Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
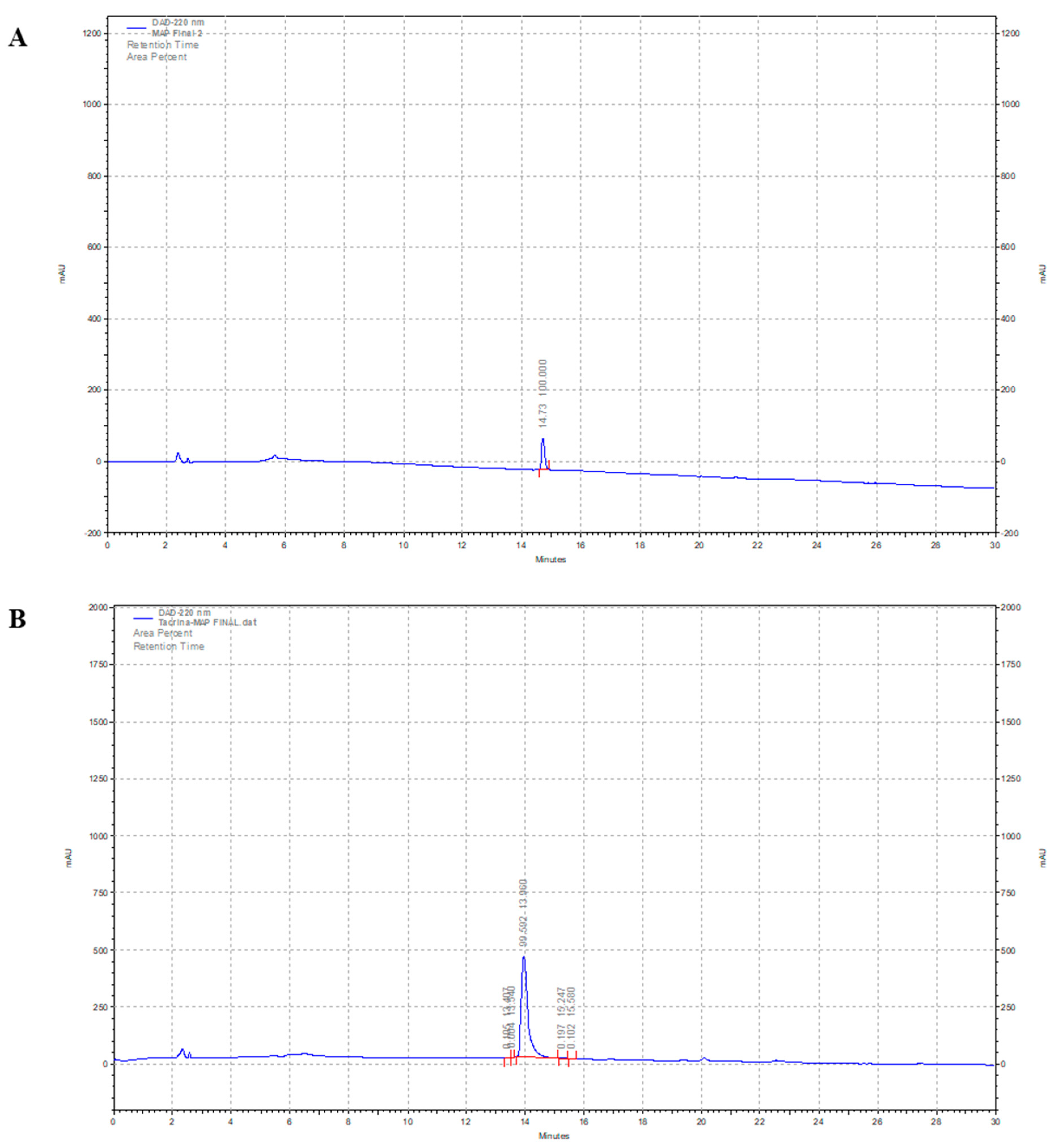
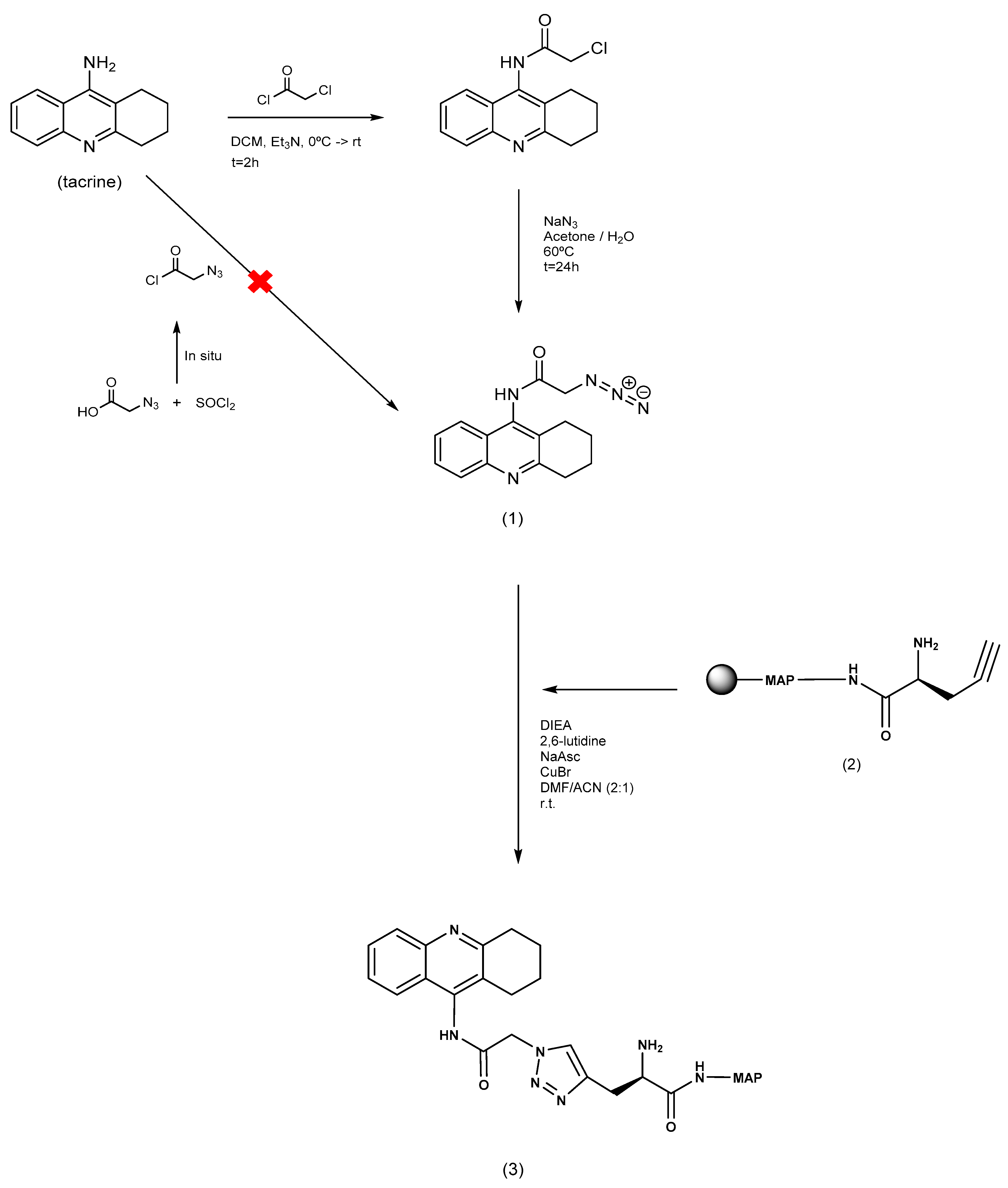
2.1. Chemical Synthesis of MAP and Its Tacrine Conjugate
2.2. Permeability Assays
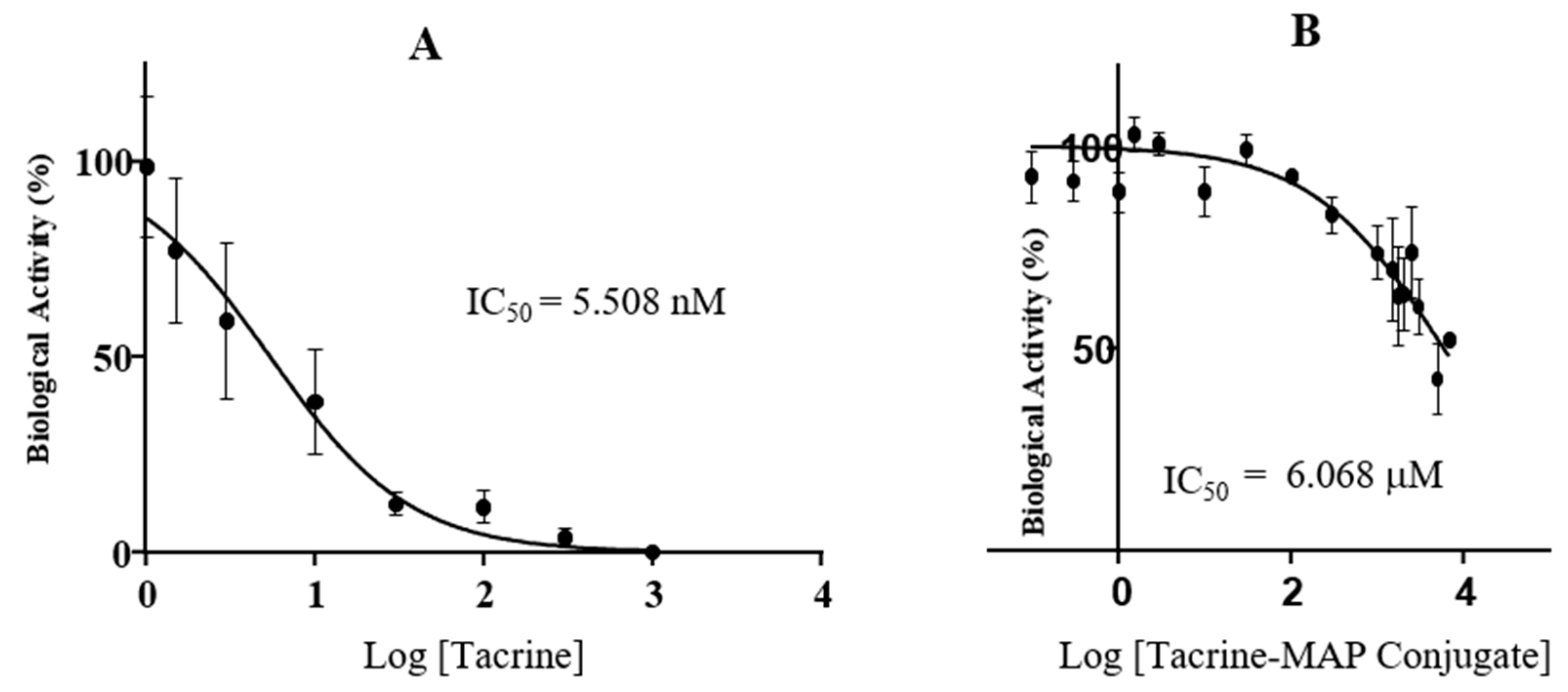
2.3. AChE In Vitro Inhibition Assay
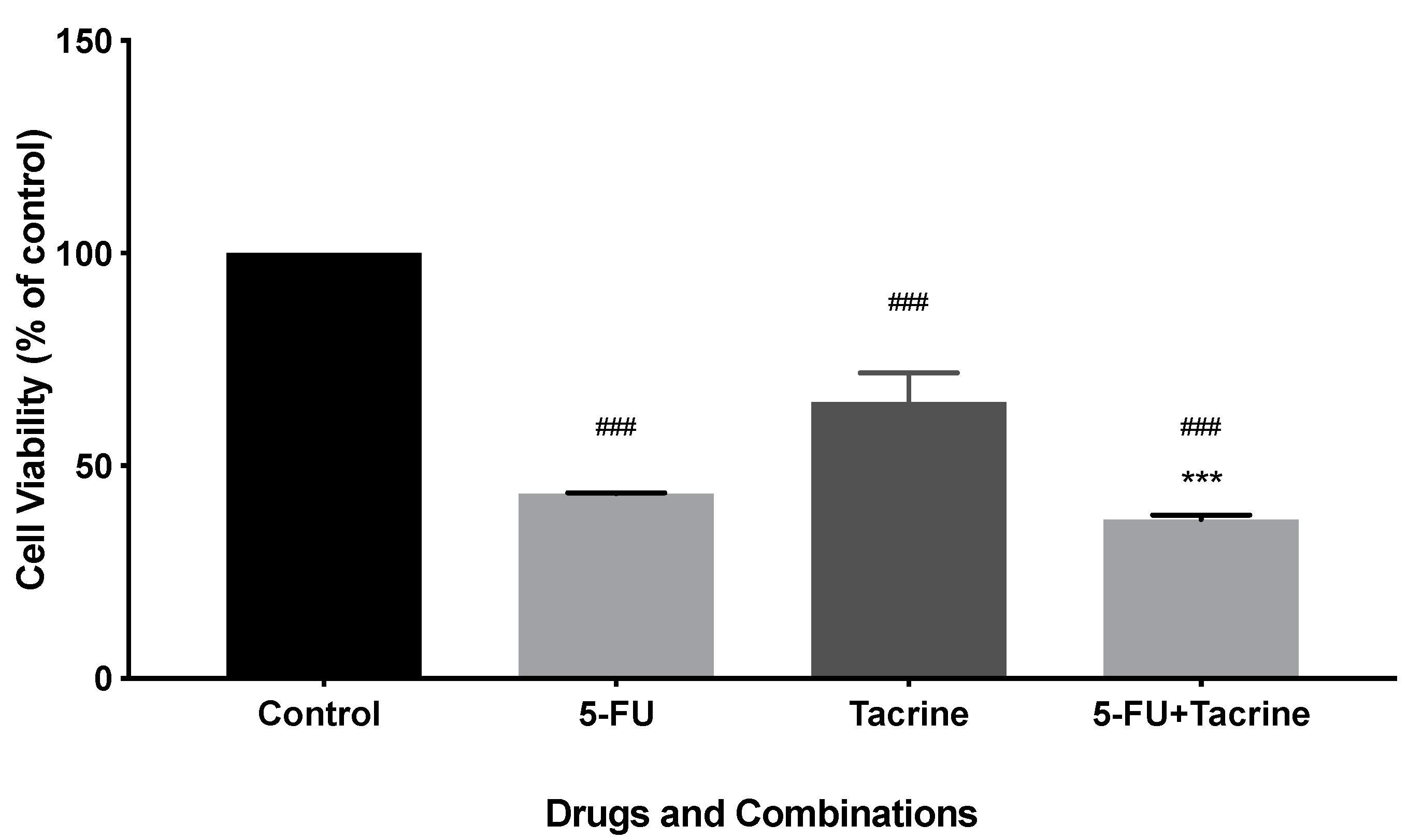
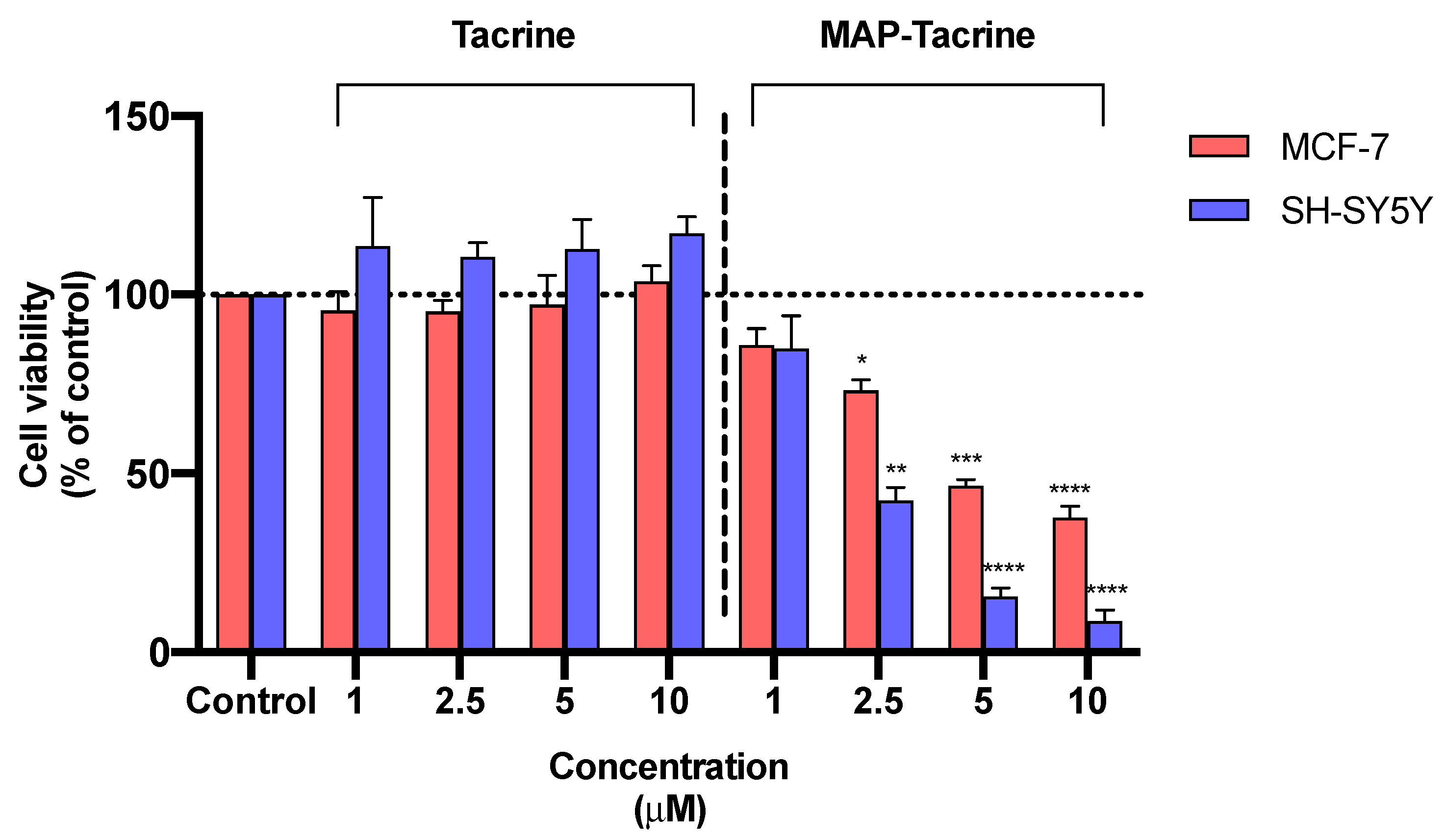
2.4. Toxicity of Tacrine and Its MAP Conjugate to MCF-7 and SH-SY5Y Cells
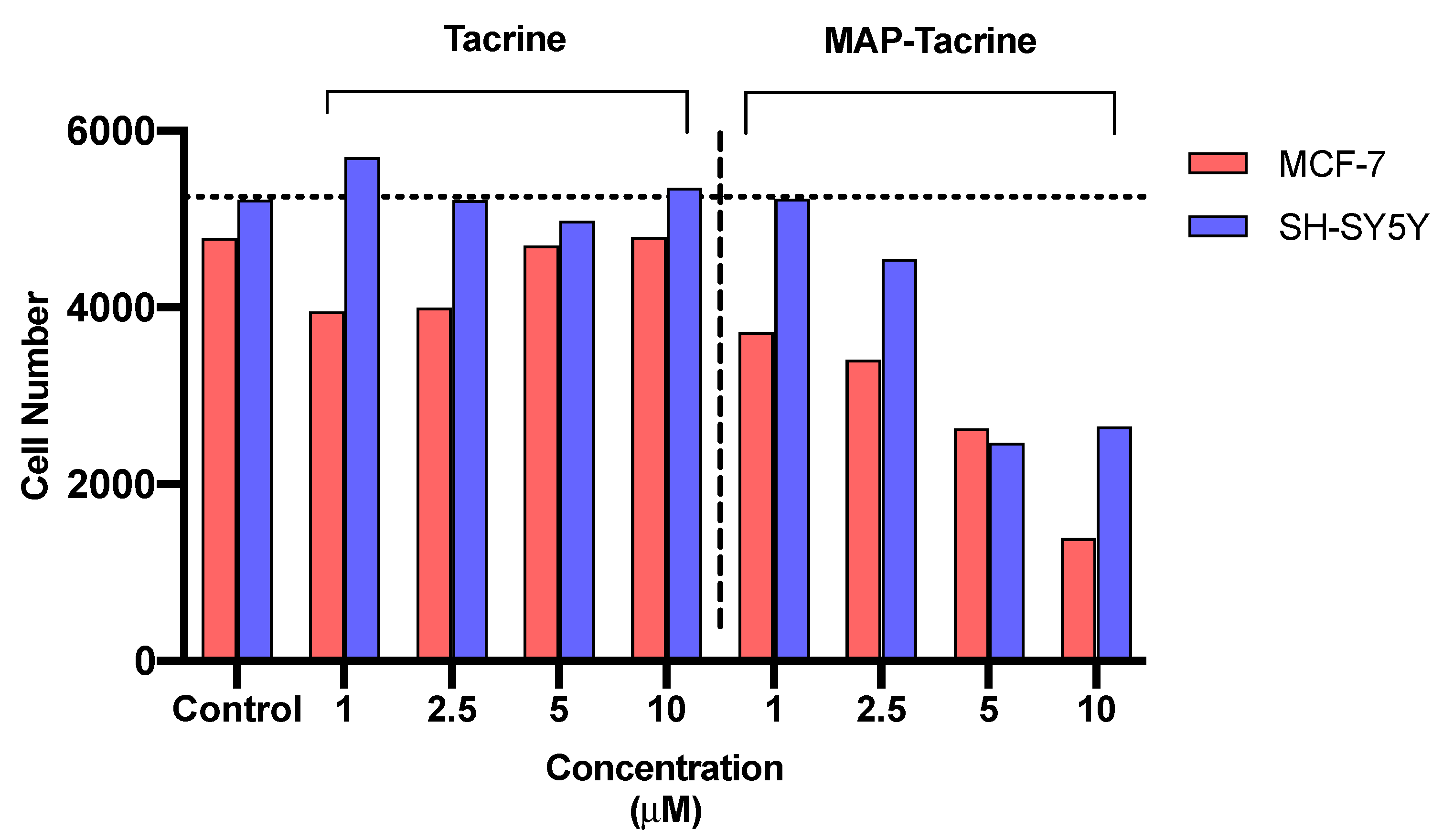
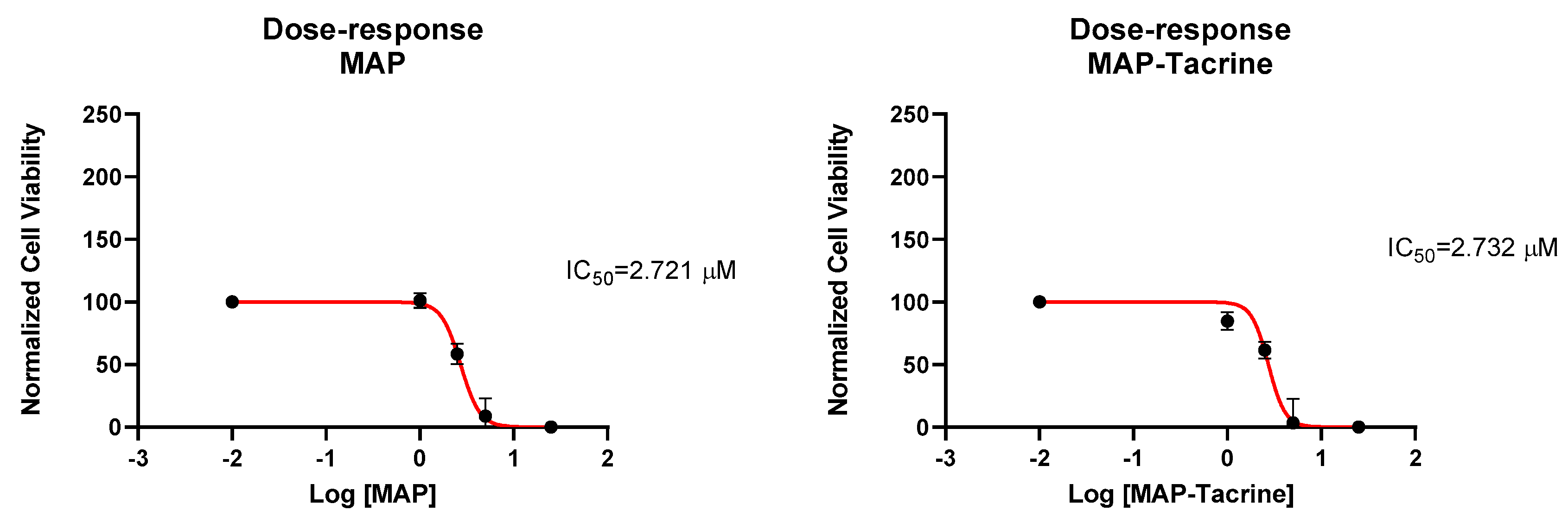
2.5. Determination of IC50 and Cell Count Analysis of Tacrine, MAP, and Tacrine-MAP against MCF-7
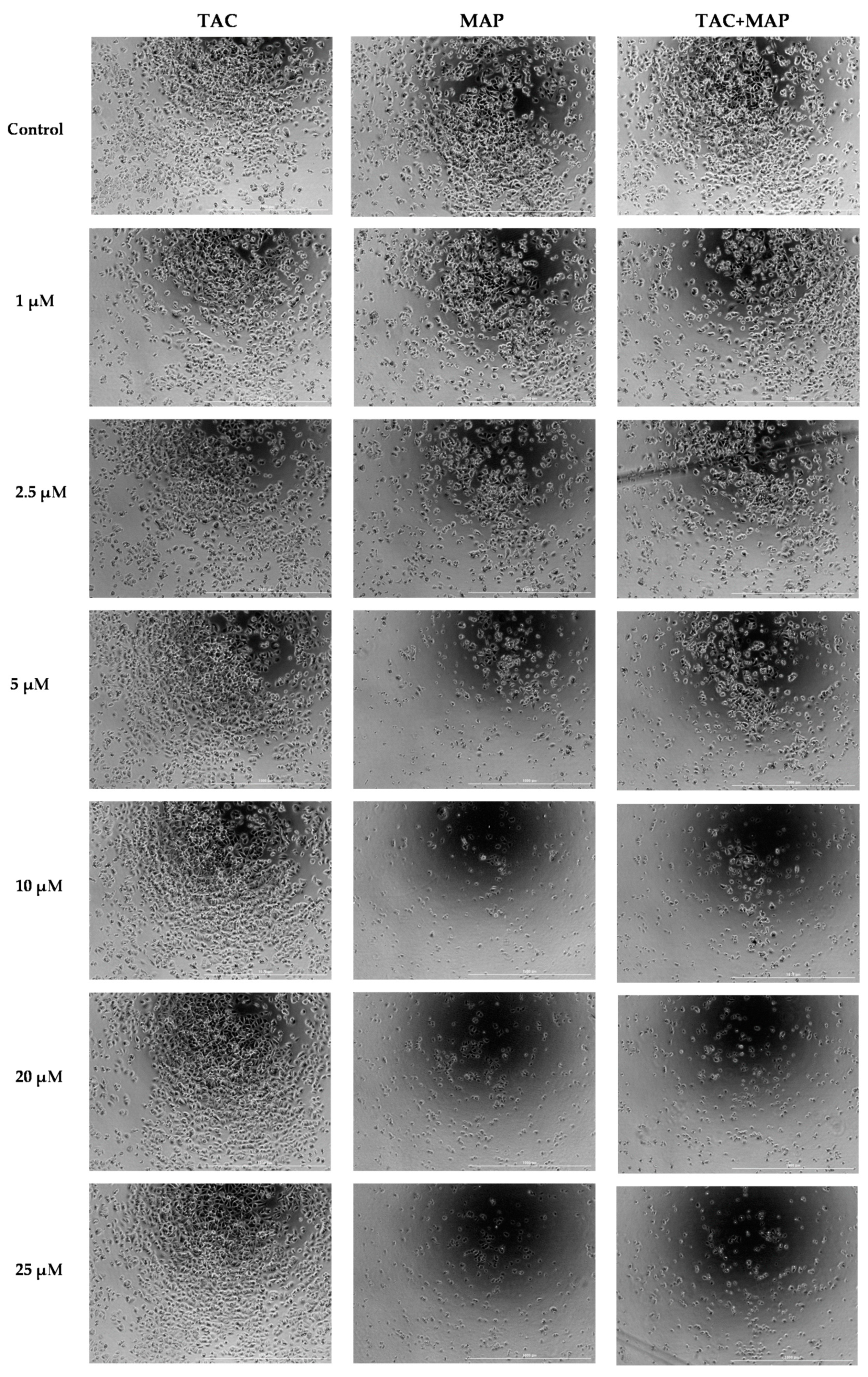
2.6. Morphological Changes Caused by Tacrine, MAP, and Tacrine-MAP on MCF-7 Cells
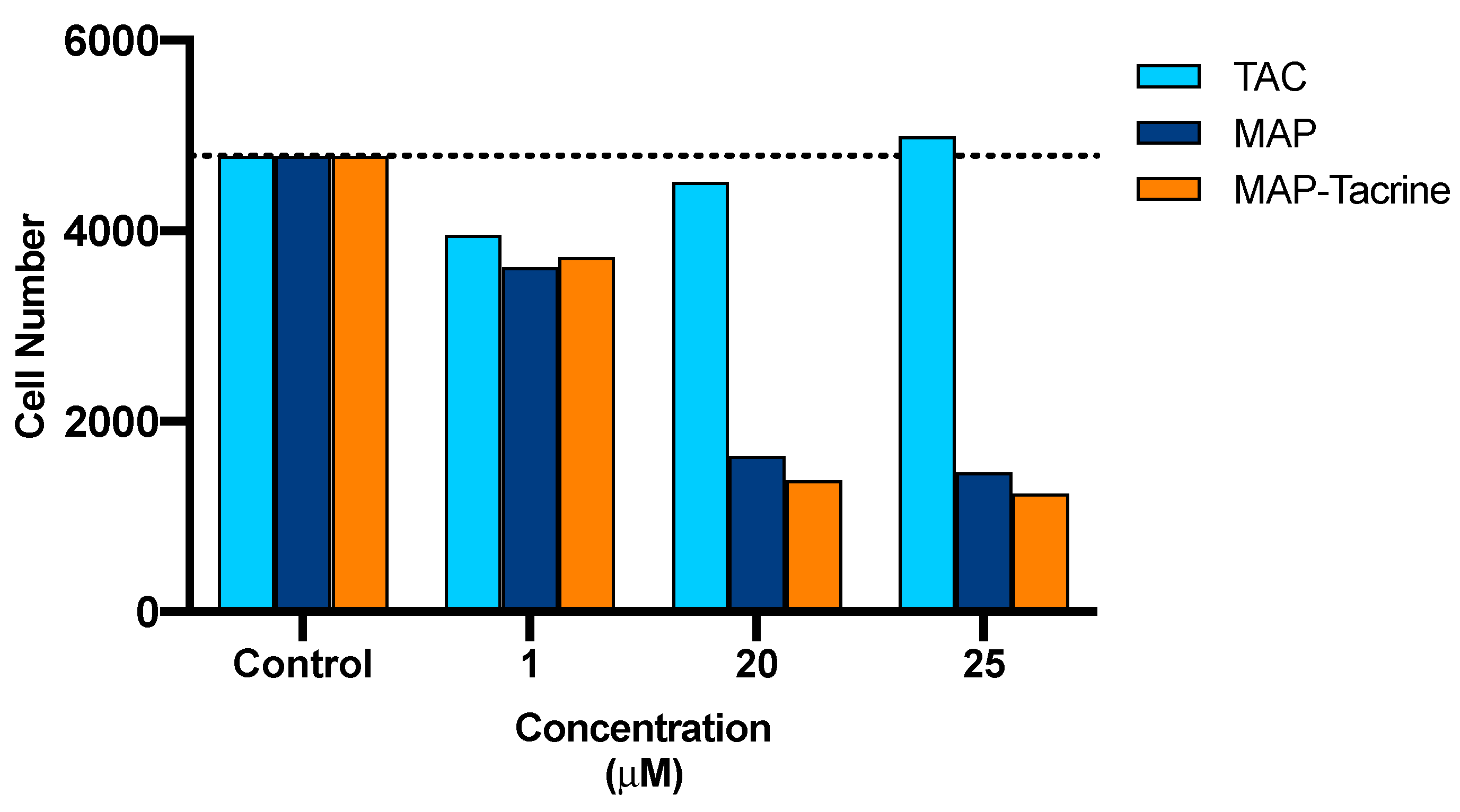
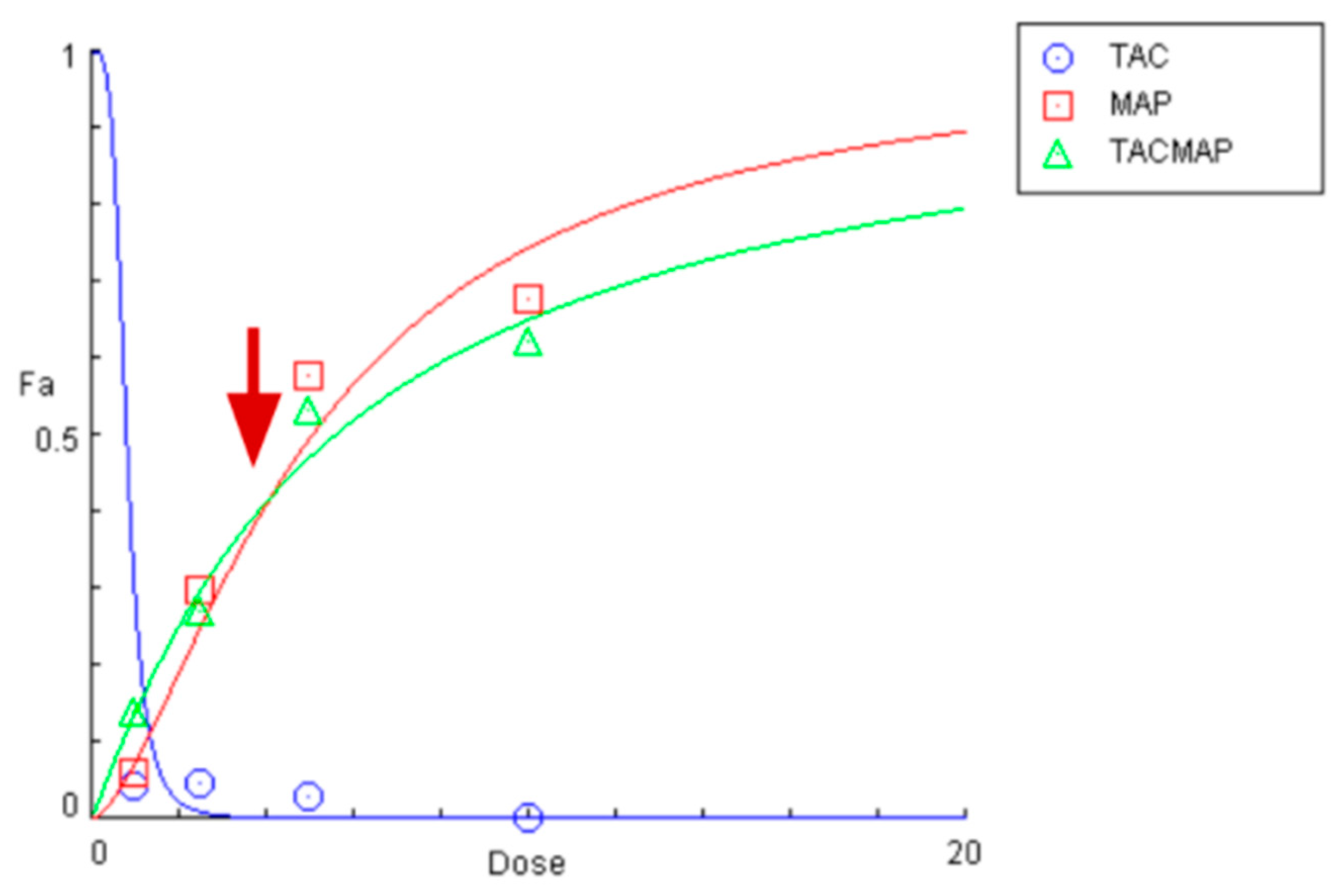
2.7. Drug Interactions between Tacrine and MAP in the Conjugate
3. Concluding Remarks
4. Materials and Methods
4.1. MAP and Conjugate Synthesis
4.1.1. General Peptide Synthesis Procedure
4.1.2. General Analysis Procedure
4.2. Cell Culture
4.2.1. Materials
4.2.2. Cell Culture Conditions
4.3. Permeability Studies
4.4. Cytotoxicity Assays
MTT Reduction Assay
