Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
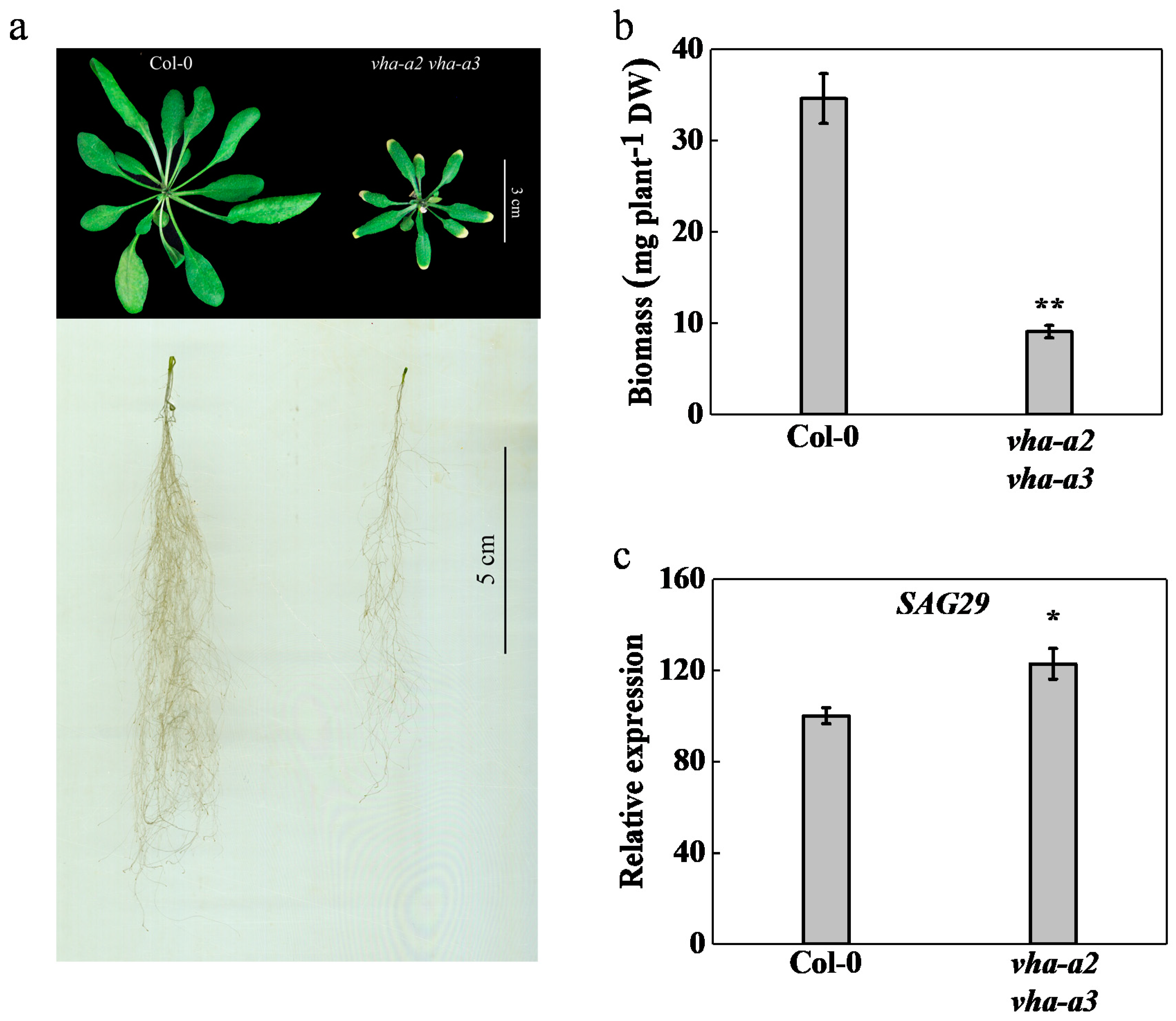
2.1. V-ATPase Activity at the Tonoplast Regulates Growth and Development
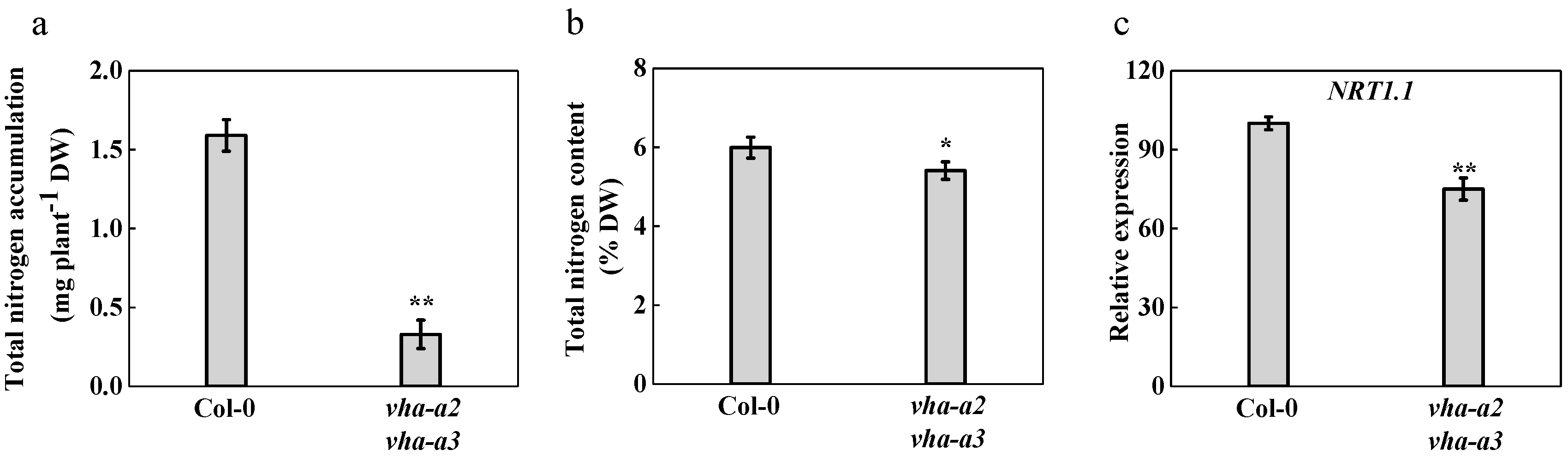
2.2. Loss of Tonoplast V-ATPase Reduces Nitrogen Absorption
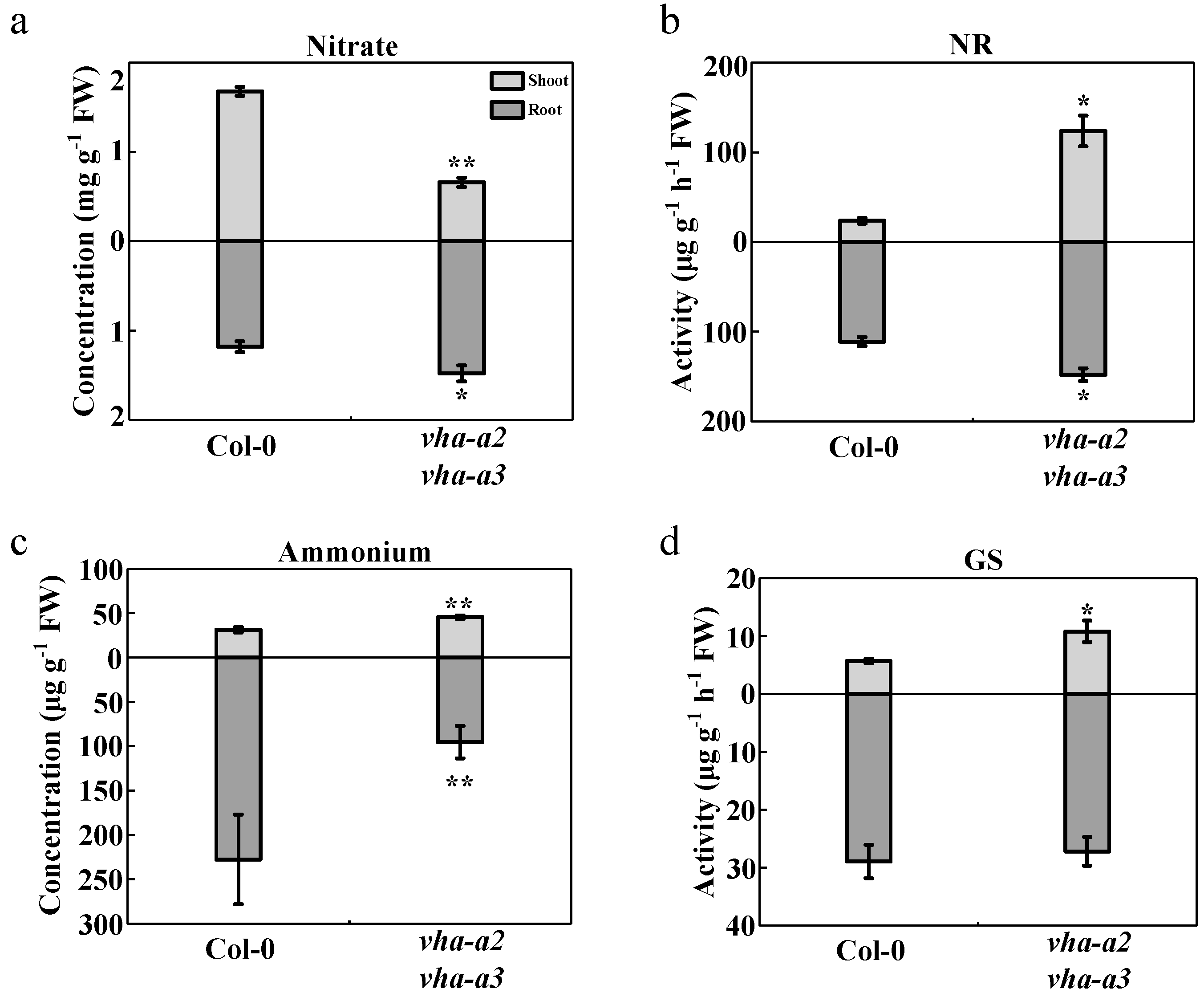
2.3. Disruption of Nitrogen Metabolism Causes Ammonium Accumulation in Shoots of Mutant Plants
2.4. Absence of Tonoplast V-ATPase Affects the Long-Distance Transport of Nitrogen between Shoots and Roots
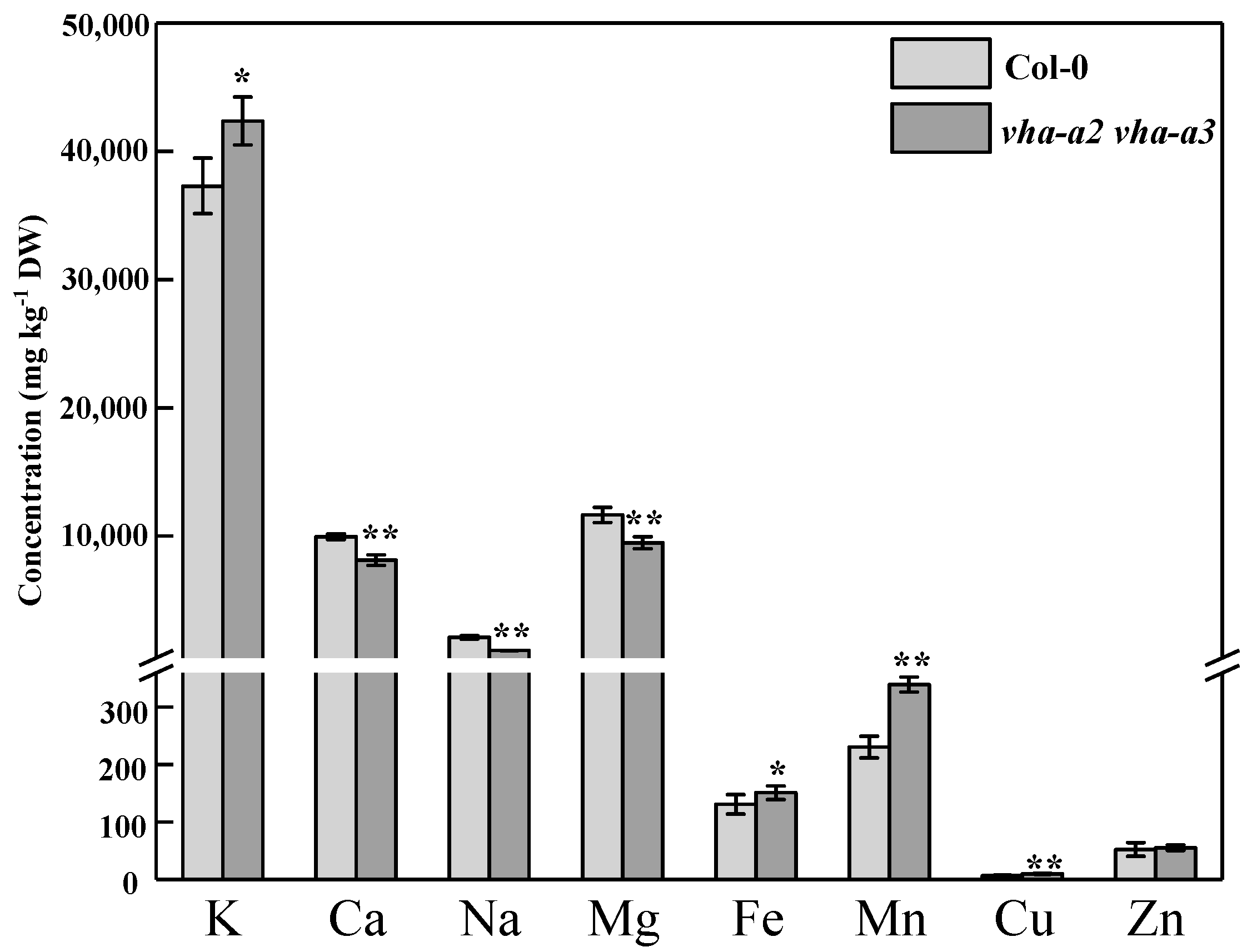
2.5. Impaired Tonoplast V-ATPase Activity Influences Ion Homeostasis
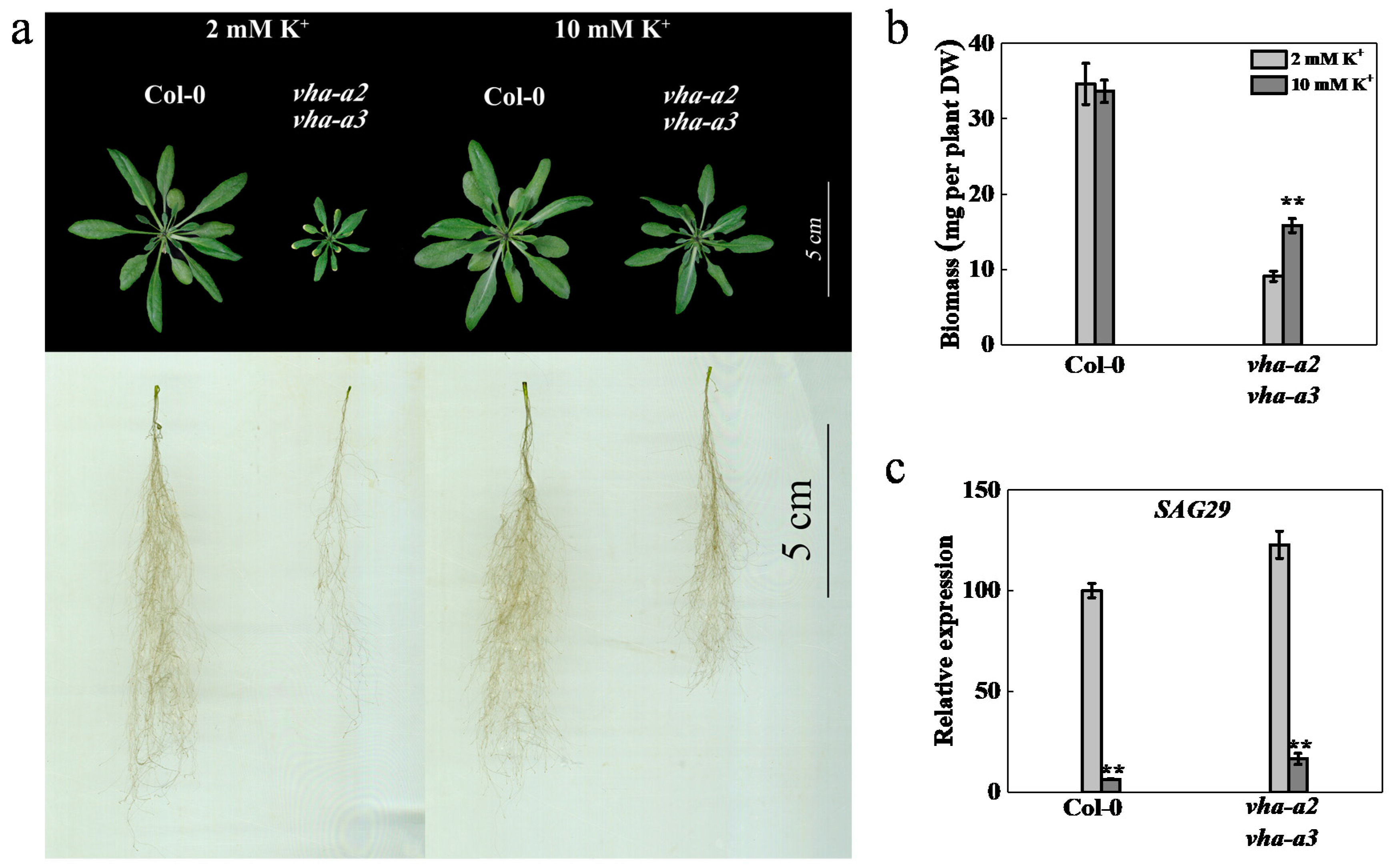
2.6. Growth Retardation is Alleviated and Senescence is Delayed by Supplementing K+ to the Mutant
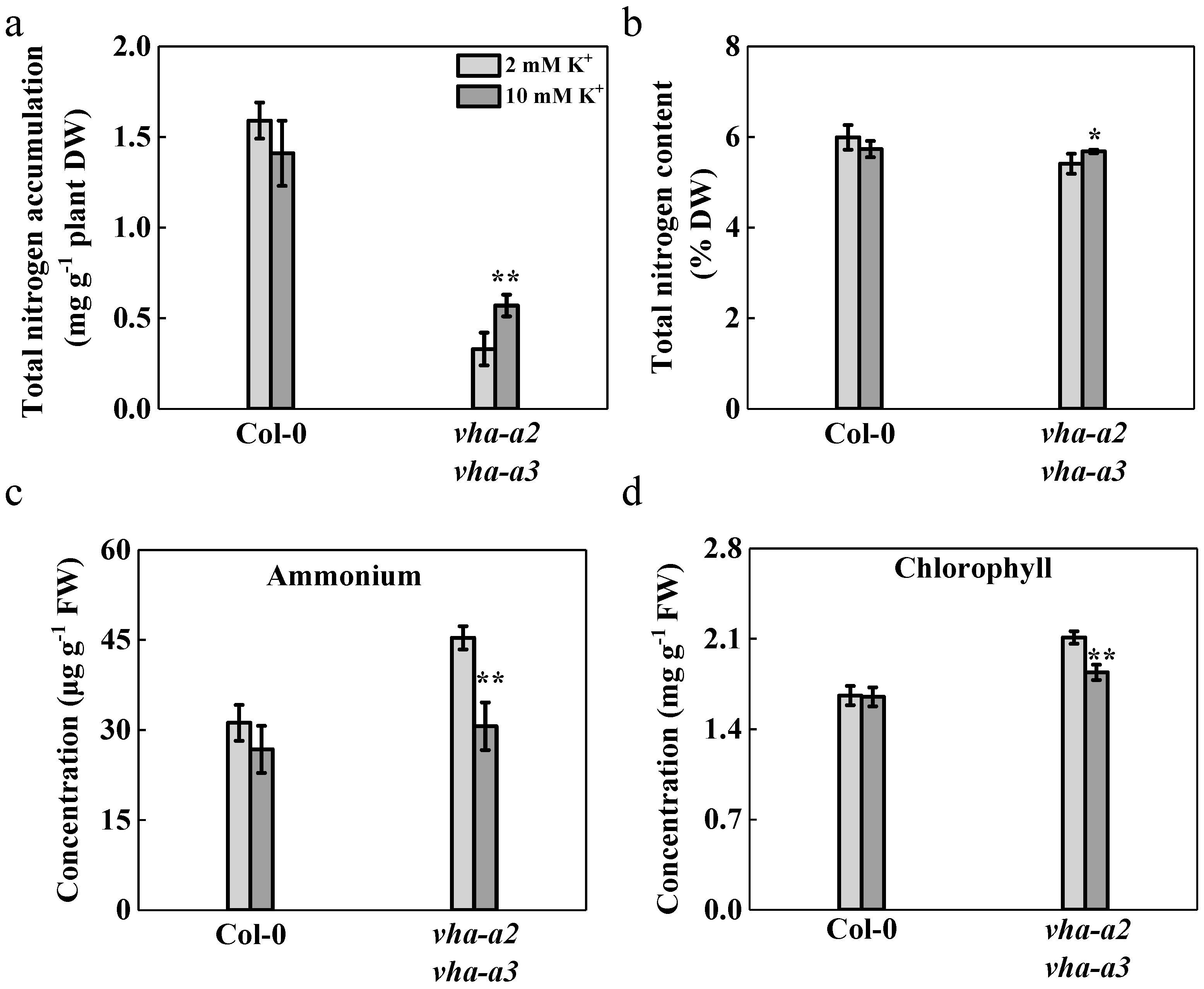
2.7. Nitrogen Absorption is Promoted and Ammonium Accumulation is Reduced with Additional K+ Provision to the Mutant
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of Leaf Rosette Areas
4.3. Measurement of Root Configuration
4.4. Determination of Nitrogen Content
4.5. Assays for NO3− Content Estimation
4.6. Determination of NH4+ Content
4.7. Determination of the Activities of Nitrate Reductase (NR) and Glutamine Synthetase (GS)
4.8. Chlorophyll Content Assays
4.9. RNA Extraction and qRT-PCR Assay
4.10. Determination of Ammonium in Xylem Sap
4.11. Determination of Cation Content
4.12. Statistical Analyses
4.13. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kriegel, A.; Andrés, Z.; Medzihradszky, A.; Krüger, F.; Scholl, S.; Delang, S.; Patir-Nebioglu, M.G.; Gute, G.; Yang, H.; Murphy, A.S.; et al. Job sharing in the endomembrane system: Vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 2015, 27, 3383–3396. [Google Scholar] [CrossRef]
- Liao, X.R.; Chen, T.; Liu, X.L. The formation and function of plant vacuole. Chin. J. Cell Biol. 2002, 24, 95–101. [Google Scholar]
