. Mol. Neurosci. 2017, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.L.; Bermúdez-Rattoni, F. Long-term potentiation in the insular cortex enhances conditioned taste aversion retention. Brain Res. 2000, 852, 208–212. [Google Scholar] [CrossRef]
- Kimura, R.; Ma, L.-Y.; Wu, C.; Turner, D.; Shen, J.-X.; Ellsworth, K.; Wakui, M.; Maalouf, M.; Wu, J. Acute Exposure to the Mitochondrial Complex I Toxin Rotenone Impairs Synaptic Long-Term Potentiation in Rat Hippocampal Slices. CNS Neurosci. Ther. 2012, 18, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.L. Taste aversion learning: A contemporary perspective. Nutrition 1999, 15, 229–234. [Google Scholar] [CrossRef]
- Yamamoto, T. Neural mechanisms of taste aversion learning. Neurosci. Res. 1993, 16, 181–185. [Google Scholar] [CrossRef]
- Sato, H.; Kawano, T.; Yin, D.X.; Kato, T.; Toyoda, H. Nicotinic activity depresses synaptic potentiation in layer V pyramidal neurons of mouse insular cortex. Neuroscience 2017, 358, 13–27. [Google Scholar] [CrossRef]
- Toyoda, H.; Zhao, M.-G.; Zhuo, M. Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. Eur. J. Neurosci. 2005, 22, 485–494. [Google Scholar] [CrossRef]
- Sharma, N.; Jamwal, S.; Kumar, P. Beneficial effect of antidepressants against rotenone induced Parkinsonism like symptoms in rats. Pathophysiology 2016, 23, 123–134. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, N.; Chergui, K. The GABAA receptor agonist muscimol induces an age- and region-dependent form of long-term depression in the mouse striatum. Learn. Mem. 2016, 23, 479–485. [Google Scholar] [CrossRef]
- Huang, C.-W.; Lin, K.-M.; Hung, T.-Y.; Chuang, Y.-C.; Wu, S.-N. Multiple Actions of Rotenone, an Inhibitor of Mitochondrial Respiratory Chain, on Ionic Currents and Miniature End-Plate Potential in Mouse Hippocampal (mHippoE-14) Neurons. Cell. Physiol. Biochem. 2018, 47, 330–343. [Google Scholar] [CrossRef]
- Yee, A.G.; Freestone, P.S.; Bai, J.-Z.; Lipski, J. Paradoxical lower sensitivity of Locus Coeruleus than Substantia Nigra pars compacta neurons to acute actions of rotenone. Exp. Neurol. 2017, 287, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Accolla, R.; Bathellier, B.; Petersen, C.C.H.; Carleton, A. Differential Spatial Representation of Taste Modalities in the Rat Gustatory Cortex. J. Neurosci. 2007, 27, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective Effect of Lycopene on Oxidative Stress and Cognitive Decline in Rotenone Induced Model of Parkinson’s Disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Castro, M.A.; Jose, E.A.; Delattre, A.M.; Dombrowski, P.A.; Da Cunha, C.; Ferraz, A.C.; Lima, M.M. REM sleep deprivation generates cognitive and neurochemical disruptions in the intranigral rotenone model of Parkinson’s disease. J. Neurosci. Res. 2013, 91, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Song, N.; Zhao, C.; Xie, J.; Jiang, H. Unexpected Improvements of Spatial Learning and Memory Abilities in Chronic Rotenone Intoxicated Mice. PLoS ONE 2014, 9, e91641. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Belcastro, V.; Tozzi, A.; Di Filippo, M.; Tantucci, M.; Siliquini, S.; Autuori, A.; Picconi, B.; Spillantini, M.G.; Fedele, E.; et al. Electrophysiology and Pharmacology of Striatal Neuronal Dysfunction Induced by Mitochondrial Complex I Inhibition. J. Neurosci. 2008, 28, 8040–8052. [Google Scholar] [CrossRef]