4.5. Ellman’s Method
4.6. Cell Morphology Visualization and Cell Count Analysis
4.7. Analysis of Drug Interactions
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| AChE | Acetylcholinesterase |
| ACN | Acetonitrile |
| AD | Alzheimer Disease |
| AMP | Antimicrobial peptide |
| BBB | Blood brain barrier |
| CPP | cell penetrating peptide |
| CuAAC | Copper-catalyzed azide−alkyne coupling |
| DAD | Diode-array detector |
| DIEA | Diisopropylethylamine |
| DMF | N,N-dimethylformamide |
| ESI-IT MS | Electrospray ionization-ion trap mass spectrometry |
| FDA | Food and Drug Administration |
| HOBt | 1-hydroxybenzotriazole |
| HPLC | High performance liquid chromatography |
| LC-MS | Liquid chromatography–mass spectrometry |
| MALDI-TOF/TOF | Matrix assisted desorption/ionization mass spectrometry |
| MAP | model amphipathic peptide |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| MW | Microwave |
| NaAsc | Sodium ascorbate |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| SPPS | solid-phase peptide synthesis |
| TEER | Trans-epithelial electrical resistance |
| TFA | Trifluoroacetic acid |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Crismon, M.L. Tacrine: First drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.J.; Knopman, D.S.; Solomon, P.R.; Pendlebury, W.W.; Davis, C.S.; Gracon, S.I.; Apter, J.T.; Lazarus, C.N.; Baker, K.E.; Barnett, M.; et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA 1994, 271, 985–991. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, S.-L.; Tan, M.-X.; Wang, Z.-F.; Luo, D.-M.; Zou, B.-Q.; Liu, Y.-C.; Yao, P.-F.; Liang, H. Novel tacrine platinum (II) complexes display high anticancer activity via inhibition of telomerase activity, dysfunction of mitochondria, and activation of the p53 signaling pathway. Eur. J. Med. Chem. 2018, 158, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Janockova, J.; Plsikova, J.; Koval, J.; Jendželovský, R.; Mikes, J.; Kasparkova, J.; Brabec, V.; Hamulakova, S.; Fedoročko, P.; Kozurkova, M. Tacrine derivatives as dual topoisomerase I and II catalytic inhibitors. Bioorganic Chem. 2015, 59, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Sabolová, D.; Kristian, P.; Kozurkova, M. Multifunctional properties of novel tacrine congeners: Cholinesterase inhibition and cytotoxic activity. J. Appl. Toxicol. 2018, 38, 1377–1387. [Google Scholar] [CrossRef]
