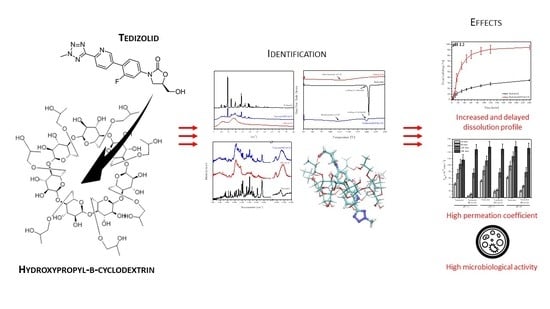
Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. System Preparation
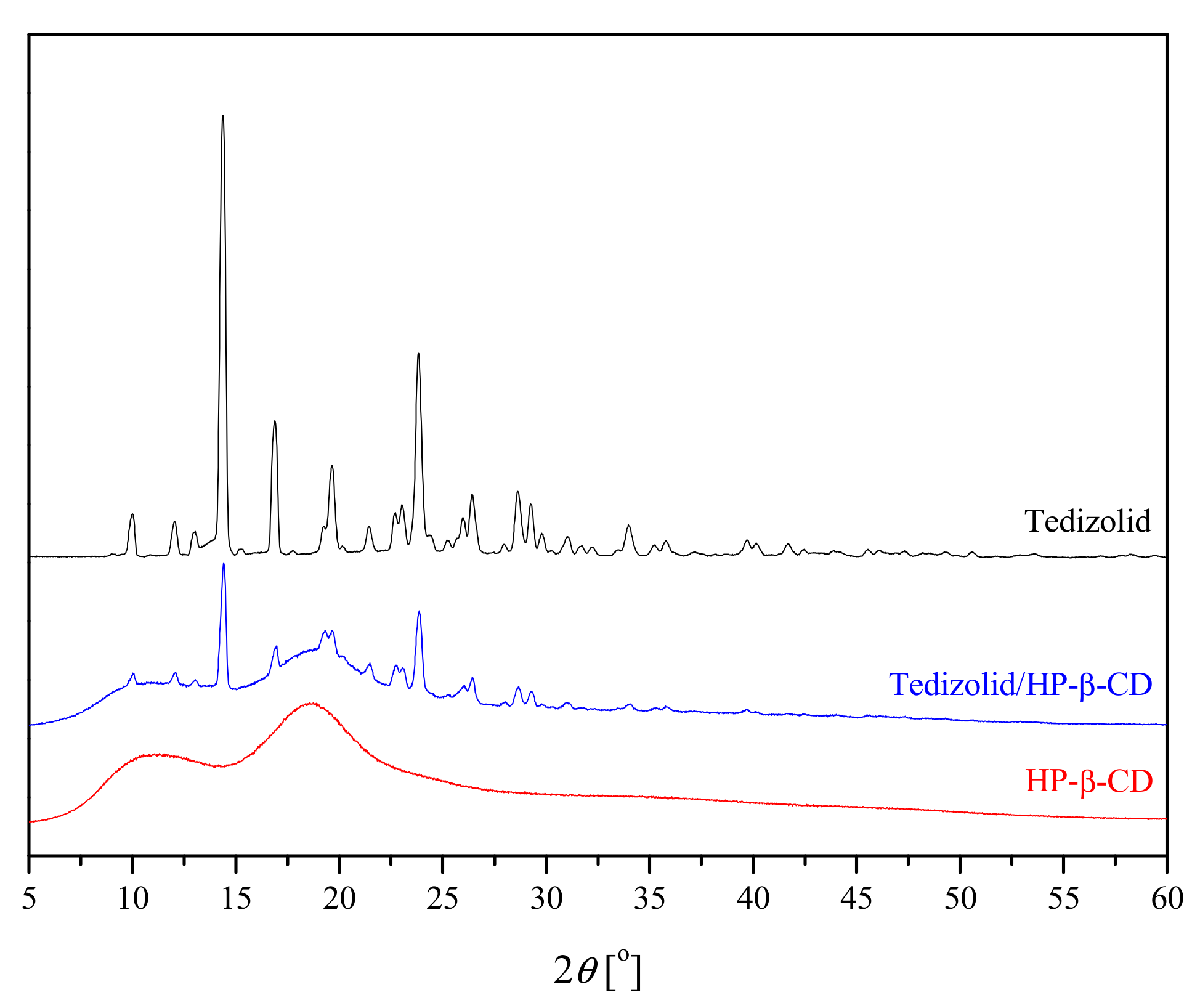
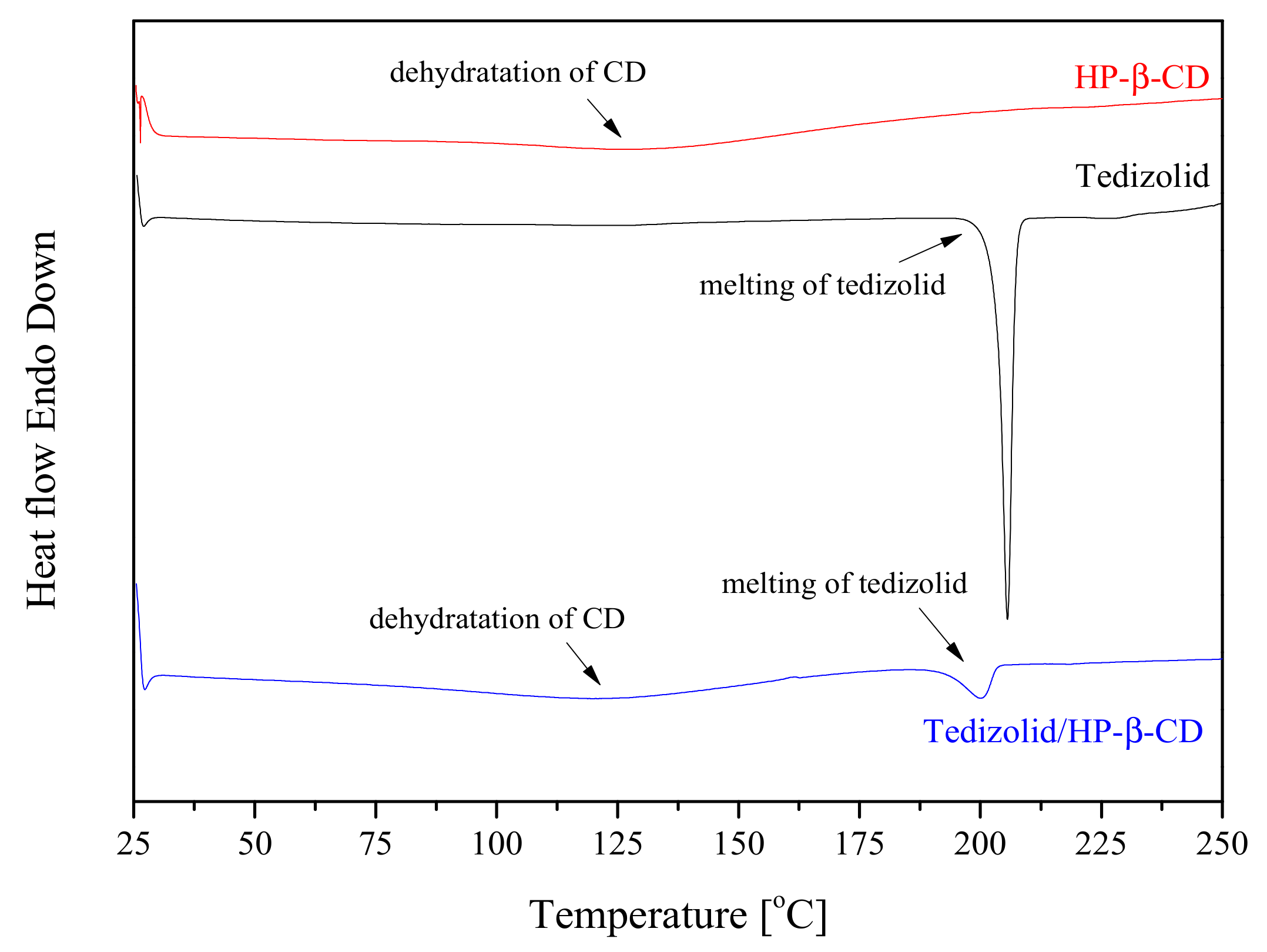
3.2. The Identification of the Systems
3.2.1. Differential Scanning Colorimetry (DSC)
3.2.2. Powder X-ray Diffraction (PXRD)
3.2.3. FT-IR Spectroscopy
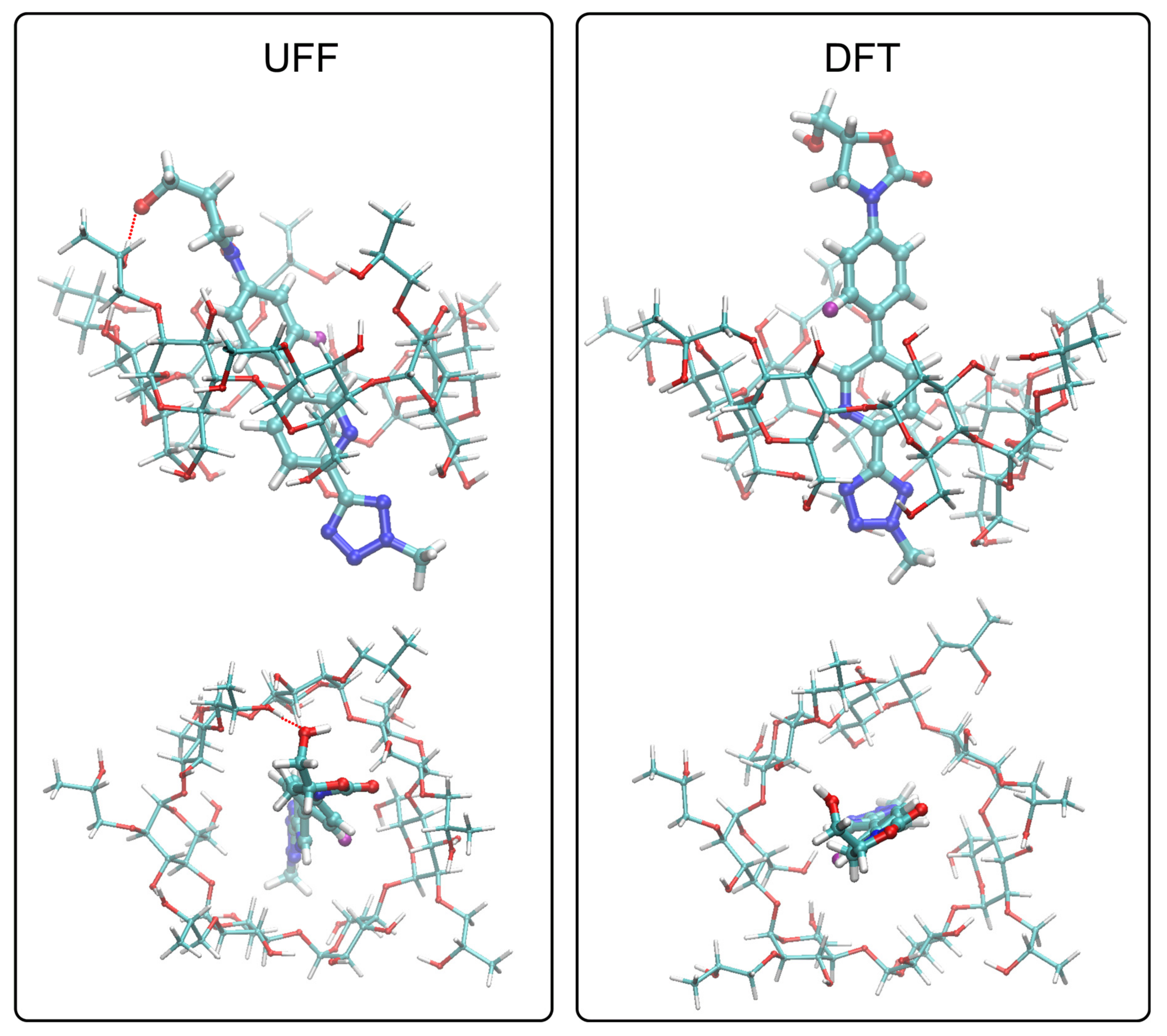
3.2.4. Molecular Docking
3.3. Studies of Physicochemical Properties of Systems
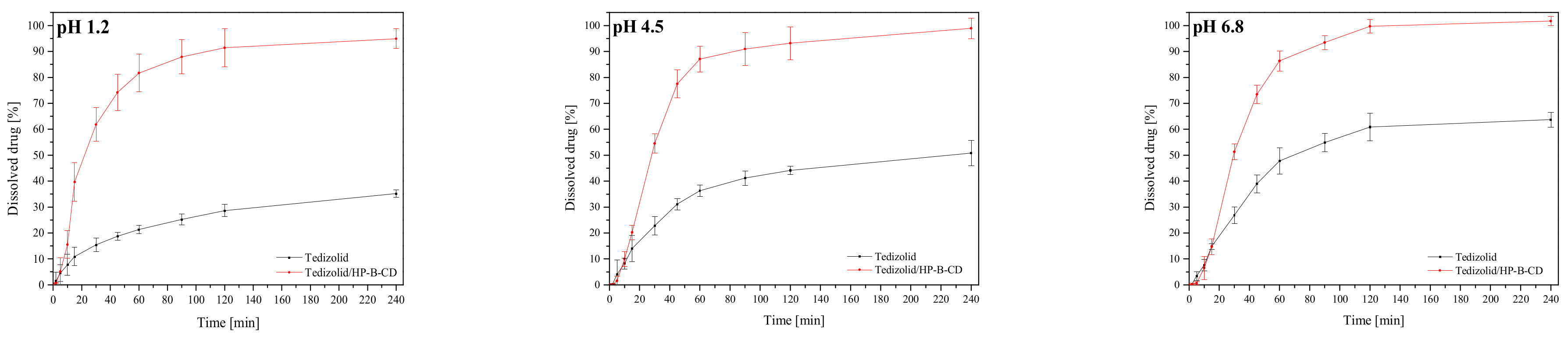
3.3.1. Dissolution of Systems