- Leng, A.; Feldon, J.; Ferger, B. Rotenone increases glutamate-induced dopamine release but does not affect hydroxyl-free radical formation in rat striatum. Synapse 2003, 50, 240–250. [Google Scholar] [CrossRef]
- Moussa, C.; Rusnák, M.; Hailu, A.; Sidhu, A.; Fricke, S.T. Alterations of striatal glutamate transmission in rotenone-treated mice: MRI/MRS in vivo studies. Exp. Neurol. 2008, 209, 224–233. [Google Scholar] [CrossRef][Green Version]
- Ren, Y.; Feng, J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J. Neurochem. 2007, 103, 303–311. [Google Scholar] [CrossRef]
- Liu, S.; Plachez, C.; Shao, Z.; Puche, A.; Shipley, M.T. Olfactory Bulb Short Axon Cell Release of GABA and Dopamine Produces a Temporally Biphasic Inhibition-Excitation Response in External Tufted Cells. J. Neurosci. 2013, 33, 2916–2926. [Google Scholar] [CrossRef]
- Ohara, P.T.; Granato, A.; Moallem, T.M.; Wang, B.-R.; Tillet, Y.; Jasmin, L. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J. Neurocytol. 2003, 32, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Bloch, B.; Moine, C. D1 and D2 Receptor Gene Expression in the Rat Frontal Cortex: Cellular Localization in Different Classes of Efferent Neurons. Eur. J. Neurosci. 1995, 7, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Richfield, E.K.; Young, A.B.; Penney, J.B. Comparative distributions of dopamine D-1 and D-2 receptors in the cerebral cortex of rats, cats, and monkeys. J. Comp. Neurol. 1989, 286, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Zagoura, D.; Canovas-Jorda, D.; Pistollato, F.; Bremer-Hoffmann, S.; Bal-Price, A. Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem. Int. 2017, 106, 62–73. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Imam, S.Z.; Dong, Z.; Jankovic, J.; Ali, S.F.; Appel, S.H.; Le, W. Role of nitric oxide in rotenone-induced nigro-striatal injury. J. Neurochem. 2003, 86, 1338–1345. [Google Scholar] [CrossRef]
- Choi, S.; Won, J.S.; Carroll, S.L.; Annamalai, B.; Singh, I.; Singh, A.K. Pathology of nNOS-Expressing GABAergic Neurons in Mouse Model of Alzheimer’s Disease. Neuroscience 2018, 384, 41–53. [Google Scholar] [CrossRef]
- Jones, M.W.; French, P.; Bliss, T.V.P.; Rosenblum, K. Molecular Mechanisms of Long-Term Potentiation in the Insular Cortex In Vivo. J. Neurosci. 1999, 19, RC36. [Google Scholar] [CrossRef]
- Rodríguez-Durán, L.F.; Martínez-Moreno, A.; Escobar, M.L. Bidirectional modulation of taste aversion extinction by insular cortex LTP and LTD. Neurobiol. Learn. Mem. 2017, 142, 85–90. [Google Scholar] [CrossRef]
- Li, W.-G.; Liu, M.-G.; Deng, S.; Liu, Y.-M.; Shang, L.; Ding, J.; Hsu, T.-T.; Jiang, Q.; Li, Y.; Li, F.; et al. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat. Commun. 2016, 7, 13770. [Google Scholar] [CrossRef]
- Leuner, B.; Falduto, J.; Shors, T.J. Associative Memory Formation Increases the Observation of Dendritic Spines in the Hippocampus. J. Neurosci. 2003, 23, 659–665. [Google Scholar] [CrossRef]
- Roberts, T.F.; Tschida, K.A.; Klein, M.E.; Mooney, R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nat. Cell Biol. 2010, 463, 948–952. [Google Scholar] [CrossRef]