- Shen, Q.R.; Tang, L.; Xu, Y.C. A review on the behavior of nitrate in vacuoles of plants. Acta Pedol. Sin. 2003, 40, 465–470. [Google Scholar]
- Krebs, M.; Beyhl, D.; Görlich, E.; Al-Rasheid, K.A.; Marten, I.; Stierhof, Y.D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef]
- Hedrich, R.; Kurkdjian, A.; Guern, J.; Flugge, U.I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989, 8, 2835–2841. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Tawada, K. Separation of myosin subfragment 1 into two fractions, one having the burst site and the other having the non-burst site. J. Biochem. 1976, 80, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef]
- Schumacher, K. pH in the plant endomembrane system—an import and export business. Curr. Opin. Plant Biol. 2014, 22, 71–76. [Google Scholar] [CrossRef]
- McGuire, C.; Stransky, L.; Cotter, K.; Forgac, M. Regulation of V-ATPase activity. Front Biosci. (Landmark Ed) 2017, 22, 609–622. [Google Scholar]
- Cipriano, D.J.; Wang, Y.; Bond, S.; Hinton, A.; Jefferies, K.C.; Qi, J.; Forgac, M. Structure and regulation of the vacuolar ATPase. Biochim. Biophys. Acta 2008, 1777, 599–604. [Google Scholar] [CrossRef]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.D.; Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006, 18, 715–730. [Google Scholar] [CrossRef] [PubMed]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Jiang, P.; Nie, L.; Chen, X.; Tai, F.; Wang, D.; Fan, P.; Feng, J.; Bao, H.; Wang, J.; et al. H+-pyrophosphatase from Salicornia europaea confers tolerance to simultaneously occurring salt stress and nitrogen deficiency in Arabidopsis and wheat. Plant Cell Environ. 2015, 38, 2433–2449. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Jiang, P.; Tai, F.; Wang, D.; Feng, J.; Fan, P.; Bao, H.; Li, Y. The V ATPase subunit A is essential for salt tolerance through participating in vacuolar Na+ compartmentalization in Salicornia europaea. Planta 2017, 246, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Berezin, I.; Mizrachy-Dagry, T.; Brook, E.; Mizrahi, K.; Elazar, M.; Zhuo, S.; Saul-Tcherkas, V.; Shaul, O. Overexpression of AtMHX in tobacco causes increased sensitivity to Mg2+, Zn2+, and Cd2+ ions, induction of V-ATPase expression, and a reduction in plant size. Plant Cell Rep. 2008, 27, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Hirschi, K.D. CAX-ing a wide net: Cation/H+ transporters in metal remediation and abiotic stress signalling. Plant Biol. 2016, 18, 741–749. [Google Scholar] [CrossRef]
- Dietz, K.J.; Tavakoli, N.; Kluge, C.; Mimura, T.; Sharma, S.S.; Harris, G.C.; Chardonnens, A.N.; Golldack, D. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. J. Exp. Bot. 2001, 52, 1969–1980. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Peer, W.A.; Richter, G.; Blakeslee, J.; Bandyopadhyay, A.; Titapiwantakun, B.; Undurraga, S.; Khodakovskaya, M.; Richards, E.L.; et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005, 310, 121–125. [Google Scholar] [CrossRef]
- Neuhaus, H.E.; Trentmann, O. Regulation of transport processes across the tonoplast. Front Plant Sci. 2014, 5, 460. [Google Scholar] [CrossRef]
- Schulze, W.X.; Schneider, T.; Starck, S.; Martinoia, E.; Trentmann, O. Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters. Plant J. 2012, 69, 529–541. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Well, D.M. Nitrate transport and signaling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Jian, S.F.; Song, H.X.; Guan, C.Y.; Lepo, J.E.; Ismail, A.M.; Zhang, Z.H. Balance between nitrogen use efficiency and cadmium tolerance in Brassica napus