Molecular Aspects of the Development and Function of Auditory Neurons
Abstract
1. Introduction
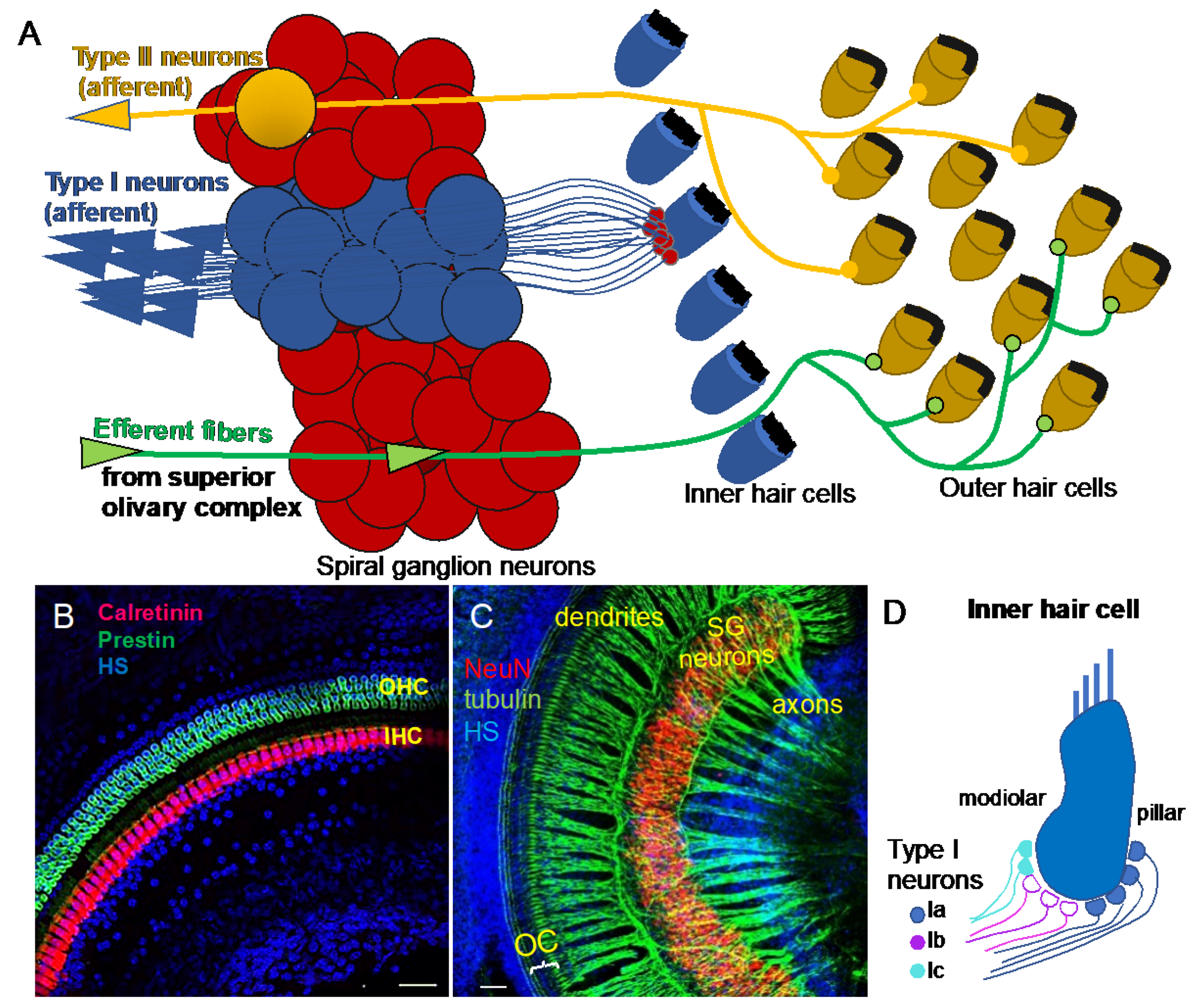
2. Functional Diversity of Auditory Neurons
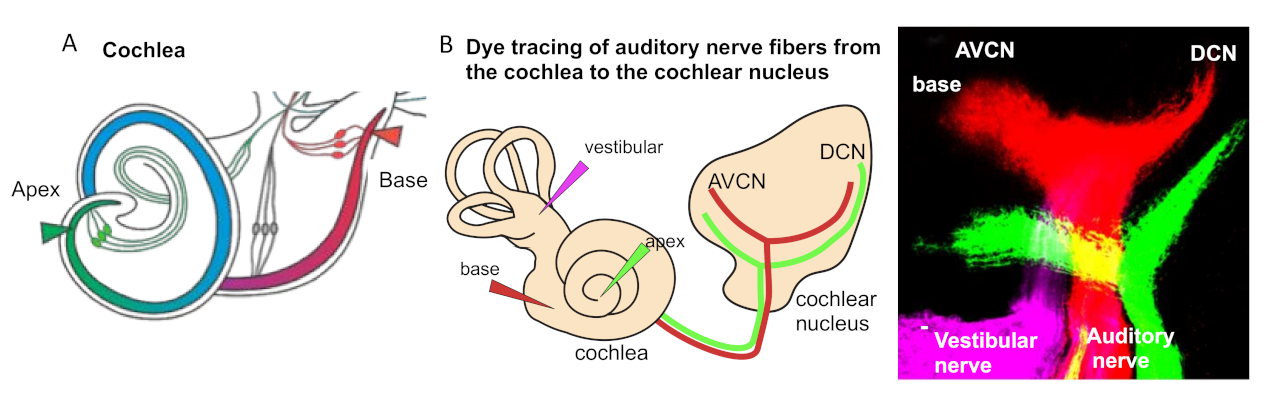
3. Tonotopic Organization of Auditory Neurons in the Cochlea
4. Molecular Diversity of Auditory Neurons in the Cochlea
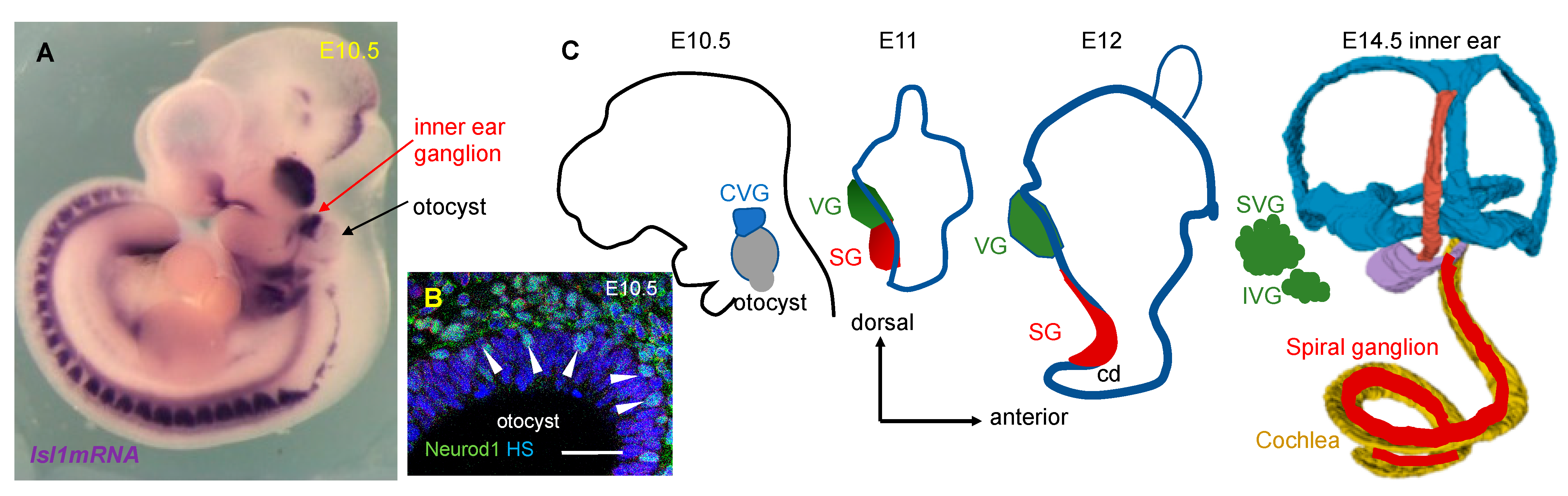
5. Neuronal Development in the Inner Ear
6. Transcriptional Network in the Development and Maintenance of Neurons in the Inner Ear
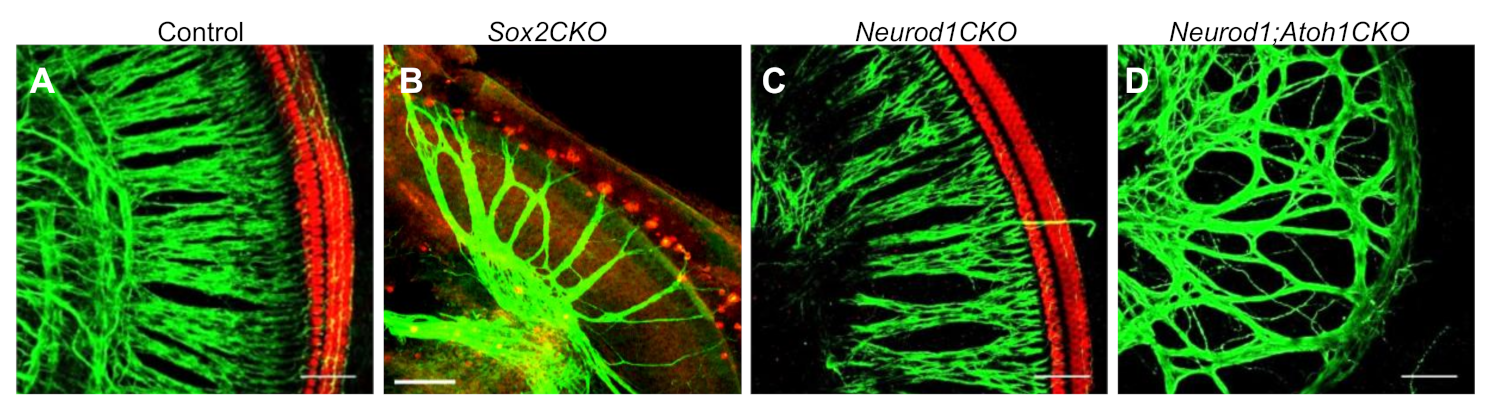
6.1. SRY (Sex-Determining Region Y)-Box 2 (SOX2) in Neurogenic and Sensory Progenitors
6.2. bHLH Transcription Factors in Neurogenesis
6.3. ISL1 in the Development of Neuronal and Sensory Lineages in the Inner Ear
6.4. Auditory Neuron Diversity Determined by Transcription Factor Combinations
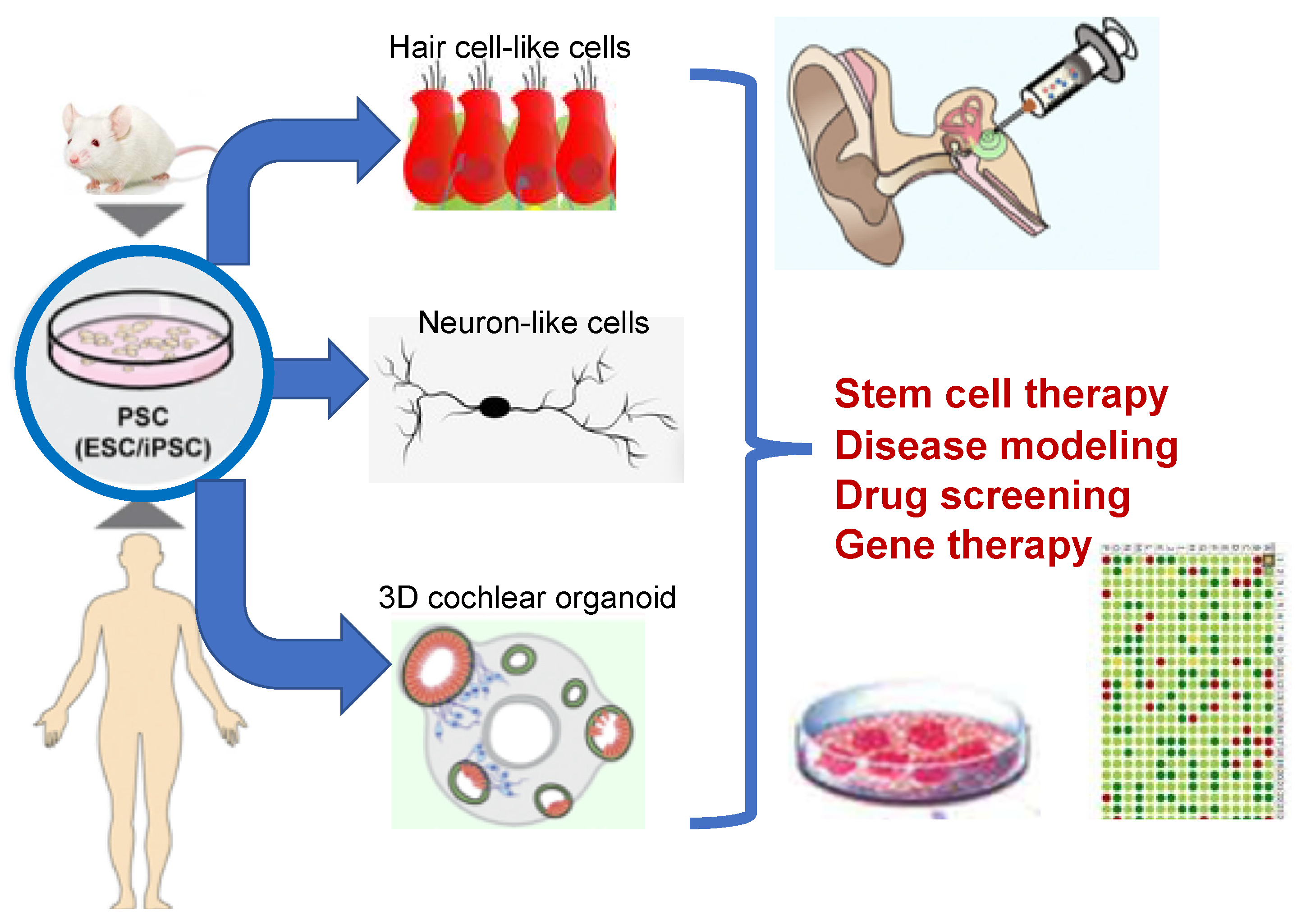
7. Cell-Based Therapy for Neuronal Replacement
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AVCN | anteroventral cochlear nucleus |
| BDNF | brain-derived neurotrophic factor |
| bHLH | basic helix-loop-helix |
| Cplx2 | complexin 2 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CVG | cochlear-vestibular ganglion |
| DCN | dorsal cochlear nucleus |
| ESCs | embryonic stem cells |
| Etv4 | ETS Variant Transcription Factor 4 |
| Grm8 | glutamate receptor, metabotropic 8 |
| Gata3 | GATA binding protein 3 |
| IHC | inner hair cell |
| iPSCs | induced pluripotent stem cells |
| Isl1 | ISL1 transcription factor, LIM/homeodomain |
| Mafb | v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B |
| Neurod1 | neurogenic differentiation 1 |
| Neurog1 | neurogenin 1 |
| Ngfr | nerve growth factor receptor |
| Ntng1 | netrin G1 |
| NT-3 | neurotrophin 3 |
| OHC | outer hair cell |
| Pcdh15 | protocadherin 15 |
| Pou4f1 | POU domain, class 4, transcription factor 1 |
| PSCs | pluripotent stem cells |
| Prox1 | prospero homeobox 1 |
| PVCN | posteroventral cochlear nucleus |
| Runx1 | runt-related transcription factor 1 |
| SG | spiral ganglion |
| Sox2 | SRY (sex determining region Y)-box 2 |
| SR | spontaneous firing rate |
| Tmie | transmembrane inner ear |
| TrkB | neurotrophic tyrosine kinase, receptor, type 2 |
| TrkC | neurotrophic tyrosine kinase, receptor, type 3 |
| VG | vestibular ganglion |
References