- Rammes, G.; Eder, M.; Dodt, H.-U.; Kochs, E.; Zieglgänsberger, W. Long-term depression in the basolateral amygdala of the mouse involves the activation of interneurons. Neuroscience 2001, 107, 85–97. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Stone, T.W. Induction of a novel form of hippocampal long-term depression by muscimol: Involvement of GABAA but not glutamate receptors. Br. J. Pharmacol. 1995, 115, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Steele, P.M.; Mauk, M.D. Inhibitory Control of LTP and LTD: Stability of Synapse Strength. J. Neurophysiol. 1999, 81, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Naka, A.; Adesnik, H. Inhibitory Circuits in Cortical Layer 5. Front. Neural Circuits 2016, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.E.; Hazvi, S.; Neduva, V.; Dudai, Y. The Role of Identified Neurotransmitter Systems in the Response of Insular Cortex to Unfamiliar Taste: Activation of ERK1–2 and Formation of a Memory Trace. J. Neurosci. 2000, 20, 7017–7023. [Google Scholar] [CrossRef]
- Morón, I.; Ramírez-Lugo, L.; Ballesteros, M.; Gutierrez, R.; Miranda, M.-I.; Gallo, M.; Bermúdez-Rattoni, F. Differential effects of bicuculline and muscimol microinjections into the nucleus basalis magnocellularis in taste and place aversive memory formation. Behav. Brain Res. 2002, 134, 425–431. [Google Scholar] [CrossRef]
- Rodríguez-García, G.; Miranda, M.-I. Opposing Roles of Cholinergic and GABAergic Activity in the Insular Cortex and Nucleus Basalis Magnocellularis during Novel Recognition and Familiar Taste Memory Retrieval. J. Neurosci. 2016, 36, 1879–1889. [Google Scholar] [CrossRef]
- Mura, E.; Taruno, A.; Yagi, M.; Yokota, K.; Hayashi, Y. Innate and acquired tolerance to bitter stimuli in mice. PLoS ONE 2018, 13, e0210032. [Google Scholar] [CrossRef]
- Rivera-Olvera, A.; Rodriguez-Duran, L.F.; Escobar, M.L. Conditioned taste aversion prevents the long-lasting BDNF-induced enhancement of synaptic transmission in the insular cortex: A metaplastic effect. Neurobiol. Learn. Mem. 2016, 130, 71–76. [Google Scholar] [CrossRef]
- Rodríguez-Durán, L.F.; Castillo, D.V.; Moguel-González, M.; Escobar, M.L. Conditioned taste aversion modifies persistently the subsequent induction of neocortical long-term potentiation in vivo. Neurobiol. Learn. Mem. 2011, 95, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Saito, M.; Sato, H.; Tanaka, T.; Ogawa, T.; Yatani, H.; Kawano, T.; Kanematsu, T.; Hirata, M.; Kang, Y. Enhanced desensitization followed by unusual resensitization in GABAA receptors in phospholipase C-related catalytically inactive protein-1/2 double-knockout mice. Pflugers Arch. 2015, 467, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-G.; Toyoda, H.; Lee, Y.-S.; Wu, L.-J.; Ko, S.W.; Zhang, X.-H.; Jia, Y.; Shum, F.; Xu, H.; Li, B.-M.; et al. Roles of NMDA NR2B Subtype Receptor in Prefrontal Long-Term Potentiation and Contextual Fear Memory. Neuron 2005, 47, 859–872. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyoda, H.; Katagiri, A.; Kato, T.; Sato, H. Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory. Int. J. Mol. Sci. 2021, 22, 259. https://doi.org/10.3390/ijms22010259
Toyoda H, Katagiri A, Kato T, Sato H. Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory. International Journal of Molecular Sciences. 2021; 22(1):259. https://doi.org/10.3390/ijms22010259
Chicago/Turabian StyleToyoda, Hiroki, Ayano Katagiri, Takafumi Kato, and Hajime Sato. 2021. "Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory" International Journal of Molecular Sciences 22, no. 1: 259. https://doi.org/10.3390/ijms22010259
APA StyleToyoda, H., Katagiri, A., Kato, T., & Sato, H. (2021). Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory. International Journal of Molecular Sciences, 22(1), 259. https://doi.org/10.3390/ijms22010259