- Janockova, J.; Korábečný, J.; Plsikova, J.; Babkova, K.; Konkolova, E.; Kucerova, D.; Vargova, J.; Koval, J.; Jendzelovsky, R.; Fedorocko, P.; et al. In vitro investigating of anticancer activity of new 7-MEOTA-tacrine heterodimers. J. Enzym. Inhib. Med. Chem. 2019, 34, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Correia, A.; Silva, D.; Correia, A.; Vilanova, M.; Gärtner, F.; Vale, N. Study of new therapeutic strategies to combat breast cancer using drug combinations. Biomolecules 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-penetrating peptides: Design, synthesis, and applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Habault, J.; Poyet, J.-L. Recent advances in cell penetrating peptide-based anticancer therapies. Molecules 2019, 24, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252–37267. [Google Scholar] [CrossRef] [Green Version]
- Oehlke, J.; Scheller, A.; Wiesner, B.; Krause, E.; Beyermann, M.; Klauschenz, E.; Melzig, M.; Bienert, M. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1414, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Oehlke, J.; Wallukat, G.; Wolf, Y.; Ehrlich, A.; Wiesner, B.; Berger, H.; Bienert, M. Enhancement of intracellular concentration and biological activity of PNA after conjugation with a cell-penetrating synthetic model peptide. JBIC J. Biol. Inorg. Chem. 2004, 271, 3043–3049. [Google Scholar] [CrossRef] [PubMed]