- Shi, F.; Edge, A.S. Prospects for replacement of auditory neurons by stem cells. Hear. Res. 2013, 297, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Elgoyhen, A.B.; Wedemeyer, C.; Guilmi, M.N.D. Efferent Innervation to the Cochlea; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Elgoyhen, A.B.; Vetter, D.E.; Katz, E.; Rothlin, C.V.; Heinemann, S.F.; Boulter, J. alpha10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Lustig, L.R.; Peng, H.; Hiel, H.; Yamamoto, T.; Fuchs, P.A. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10). Genomics 2001, 73, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Maison, S.F.; Liberman, M.C. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J. Neurosci. 2000, 20, 4701–4707. [Google Scholar] [CrossRef] [PubMed]
- Macova, I.; Pysanenko, K.; Chumak, T.; Dvorakova, M.; Bohuslavova, R.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Neurod1 Is Essential for the Primary Tonotopic Organization and Related Auditory Information Processing in the Midbrain. J. Neurosci. 2019, 39, 984–1004. [Google Scholar] [CrossRef]
- Perkins, R.E.; Morest, D.K. A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarkski Optics. J. Comp. Neurol. 1975, 163, 129–158. [Google Scholar] [CrossRef]
- Ryugo, D.K. The Auditory Nerve: Peripheral Innervatio Cell Body Morphology, and Central Projections; Springer: New York, NY, USA, 1992. [Google Scholar]
- Fechner, F.P.; Nadol, J.J.; Burgess, B.J.; Brown, M.C. Innervation of supporting cells in the apical turns of the guinea pig cochlea is from type II afferent fibers. J. Comp. Neurol. 2001, 429, 289–298. [Google Scholar] [CrossRef]
- Reid, M.A.; Flores-Otero, J.; Davis, R.L. Firing patterns of type II spiral ganglion neurons in vitro. J. Neurosci. 2004, 24, 733–742. [Google Scholar] [CrossRef]
- Flores, E.N.; Duggan, A.; Madathany, T.; Hogan, A.K.; Marquez, F.G.; Kumar, G.; Seal, R.P.; Edwards, R.H.; Liberman, M.C.; Garcia-Anoveros, J. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr. Biol. 2015, 25, 606–612. [Google Scholar] [CrossRef]
- Liu, C.; Glowatzki, E.; Fuchs, P.A. Unmyelinated type II afferent neurons report cochlear damage. Proc. Natl. Acad. Sci. USA 2015, 112, 14723–14727. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Chia, C.; Wu, L.; Kujawa, S.G.; Liberman, M.C.; Goodrich, L.V. Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity. Cell 2018, 174, 1229–1246.e17. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Babola, T.; Pregernig, G.; So, K.S.; Nguyen, M.; Su, S.M.; Palermo, A.T.; Bergles, D.E.; Burns, J.C.; Muller, U. Hair Cell Mechanotransduction Regulates Spontaneous Activity and Spiral Ganglion Subtype Specification in the Auditory System. Cell 2018, 174, 1247–1263.e15. [Google Scholar] [CrossRef] [PubMed]