and Arabidopsis thaliana. Plant Sci. 2019, 284, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, S.L.; Dai, S.X.; Wang, R.G.; Li, N.Y.; Shen, X.; Zhou, X.Y.; Lu, C.F.; Zheng, X.J.; Hu, Z.M.; et al. Ion flux profiles and plant ion homeostasis control under salt stress. Plant Signal Behav. 2009, 4, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Chanroj, S. Plant endomembrane dynamics: Studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; de Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Du, X.Q.; Wang, Z.F.; Wu, W.H.; Quintero, F.J.; Jin, X.H.; Li, H.D.; Wang, Y. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell 2017, 29, 2016–2026. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Véry, A.A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environment. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef]
- Schachtman, D.P. Molecular insights into the structure and function of plant K+ transport mechanisms. Biochim. Biophys. Acta 2000, 1465, 127–139. [Google Scholar] [CrossRef][Green Version]
- Blevins, D.G.; Barnett, N.M.; Frost, W.B. Role of potassium and malate in nitrate uptake and translocation by wheat seedlings. Plant Physiol. 1978, 62, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Triplett, E.W.; Barnett, N.M.; Blevins, D.G. Organic acids and ionic balance in xylem exudate of wheat during nitrate or sulfate absorption. Plant Physiol. 1980, 65, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Li, M.; Oh, S.; Kronzucker, H.J. Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol. 2013, 162, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Fan, X.; Wei, J.; Feng, H.; Qu, H.; Xie, D.; Miller, A.J.; Xu, G. Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and long-distance transport. J. Exp. Bot. 2015, 66, 317–331. [Google Scholar] [CrossRef]