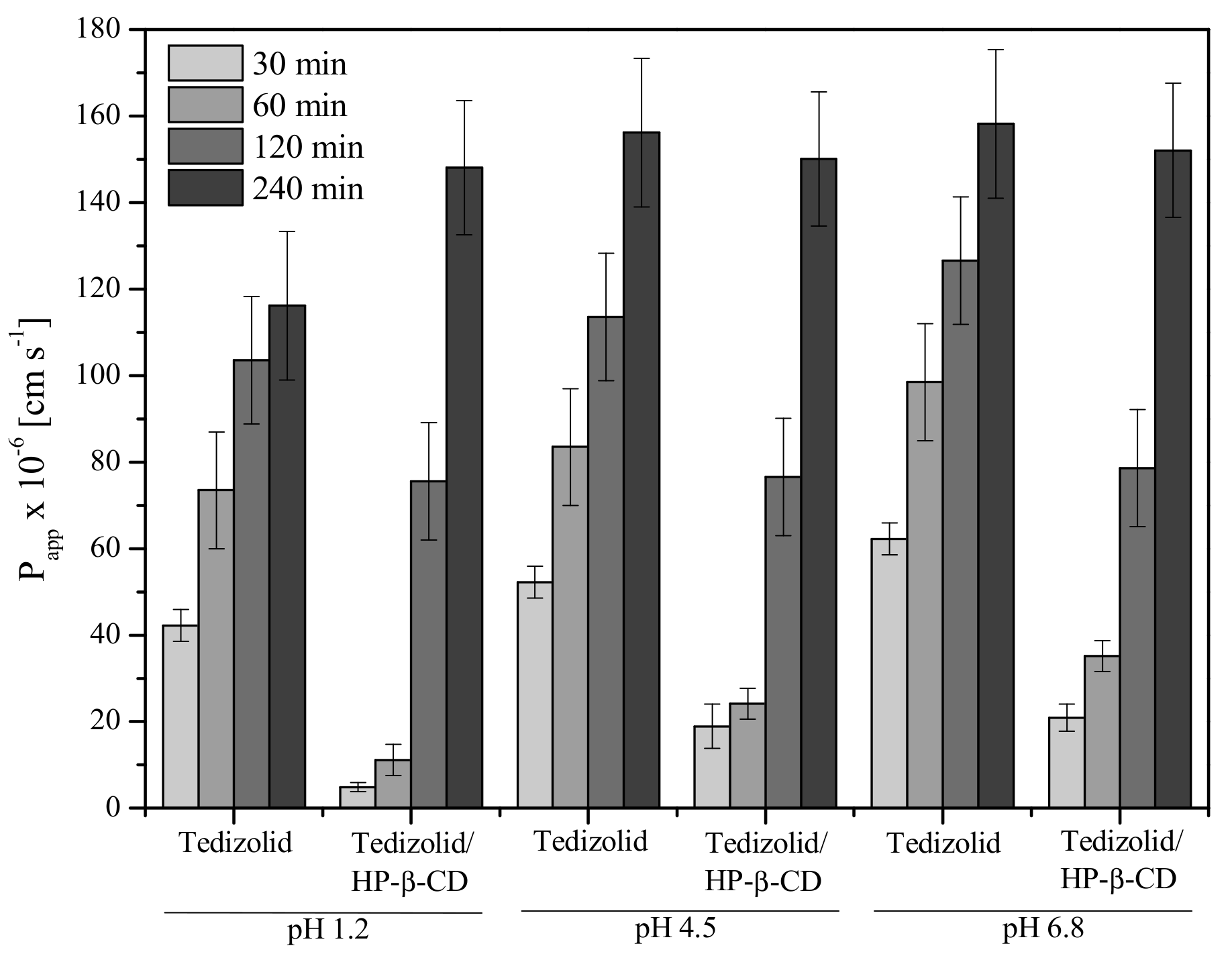
3.3.2. Permeability Studies of Systems
3.3.3. Studies of Bactericidal Activity of Tedizolid in Tedizolid/HP-β-CD Inclusion Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FDA. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/25/infections-and-infectious-diseases (accessed on 6 March 2020).
- Rumondor, A.C.F.; Dhareshwar, S.S.; Kesisoglou, F. Amorphous Solid Dispersions or Prodrugs: Complementary Strategies to Increase Drug Absorption. J. Pharm. Sci. 2016, 105, 2498–2508. [Google Scholar] [CrossRef]