- Petitpre, C.; Wu, H.; Sharma, A.; Tokarska, A.; Fontanet, P.; Wang, Y.; Helmbacher, F.; Yackle, K.; Silberberg, G.; Hadjab, S.; et al. Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat. Commun. 2018, 9, 3691. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.D.; Wang, H.; Liberman, M.C. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J. Neurosci. 2011, 31, 801–808. [Google Scholar] [CrossRef]
- Wu, J.S.; Young, E.D.; Glowatzki, E. Maturation of Spontaneous Firing Properties after Hearing Onset in Rat Auditory Nerve Fibers: Spontaneous Rates, Refractoriness, and Interfiber Correlations. J. Neurosci. 2016, 36, 10584–10597. [Google Scholar] [CrossRef]
- Liberman, M.C. Single-neuron labeling in the cat auditory nerve. Science 1982, 216, 1239–1241. [Google Scholar] [CrossRef]
- Rubel, E.W.; Fritzsch, B. Auditory system development: Primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002, 25, 51–101. [Google Scholar] [CrossRef]
- Muniak, M.; Connelly, C.J.; Suthakar, K.; Milinkeviciute, G.; Ayeni, F.; Ryugo, D.K. Central Projections of Spiral Ganglion Neurons. In The Primary Auditory Neurons of the Mammalian Cochlea; Springer: New York, NY, USA, 2016; pp. 157–190. [Google Scholar]
- Fariñas, I.; Jones, K.R.; Tessarollo, L.; Vigers, A.J.; Huang, E.; Kirstein, M.; De Caprona, D.C.; Coppola, V.; Backus, C.; Reichardt, L.F. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. 2001, 21, 6170–6180. [Google Scholar] [CrossRef]
- Yang, T.; Kersigo, J.; Jahan, I.; Pan, N.; Fritzsch, B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res. 2011, 278, 21–33. [Google Scholar] [CrossRef]
- Schmiedt, R.A. Spontaneous rates, thresholds and tuning of auditory-nerve fibers in the gerbil: Comparisons to cat data. Hear. Res. 1989, 42, 23–35. [Google Scholar] [CrossRef]