- Kellermeier, F.; Armengaud, P.; Seditas, T.J.; Danku, J.; Salt, D.E.; Amtmann, A. Analysis of the Root System Architecture of Arabidopsis Provides a Quantitative Readout of Crosstalk between Nutritional Signals. Plant Cell 2014, 26, 1480–1496. [Google Scholar] [CrossRef]
- Zhu, J.K.; Liu, J.; Xiong, L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1191. [Google Scholar] [CrossRef]
- Wang, B.; Lüttge, U.; Ratajczak, R. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J. Exp. Bot. 2001, 52, 2355–2365. [Google Scholar] [CrossRef]
- Pérez-Castiñeira, J.R.; López-Marqués, R.L.; Losada, M.; Serrano, A. A thermostable K(+)-stimulated vacuolar-type pyrophosphatase from the hyperthermophilic bacterium Thermotoga maritima. FEBS Lett. 2001, 496, 6–11. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Shabana, A.A.; Balkos, K.D.; Kronzucker, H.J. NH4+ stimulated and inhibited components of K+ transport in rice (Oryza sativa L.). J. Exp. Bot. 2008, 59, 3415–3423. [Google Scholar] [CrossRef]
- Ten Hoopen, F.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S.; Schjoerring, J.K.; Jahn, T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef]
- Sanchez-Zabala, J.; González-Murua, C.; Marino, D. Mild ammonium stress increases chlorophyll content in Arabidopsis thaliana. Plant Signal Behav. 2015, 10, e991596. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Percival, F.; Merino, J.; Mooney, H.A. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ. 1987, 10, 725–734. [Google Scholar]
- Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the soil to the seeds: The long journey of nitrate in plants. J. Exp. Bot. 2011, 62, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Kuo, H.F.; Canivenc, G.; Lin, C.S.; Lepetit, M.; Hsu, P.K.; Tillard, P.; Lin, H.L.; Wang, Y.Y.; Tsai, C.B.; et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M.; Huang, J.