- Scheller, A.; Melzig, M.; Oehlke, J. Induction of caspase-8 in human cells by the extracellular administration of peptides containing a C-terminal SLV sequence. Int. J. Pept. Res. Ther. 2001, 8, 29–34. [Google Scholar] [CrossRef]
- Kenien, R.; Shen, W.-C.; Zaro, J.L. Vesicle-to-cytosol transport of disulfide-linked cargo mediated by an amphipathic cell-penetrating peptide. J. Drug Target. 2012, 20, 793–800. [Google Scholar] [CrossRef]
- Hällbrink, M.; Florén, A.; Elmquist, A.; Pooga, M.; Bartfai, T.; Langel, Ü. Cargo delivery kinetics of cell-penetrating peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2001, 1515, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Weksler, B.; Romero, I.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Araújo, A.F.; Gomes, M.J.; Fernandes, C.; Nunes, R.; Li, W.; Santos, H.A.; Borges, F.; Sarmento, B. Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur. J. Pharm. Biopharm. 2019, 138, 111–124. [Google Scholar] [CrossRef]
- Cayero-Otero, M.; Gomes, M.J.; Martins, C.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Sarmento, B.; Martín-Banderas, L. In vivo biodistribution of venlafaxine-PLGA nanoparticles for brain delivery: Plain vs. functionalized nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 1413–1427. [Google Scholar] [CrossRef]
- Presgraves, S.P.; Ahmed, T.; Borwege, S.; Joyce, J.N. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox. Res. 2003, 5, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Ferreira, L.M.; Branco, P.; Fernandes, E.; Bastos, M.D.L.; Carvalho, F. “Ecstasy”-induced toxicity in SH-SY5Y differentiated cells: Role of hyperthermia and metabolites. Arch. Toxicol. 2013, 88, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.J.; Capela, J.P.; Silva, R.; Vilas-Boas, V.; Ferreira, L.M.; Branco, P.; Fernandes, E.; Bastos, M.D.L.; Carvalho, F. The mixture of “ecstasy” and its metabolites is toxic to human SH-SY5Y differentiated cells at in vivo relevant concentrations. Arch. Toxicol. 2013, 88, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Palm-Apergi, C.; Lorents, A.; Padari, K.; Pooga, M. The membrane repair response masks membrane disturbances caused by cell-penetrating peptide uptake. FASEB J. 2008, 23, 214–223. [Google Scholar] [CrossRef]