- Kandler, K.; Clause, A.; Noh, J. Tonotopic reorganization of developing auditory brainstem circuits. Nat. Neurosci. 2009, 12, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Muniak, M.A.; Rivas, A.; Montey, K.L.; May, B.J.; Francis, H.W.; Ryugo, D.K. 3D model of frequency representation in the cochlear nucleus of the CBA/J mouse. J. Comp. Neurol. 2013, 521, 1510–1532. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Wu, J.S.; Jimenez, A.; Glowatzki, E.; Fuchs, P.A. Characterization of transgenic mouse lines for labeling type I and type II afferent neurons in the cochlea. Sci. Rep. 2019, 9, 5549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, Z.; Grillet, N.; Yan, L.; Xiong, W.; Harkins-Perry, S.; Muller, U. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 2014, 84, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Alagramam, K.N.; Goodyear, R.J.; Geng, R.; Furness, D.N.; van Aken, A.F.; Marcotti, W.; Kros, C.J.; Richardson, G.P. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS ONE 2011, 6, e19183. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Fekete, D.M. Shaping sound in space: The regulation of inner ear patterning. Development 2012, 139, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Barald, K.F.; Kelley, M.W. From placode to polarization: New tunes in inner ear development. Development 2004, 131, 4119–4130. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Anderson, D.J.; Fritzsch, B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res. Otolaryngol. 2000, 1, 129–143. [Google Scholar] [CrossRef]
- Liu, M.; Pereira, F.A.; Price, S.D.; Chu, M.-j.; Shope, C.; Himes, D.; Eatock, R.A.; Brownell, W.E.; Lysakowski, A.; Tsai, M.-J. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000, 14, 2839–2854. [Google Scholar] [CrossRef]
- Jahan, I.; Pan, N.; Kersigo, J.; Fritzsch, B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS ONE 2010, 5, e11661. [Google Scholar] [CrossRef]
- Fritzsch, B.; Straka, H. Evolution of vertebrate mechanosensory hair cells and inner ears: Toward identifying stimuli that select mutation driven altered morphologies. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014, 200, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A. Comparative Auditory Neuroscience: Understanding the Evolution and Function of Ears. J. Assoc. Res. Otolaryngol. 2017, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Appler, J.M.; Goodrich, L.V. Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Prog. Neurobiol. 2011, 93, 488–508. [Google Scholar] [CrossRef]