- Ruiz, P.; Causse, M.; Vaquero, M.; Casal, M. In vitro activity of Tedizolid against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e01939-18. [Google Scholar] [CrossRef]
- Michalska, K.; Karpiuk, I.; Król, M.; Tyski, S. Recent development of potent analogues of oxazolidinone antibacterial agents. Bioorg. Med. Chem. 2013, 21, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Love, R.; Adam, H.; Golden, A.; Zelenitsky, S.; Schweizer, F.; Gorityala, B.; Lagacé-Wiens, P.R.; Rubinstein, E.; Walkty, A.; et al. Tedizolid: A novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens. Drugs 2015, 75, 253–270. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon PASS. Available online: https://chemicalize.com/app/calculation/tedizolid (accessed on 17 December 2020).
- Im, W.B.; Choi, S.H.; Park, J.-Y.; Choi, S.H.; Finn, J.; Yoon, S.-H. Discovery of torezolid as a novel 5-hydroxymethyl-oxazolidinone antibacterial agent. Eur J. Med. Chem. 2011, 46, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef]
- Shaw, K.J.; Barbachyn, M.R. The oxalidinones: Past, present and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef]
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; Ferreira de Melo, T.R.; dos Santos, J.L.; Chung, M.C. The prodrug approach: A successful tool for improving drug solubility. Molecules 2016, 21, 1–31. [Google Scholar] [CrossRef]
- Kidney Health. Calcium and Phosphate Balance with Kidney Disease. Available online: https://kidney.org.au/uploads/resources/calcium-and-phosphate-balance-with-kidney-disease-kidney-health-australia-fact-sheet.pdf (accessed on 20 November 2020).