- Saar, K.; Lindgren, M.; Hansen, M.; Eiríksdóttir, E.; Jiang, Y.; Rosenthal-Aizman, K.; Sassian, M.; Langel, Ü. Cell-penetrating peptides: A comparative membrane toxicity study. Anal. Biochem. 2005, 345, 55–65. [Google Scholar] [CrossRef]
- Pannecouque, C.; Daelemans, D.; De Clercq, E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: Revisited 20 years later. Nat. Protoc. 2008, 3, 427–434. [Google Scholar] [CrossRef]
- Aguiar, L.; Biosca, A.; Lantero, E.; Gut, J.; Vale, N.; Rosenthal, P.J.; Nogueira, F.; Andreu, D.; Fernàndez-Busquets, X.; Gomes, P. Coupling the antimalarial cell penetrating peptide TP10 to classical antimalarial drugs primaquine and chloroquine produces strongly hemolytic conjugates. Molecules 2019, 24, 4559. [Google Scholar] [CrossRef] [Green Version]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Dębowski, D.; Gitlin-Domagalska, A.; Lica, J.; et al. Conjugates of ciprofloxacin and levofloxacin with cell-penetrating peptide exhibit antifungal activity and mammalian cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mao, F.; Chen, J.; Zhou, Q.; Luo, Z.; Huang, L.; Li, X. Novel tacrine-ebselen hybrids with improved cholinesterase inhibitory, hydrogen peroxide and peroxynitrite scavenging activity. Bioorganic Med. Chem. Lett. 2013, 23, 6737–6742. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Alves, C.; Duarte, D.; Costa, A.; Sarmento, B.; Almeida, A.J.; Gomes, P.; Vale, N. Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity. Int. J. Mol. Sci. 2021, 22, 242. https://doi.org/10.3390/ijms22010242
Silva S, Alves C, Duarte D, Costa A, Sarmento B, Almeida AJ, Gomes P, Vale N. Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity. International Journal of Molecular Sciences. 2021; 22(1):242. https://doi.org/10.3390/ijms22010242
Chicago/Turabian StyleSilva, Sara, Cláudia Alves, Diana Duarte, Ana Costa, Bruno Sarmento, António J. Almeida, Paula Gomes, and Nuno Vale. 2021. "Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity" International Journal of Molecular Sciences 22, no. 1: 242. https://doi.org/10.3390/ijms22010242
APA StyleSilva, S., Alves, C., Duarte, D., Costa, A., Sarmento, B., Almeida, A. J., Gomes, P., & Vale, N. (2021). Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity. International Journal of Molecular Sciences, 22(1), 242. https://doi.org/10.3390/ijms22010242