- Matei, V.; Pauley, S.; Kaing, S.; Rowitch, D.; Beisel, K.W.; Morris, K.; Feng, F.; Jones, K.; Lee, J.; Fritzsch, B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 2005, 234, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Pan, N.; Jahan, I.; Elliott, K.L. Inner ear development: Building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Rese. 2015, 361, 7–24. [Google Scholar] [CrossRef][Green Version]
- Dvorakova, M.; Macova, I.; Bohuslavova, R.; Anderova, M.; Fritzsch, B.; Pavlinkova, G. Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev. Biol. 2020, 457, 43–56. [Google Scholar] [CrossRef]
- Appler, J.M.; Lu, C.C.; Druckenbrod, N.R.; Yu, W.M.; Koundakjian, E.J.; Goodrich, L.V. Gata3 is a critical regulator of cochlear wiring. J. Neurosci. 2013, 33, 3679–3691. [Google Scholar] [CrossRef]
- Dvorakova, M.; Jahan, I.; Macova, I.; Chumak, T.; Bohuslavova, R.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Sci. Rep. 2016, 6, 38253. [Google Scholar] [CrossRef]
- Kempfle, J.S.; Turban, J.L.; Edge, A.S. Sox2 in the differentiation of cochlear progenitor cells. Sci. Rep. 2016, 6, 23293. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.; Tang, A.S.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–1035. [Google Scholar] [CrossRef]
- Dabdoub, A.; Puligilla, C.; Jones, J.M.; Fritzsch, B.; Cheah, K.S.; Pevny, L.H.; Kelley, M.W. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 2008, 105, 18396–18401. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Noda, T.; Dabdoub, A. Dynamic expression of Sox2, Gata3, and Prox1 during primary auditory neuron development in the mammalian cochlea. PLoS ONE 2017, 12, e0170568. [Google Scholar] [CrossRef] [PubMed]
- Puligilla, C.; Dabdoub, A.; Brenowitz, S.D.; Kelley, M.W. Sox2 induces neuronal formation in the developing mammalian cochlea. J. Neurosci. 2010, 30, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Steevens, A.R.; Sookiasian, D.L.; Glatzer, J.C.; Kiernan, A.E. SOX2 is required for inner ear neurogenesis. Sci. Rep. 2017, 7, 4086. [Google Scholar] [CrossRef]
- Steevens, A.R.; Glatzer, J.C.; Kellogg, C.C.; Low, W.C.; Santi, P.A.; Kiernan, A.E. SOX2 is required for inner ear growth and cochlear nonsensory formation before sensory development. Development 2019, 146, dev170522. [Google Scholar] [CrossRef]
- Ahmed, M.; Wong, E.Y.; Sun, J.; Xu, J.; Wang, F.; Xu, P.X. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 2012, 22, 377–390. [Google Scholar] [CrossRef]