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Song, H.X.; Liu, Q. Distribution Characters of Absorption Nitrogen in Oilseed Rape (Brassica napus L.) at Different Growth Stages. J. Plant Nutr. 2014, 37, 1648–1660. [Google Scholar] [CrossRef]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef]
- Ludewig, U.; Wirén, N.; Rentsch, D.; Frommer, W.B. Rhesus factors and ammonium: A function in efflux? Genome Biol. 2001, 2, 1–5. [Google Scholar] [CrossRef]
- Neuhäuser, B.; Dynowski, M.; Ludewig, U. Channel-like NH3 flux by ammonium transporter AtAMT2. FEBS Lett. 2009, 583, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Wood, C.C.; Roeb, G.W.; Udvardi, M.K. Characterization of Arabidopsis AtAMT2, a high-affinity ammonium transporter of the plasma membrane. Plant Physiol. 2002, 130, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; Laginha, A.M.; Duan, F.; Rentsch, D.; Yuan, L.; von Wirén, N. A Critical Role of AMT2;1 in Root-To-Shoot Translocation of Ammonium in Arabidopsis. Mol. Plant 2017, 10, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 2003, 15, 347–364. [Google Scholar] [CrossRef]
- Cheng, N.H.; Pittman, J.K.; Shigaki, T.; Lachmansingh, J.; LeClere, S.; Lahner, B.; Salt, D.E.; Hirschi, K.D. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005, 138, 2048–2060. [Google Scholar] [CrossRef]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferrière, N.; Thibaud, J.B.; Sentenac, H. Identification and disruption of a plant shakerlike outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
- Hamburger, D.; Rezzonico, E.; Petétot, J.M.-C.; Somerville, C.; Poirier, Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 2002, 14, 889–902. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Ródenas, R.; Martínez, V.; Nieves-Cordones, M.; Rubio, F. High external K+ concentrations impair Pi nutrition, induce the phosphate starvation response, and reduce arsenic toxicity in Arabidopsis plants. Int. J. Mol. Sci. 2019, 20, 2237. [Google Scholar] [CrossRef]