- Bassetti, M.; Castaldo, N.; Carnelutti, A.; Peghin, M.; Giacobbe, D.R. Tedizolid phosphate for the treatment of acute bacterial skin and skin-structure infections: An evidence-based review of its place in therapy. Core Evid. 2019, 14, 31–40. [Google Scholar] [CrossRef]
- Dixit, M.; Kini, A.G.; Kulkarni, P.K. Enhancing Solubility and Dissolution of Celecoxib by Spray Drying using Pluronic F 127. Indian J. Pharm. Educ. Res. 2011, 45, 346–352. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Scien. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Michalska, K.; Gruba, E.; Cielecka-Piontek, J.; Bednarek, E. Chiral separation of tedizolid using charge single isomer derivatives of cyclodextrins by capillary electrokinetic chromatography. J. Pharm. Biomed. Anal. 2016, 120, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Gruba, E.; Bocian, W.; Cielecka-Piontek, J. Enantioselective recognition of radezolid by cyclodextrin modified capillary electrokinetic chromatography and electronic circular dichroism. J. Pharm. Biomed. Anal. 2017, 139, 98–108. [Google Scholar] [CrossRef]
- Michalska, K.; Pajchel, G.; Tyski, S. Determination of enantiomeric impurity of linezolid by capillary electrophoresis using heptakis-(2,3-diacetyl-6-sulfato)-beta-cyclodextrin. J. Chromatogr. A 2008, 1180, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Bocian, W.; Bednarek, E.; Pałys, B.; Cielecka-Piontek, J. Enantioselective recognition of sutezolid by cyclodextrin modified non-aqueous capillary electrophoresis and explanation of complex formation by means of infrared spectroscopy, NMR and molecular modelling. J. Pharm. Biomed. Anal. 2019, 169, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Cielecka-Piontek, J. Enhanced tedizolid solubility and permeability due to its interactions with hydrophilic biopolymers. Acta Pharm. Hung. 2018, 88, 195. [Google Scholar]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D.; Lewandowska, K.; Błaszczak, W.; Gościańska, J.; Pietrzak, R.; Cielecka-Piontek, J. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Paczkowska, M.; Szymanowska-Powałowska, D.; Mizera, M.; Siąkowska, D.; Błaszczak, W.; Piotrowska-Kempisty, H.; Cielecka-Piontek, J. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS ONE 2019, 14, e0210694. [Google Scholar] [CrossRef]
- Mizera, M.; Szymanowska, D.; Stasiłowicz, A.; Siąkowska, D.; Lewandowska, K.; Miklaszewski, A.; Plech, T.; Tykarska, E.; Cielecka-Piontek, J. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity. Biomolecules 2020, 10, 24. [Google Scholar] [CrossRef]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- EMA. Cyclodextrins Used as Excipients. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 20 November 2020).
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, E.; Bocian, W.; Michalska, K. Nuclear magnetic resonance spectroscopic study of the inclusion complex of (R)-tedizolid with HDAS-β-CD, β-CD, and γ-cyclodextrin in aqueous solution. J. Pharm. Biomed. Anal. 2019, 169, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Mizera, M.; Lewandowska, K.; Cielecka-Piontek, J. Infrared, Raman and ultraviolet with circular dichroism analysis and theoretical calculations of tedizolid. J. Mol. Struct. 2016, 1115, 136–143. [Google Scholar] [CrossRef]
- Flourence, T.; Malyadri, T.; Thejomoorthy, K. Enhancement of dissolution of Cilostazole by complexation method using Cyclodextrins. World J. Curr. Med. Pharm. Res. 2020, 2, 166–170. [Google Scholar] [CrossRef]
- Gu, W.; Liu, Y. Characterization and stability of beta-acids/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1201, 127159. [Google Scholar] [CrossRef]
- Li, W.; Ran, L.; Liu, F.; Hou, R.; Zhao, W.; Li, Y.; Wang, C.; Dong, J. Preparation and Characterisation of Polyphenol-HP-β-Cyclodextrin Inclusion Complex that Protects Lamb Tripe Protein against Oxidation. Molecules 2019, 24, 4487. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Torrado-Agrasar, A.; Simal-Gandara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hydroxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA J. Food 2017, 15, 409–417. [Google Scholar] [CrossRef]
- Lo Nostro, P.; Santoni, I.; Bonini, M.; Baglioni, P. Inclusion compound from a semifluorinated alkane and β-cyclodextrin. Langmuir 2013, 19, 2313–2317. [Google Scholar] [CrossRef]
- Hu, S.C.; Lai, Y.C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; Silva, S.S.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [PubMed]
- EMA. Sivextro: EPAR- Public Assessment Report. 2015. Available online: https://www.ema.europa.eu/en/documents/assessment-report/sivextro-epar-public-assessment-report_en.pdf (accessed on 17 December 2020).