- Ahmed, M.; Xu, J.; Xu, P.X. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development 2012, 139, 1965–1977. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Shen, J.; Corey, D.P. C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem Cell Rep. 2015, 4, 47–60. [Google Scholar] [CrossRef]
- Filova, I.; Dvorakova, M.; Bohuslavova, R.; Pavlinek, A.; Elliott, K.L.; Vochyanova, S.; Fritzsch, B.; Pavlinkova, G. Combined Atoh1 and Neurod1 Deletion Reveals Autonomous Growth of Auditory Nerve Fibers. Mol. Neurobiol. 2020, 57, 5307–5323. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z.; del Barco Barrantes, I.; de la Pompa, J.L.; Anderson, D.J. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 1998, 20, 469–482. [Google Scholar] [CrossRef]
- Raft, S.; Koundakjian, E.J.; Quinones, H.; Jayasena, C.S.; Goodrich, L.V.; Johnson, J.E.; Segil, N.; Groves, A.K. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 2007, 134, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-Y.; Fritzsch, B.; Serls, A.; Bakel, L.A.; Huang, E.J.; Reichardt, L.F.; Barth, D.S.; Lee, J.E. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 2001, 128, 417–426. [Google Scholar] [PubMed]
- Bhati, M.; Lee, C.; Nancarrow, A.L.; Lee, M.; Craig, V.J.; Bach, I.; Guss, J.M.; Mackay, J.P.; Matthews, J.M. Implementing the LIM code: The structural basis for cell type-specific assembly of LIM-homeodomain complexes. EMBO J. 2008, 27, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dykes, I.M.; Liang, X.; Eng, S.R.; Evans, S.M.; Turner, E.E. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat. Neurosci. 2008, 11, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Bohuslavova, R.; Cerychova, R.; Papousek, F.; Olejnickova, V.; Bartos, M.; Gorlach, A.; Kolar, F.; Sedmera, D.; Semenza, G.L.; Pavlinkova, G. HIF-1alpha is required for development of the sympathetic nervous system. Proc. Natl. Acad. Sci. USA 2019, 116, 13414–13423. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, U.; Pfaff, S.L.; Jessell, T.M.; Edlund, T.; Edlund, H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 1997, 385, 257–260. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Radde-Gallwitz, K.; Pan, L.; Gan, L.; Lin, X.; Segil, N.; Chen, P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J. Comp. Neurol. 2004, 477, 412–421. [Google Scholar] [CrossRef]