- Tsay, Y.F.; Ho, C.H.; Chen, H.Y.; Lin, S.H. Integration of Nitrogen and Potassium Signaling. Ann. Rev. Plant Biol. 2011, 62, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Liao, Q.; Song, H.; Liu, Q.; Lepo, J.E.; Guan, C.; Zhang, J.; Ismail, A.M.; Zhang, Z. NRT1.1-related NH4+ toxicity is associated with a disturbed balance between NH4+ uptake and assimilation. Plant Physiol. 2018, 178, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Zhou, T.; Yao, J.Y.; Han, Q.F.; Song, H.X.; Guan, C.Y.; Hua, Y.P.; Zhang, Z.H. Genome-scale characterization of the vacuole nitrate transporter Chloride Channel (CLC) genes and their transcriptional responses to diverse nutrient stresses in allotetraploid rapeseed. PLoS ONE 2008. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.M.; Lee, D.A.; Schroeder, J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 10118–10123. [Google Scholar] [CrossRef]
- Han, Y.L.; Song, H.X.; Liao, Q.; Yu, Y.; Jian, S.F.; Lepo, J.E.; Liu, Q.; Rong, X.M.; Tian, C.; Zeng, J. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 2016, 170, 1684–1698. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Santoni, S.; Bonifacio, E.; Zanini, E. Indophenol blue colorimetric method for measuring cation exchange capacity in sandy soils. Commun. Soil Sci. Plant Anal. 2001, 32, 2519–2530. [Google Scholar] [CrossRef]
- Fan, X.; Jia, L.; Li, Y.; Smith, S.J.; Miller, A.J.; Shen, Q. Comparing nitrate storage and remobilization in two rice cultivars that differ in their nitrogen use efficiency. J. Exp. Bot. 2007, 58, 1729–1740. [Google Scholar] [CrossRef]