- Ong, V.; Flanagan, S.; Fang, E.; Dreskin, H.J.; Locke, J.B.; Bartizal, K.; Prokocimer, P. Absorption, distribution, metabolism, and excretion of the novel antibacterial prodrug tedizolid phosphate. Drug Metab. Dispos. 2014, 42, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco3 cells (colonic) can predict in vivo (small intestinal) absorption in man–Fact or myth. Pharm Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- FDA. Clinical Pharmacology Review of Tedizolid Phosphate (SIVEXTRO). 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205435Orig1s000ClinPharmR.pdf (accessed on 17 December 2020).
- Flanagan, S.D.; Bien, P.A.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Pharmacokinetics of tedizolid following oral administration: Single and multiple dose, effect of food, and comparison of two solid forms of the prodrug. Pharmacotherapy 2014, 34, 240–250. [Google Scholar] [CrossRef]
- Shah, M.; Shah, H.D. Acute bacterial skin and skin structure infections: Current perspective. Indian J. Dermatol. 2011, 56, 510–512. [Google Scholar] [CrossRef]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of β-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef]
- Paczkowska, M.; McDonagh, A.; Bialek, K.; Tajberm, L.; Cielecka-Piontek, J. Mechanochemical activation with cyclodextrins followed by compaction as an approach to improve dissolution of rutin. Int. J. Pharm. 2020, 581, 119294. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Gebhardt, J.; Kleist, C.; Jakobtorweihen, S.; Hansen, N. Validation and Comparison of Force Fields for Native Cyclodextrins in Aqueous Solution. J. Phys. Chem. B 2018, 122, 1608–1626. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Commission of the European Communities. Validation of Analytical Procedures Q2(R2). Proceedings of the International Conference of Harmonisation (ICH). 2018. Available online: https://database.ich.org/sites/default/files/Q2R2-Q14_EWG_Concept_Paper.pdf (accessed on 17 December 2020).
- European Pharmacopeia 9.0. 2.9.3. Dissolution Test for Solid Dosage Forms; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2016. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]

| Tedizolid | Tedizolid/HP-β-CD | |
|---|---|---|
| MIC [mg L−1] | ||
| Enterococcus faecalis ATTC 29212 | 0.5 ± 0.1 | 0.25 ± 0.1 ↓ |
| E. faecalis clinical isolates | 1 ± 0.2 | 0.25 ± 0.1 ↓ |
| Enterococcus faecium ATCC 27270 | 0.5 ± 0.1 | 0.25 ± 0.1 ↓ |
| E. faecium clinical isolates | 1 ± 0.2 | 0.5 ± 0.1 ↓ |
| Staphylococcus aureus ATCC 25923 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| S. aureus clinical isolates | 2 ± 0.2 | 2 ± 0.2 |
| Staphylococcus epidermidis ATCC 12228 | 1 ± 0.2 | 1 ± 0.2 |
| S. epidermidis clinical isolates | 2 ± 0.2 | 2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczkowska-Walendowska, M.; Rosiak, N.; Tykarska, E.; Michalska, K.; Płazińska, A.; Płaziński, W.; Szymanowska, D.; Cielecka-Piontek, J. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. Int. J. Mol. Sci. 2021, 22, 115. https://doi.org/10.3390/ijms22010115
Paczkowska-Walendowska M, Rosiak N, Tykarska E, Michalska K, Płazińska A, Płaziński W, Szymanowska D, Cielecka-Piontek J. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. International Journal of Molecular Sciences. 2021; 22(1):115. https://doi.org/10.3390/ijms22010115
Chicago/Turabian StylePaczkowska-Walendowska, Magdalena, Natalia Rosiak, Ewa Tykarska, Katarzyna Michalska, Anita Płazińska, Wojciech Płaziński, Daria Szymanowska, and Judyta Cielecka-Piontek. 2021. "Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity" International Journal of Molecular Sciences 22, no. 1: 115. https://doi.org/10.3390/ijms22010115
APA StylePaczkowska-Walendowska, M., Rosiak, N., Tykarska, E., Michalska, K., Płazińska, A., Płaziński, W., Szymanowska, D., & Cielecka-Piontek, J. (2021). Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. International Journal of Molecular Sciences, 22(1), 115. https://doi.org/10.3390/ijms22010115