- Chumak, T.; Bohuslavova, R.; Macova, I.; Dodd, N.; Buckiova, D.; Fritzsch, B.; Syka, J.; Pavlinkova, G. Deterioration of the Medial Olivocochlear Efferent System Accelerates Age-Related Hearing Loss in Pax2-Isl1 Transgenic Mice. Mol. Neurobiol. 2016, 53, 2368–2383. [Google Scholar] [CrossRef]
- Bohuslavova, R.; Dodd, N.; Macova, I.; Chumak, T.; Horak, M.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Pax2-Islet1 Transgenic Mice Are Hyperactive and Have Altered Cerebellar Foliation. Mol. Neurobiol. 2017, 54, 1352–1368. [Google Scholar] [CrossRef]
- Huang, M.; Kantardzhieva, A.; Scheffer, D.; Liberman, M.C.; Chen, Z.Y. Hair cell overexpression of Islet1 reduces age-related and noise-induced hearing loss. J. Neurosci. 2013, 33, 15086–15094. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Liu, W.; Fritzsch, B.; Bianchi, L.M.; Reichardt, L.F.; Xiang, M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development 2001, 128, 2421–2432. [Google Scholar] [PubMed]
- Sherrill, H.E.; Jean, P.; Driver, E.C.; Sanders, T.R.; Fitzgerald, T.S.; Moser, T.; Kelley, M.W. Pou4f1 Defines a Subgroup of Type I Spiral Ganglion Neurons and Is Necessary for Normal Inner Hair Cell Presynaptic Ca(2+) Signaling. J. Neurosci. 2019, 39, 5284–5298. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Runker, A.; Fabel, K.; Buchholz, F.; Kempermann, G. Transcription factor Runx1 is pro-neurogenic in adult hippocampal precursor cells. PLoS ONE 2018, 13, e0190789. [Google Scholar] [CrossRef]
- Kramer, I.; Sigrist, M.; de Nooij, J.C.; Taniuchi, I.; Jessell, T.M.; Arber, S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 2006, 49, 379–393. [Google Scholar] [CrossRef]
- Duncan, J.S.; Fritzsch, B. Continued expression of GATA3 is necessary for cochlear neurosensory development. PLoS ONE 2013, 8, e62046. [Google Scholar] [CrossRef]
- Martinez-Monedero, R.; Yi, E.; Oshima, K.; Glowatzki, E.; Edge, A.S. Differentiation of inner ear stem cells to functional sensory neurons. Dev. Neurobiol. 2008, 68, 669–684. [Google Scholar] [CrossRef]
- Shi, F.; Kempfle, J.S.; Edge, A.S. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 2012, 32, 9639–9648. [Google Scholar] [CrossRef]
- Yin, J.C.; Zhang, L.; Ma, N.X.; Wang, Y.; Lee, G.; Hou, X.Y.; Lei, Z.F.; Zhang, F.Y.; Dong, F.P.; Wu, G.Y.; et al. Chemical Conversion of Human Fetal Astrocytes into Neurons through Modulation of Multiple Signaling Pathways. Stem Cell Rep. 2019, 12, 488–501. [Google Scholar] [CrossRef]