- Huang, C.B.; Wang, Z.H.; Wang, X.Y.; Li, S.X. Nitrate accumulation and reduction in Spinach and their relations to plant growth. J. Agroc. Environ. Sci. 2011, 30, 613–618. [Google Scholar]
- Zhang, C.F.; Peng, S.B.; Peng, X.X.; Chavez, A.Q.; Bennett, J. Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza sativa L.) roots. Plant Sci. 1997, 125, 163–170. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H.K. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Adv. Photosynth. Res. 1984, 2, 9–12. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Fang, H.; Shi, H.; Chen, K.; Zhang, Z.; Tan, X. Selection of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in Brassica napus under various stress conditions. Mol. Genet. Genom. 2014, 289, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant. 2016, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Peng, J.S.; Zhong, C.; Yi, H.Y.; Ow, D.W.; Gong, J.M. Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in Arabidopsis. Plant Physiol. 2012, 158, 1779–1788. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, G.; Song, H.; Xiao, Y.; Zhang, Z. Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 2. https://doi.org/10.3390/ijms22010002
Liang G, Song H, Xiao Y, Zhang Z. Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana. International Journal of Molecular Sciences. 2021; 22(1):2. https://doi.org/10.3390/ijms22010002
Chicago/Turabian StyleLiang, Guihong, Haixing Song, Yan Xiao, and Zhenhua Zhang. 2021. "Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana" International Journal of Molecular Sciences 22, no. 1: 2. https://doi.org/10.3390/ijms22010002
APA StyleLiang, G., Song, H., Xiao, Y., & Zhang, Z. (2021). Ammonium Accumulation Caused by Reduced Tonoplast V-ATPase Activity in Arabidopsis thaliana. International Journal of Molecular Sciences, 22(1), 2. https://doi.org/10.3390/ijms22010002