- Tang, P.C.; Hashino, E.; Nelson, R.F. Progress in Modeling and Targeting Inner Ear Disorders with Pluripotent Stem Cells. Stem Cell Rep. 2020, 14, 996–1008. [Google Scholar] [CrossRef]
- Coleman, B.; Hardman, J.; Coco, A.; Epp, S.; de Silva, M.; Crook, J.; Shepherd, R. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006, 15, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Corrales, C.E.; Pan, L.; Li, H.; Liberman, M.C.; Heller, S.; Edge, A.S. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of Corti. J. Neurobiol. 2006, 66, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, A.J.; Morrissey, Z.D.; Zhang, C.; Homma, K.; Belmadani, A.; Miller, C.A.; Chadly, D.M.; Kobayashi, S.; Edelbrock, A.N.; Tanaka-Matakatsu, M.; et al. Directed Differentiation of Human Embryonic Stem Cells Toward Placode-Derived Spiral Ganglion-Like Sensory Neurons. Stem Cells Transl. Med. 2017, 6, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Perny, M.; Ting, C.C.; Kleinlogel, S.; Senn, P.; Roccio, M. Generation of Otic Sensory Neurons from Mouse Embryonic Stem Cells in 3D Culture. Front. Cell Neurosci. 2017, 11, 409. [Google Scholar] [CrossRef]
- Shi, F.; Corrales, C.E.; Liberman, M.C.; Edge, A.S. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 2007, 26, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Benvenisty, N. Defining Human Pluripotency. Cell Stem Cell 2019, 25, 9–22. [Google Scholar] [CrossRef]
- Roccio, M.; Edge, A.S.B. Inner ear organoids: New tools to understand neurosensory cell development, degeneration and regeneration. Development 2019, 146, dev177188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlinkova, G. Molecular Aspects of the Development and Function of Auditory Neurons. Int. J. Mol. Sci. 2021, 22, 131. https://doi.org/10.3390/ijms22010131
Pavlinkova G. Molecular Aspects of the Development and Function of Auditory Neurons. International Journal of Molecular Sciences. 2021; 22(1):131. https://doi.org/10.3390/ijms22010131
Chicago/Turabian StylePavlinkova, Gabriela. 2021. "Molecular Aspects of the Development and Function of Auditory Neurons" International Journal of Molecular Sciences 22, no. 1: 131. https://doi.org/10.3390/ijms22010131
APA StylePavlinkova, G. (2021). Molecular Aspects of the Development and Function of Auditory Neurons. International Journal of Molecular Sciences, 22(1), 131. https://doi.org/10.3390/ijms22010131




