Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support
Abstract
1. Introduction
2. Results and Discussion
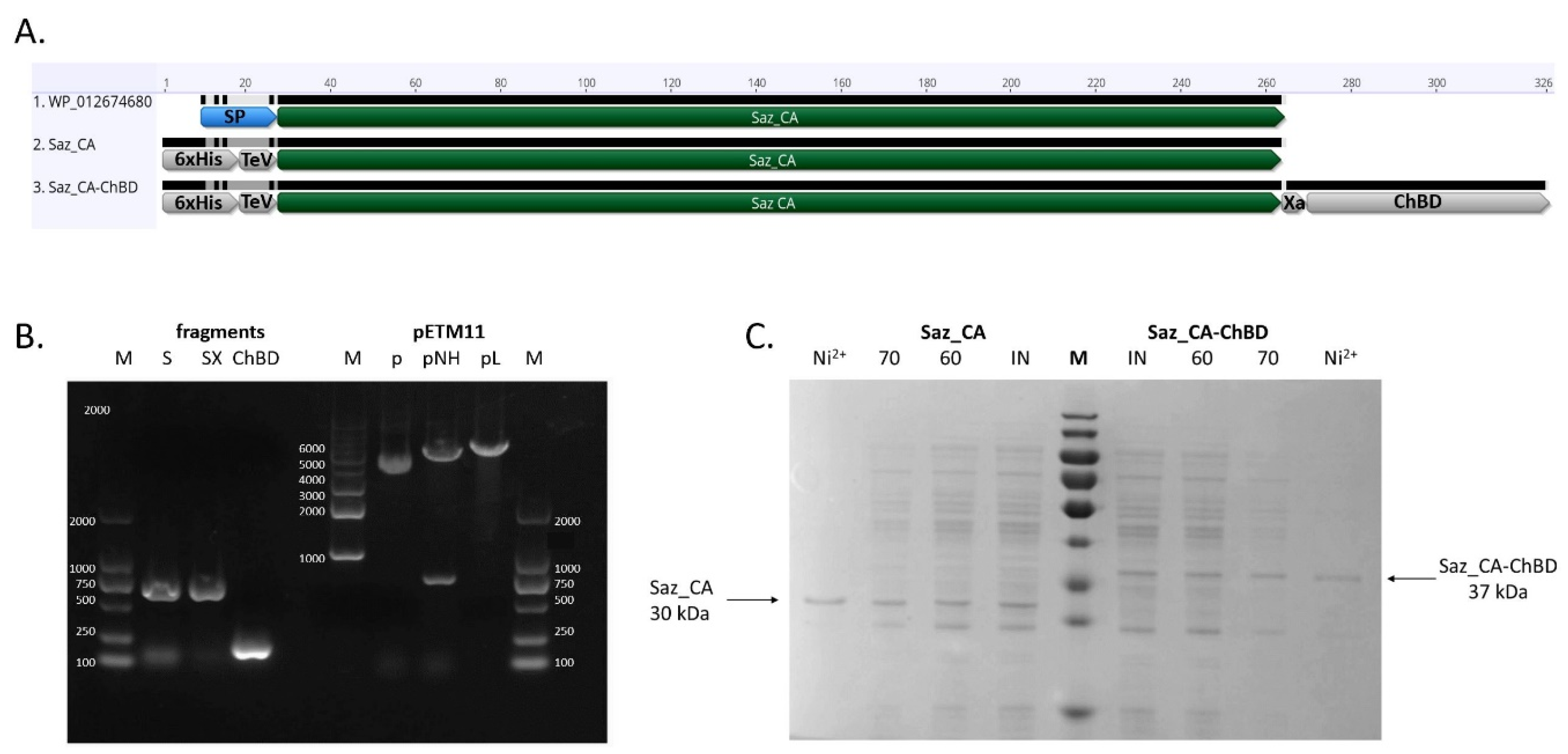
2.1. Expression and Purification of Saz_CA Variants
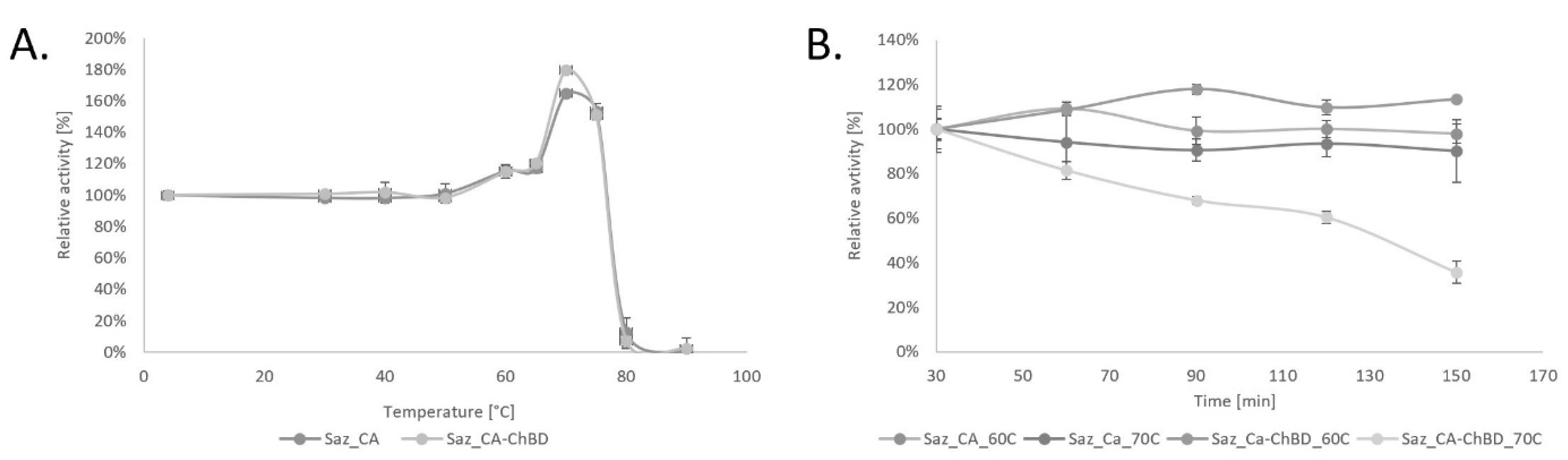
2.2. Properties of Saz_CA Variants
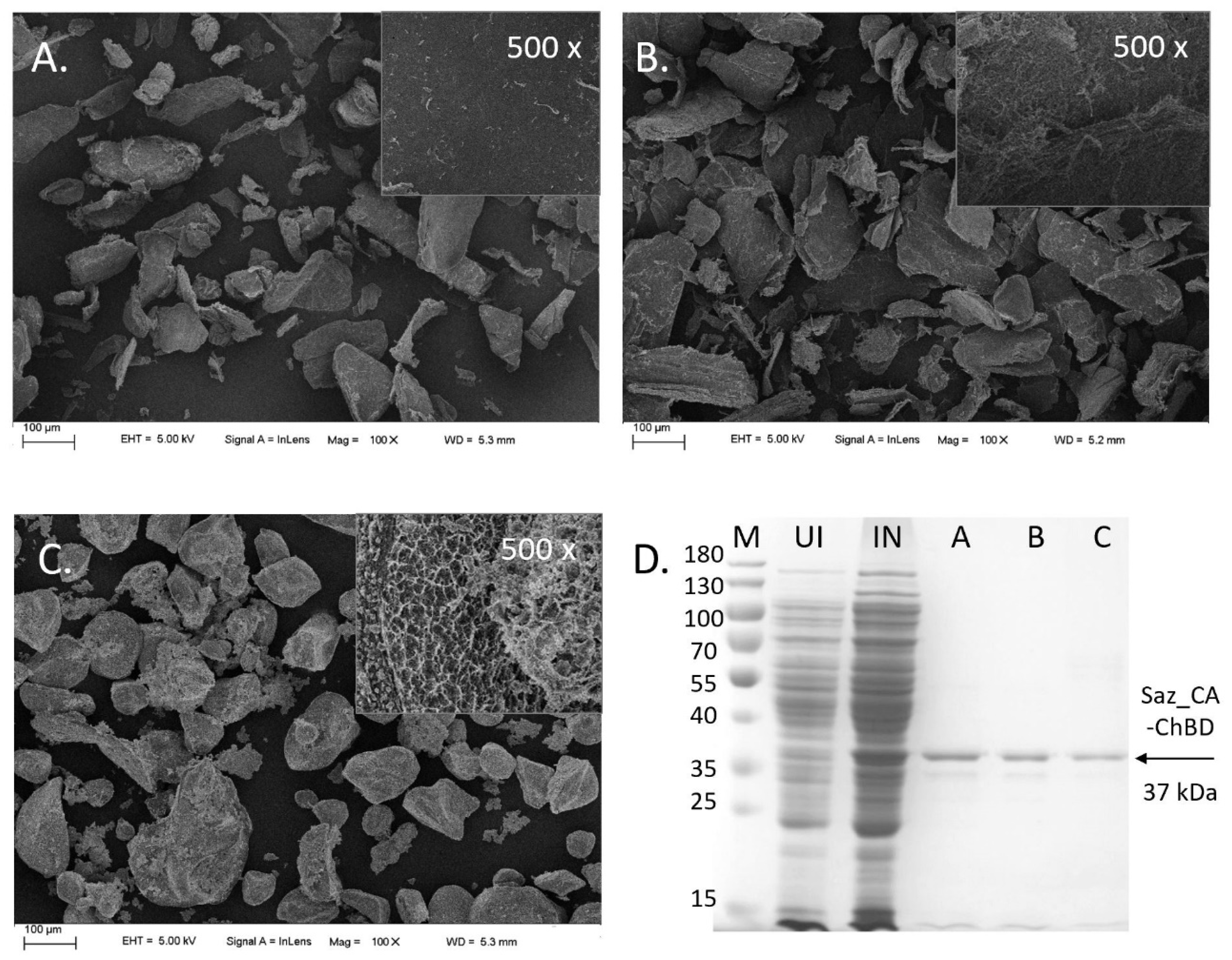
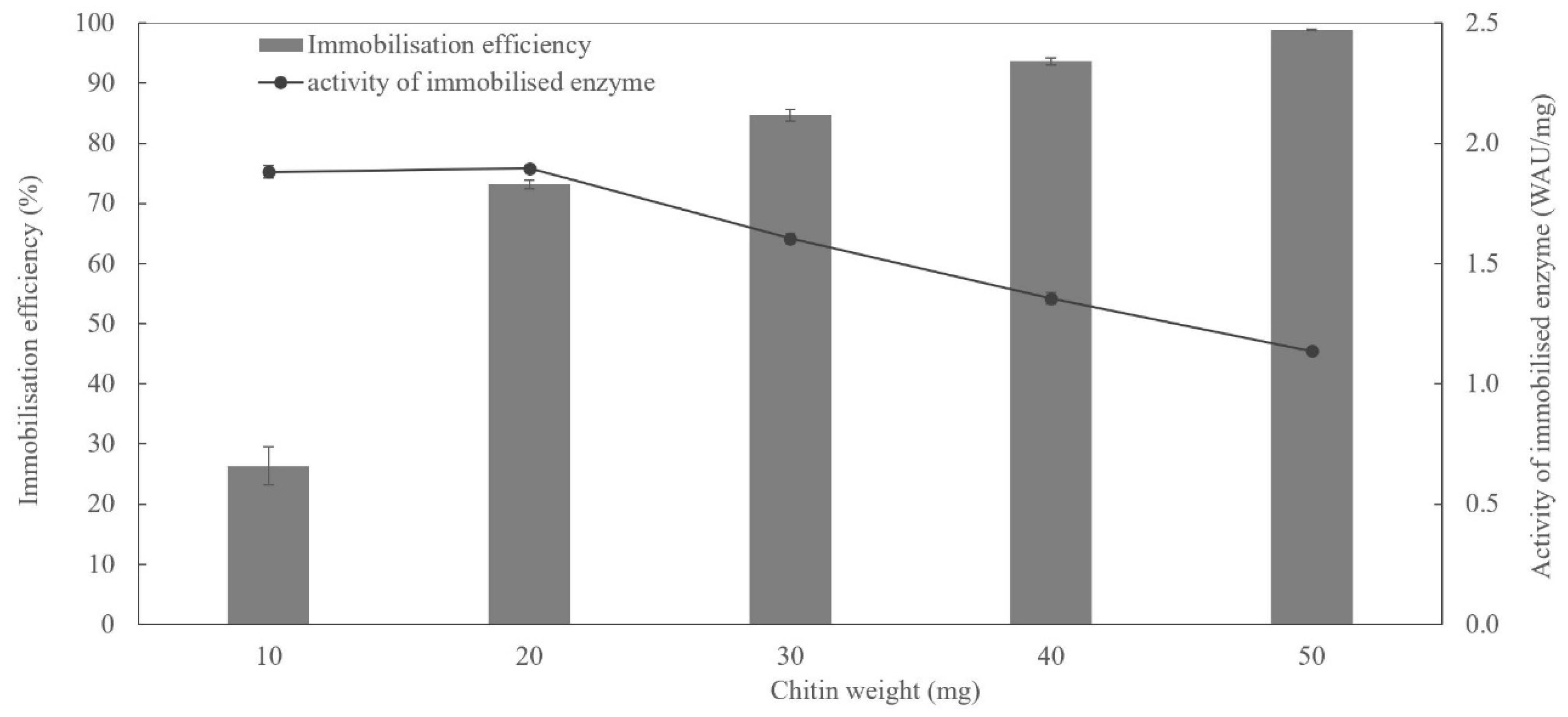
2.3. Immobilisation of Saz_CA Variants on Chitin Support
3. Materials and Methods
3.1. DNA Manipulations and Construction of Vectors
3.2. Protein Expression and Purification
3.3. Enzyme Immobilisation
3.3.1. Preparation of Chitin Immobilisation Supports
3.3.2. Enzyme Immobilisation on Chitin Supports
3.3.3. Analysis of Chitin Immobilisation Supports
3.4. CO2 Hydration Assay
3.5. Protein Properties
3.5.1. Thermostability of Saz_CA Variants
3.5.2. Time Course Thermostability of Saz_CA Variants
3.5.3. The Effect of Salts and Inhibitors on Saz_CA Variants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA | Carbonic Anhydrase |
| ChBD | Chitin Binding Domain |
| NCBI | National Center for Biotechnology Information |
| WAU | Wilbur–Anderson Unit |
| XRD | X-Ray Diffraction |
References
- Kamennaya, N.; Ajo-Franklin, C.; Northen, T.; Jansson, C. Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.B.; Jeong, S.K.; Lim, K.S.; Lee, S.H.; Trachtenberg, M.C. On carbon dioxide storage based on biomineralization strategies. Micron 2010, 41, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Han, Y.; Yu, Y.; Chiu, C.; Chang, Y. Efficient carbon dioxide sequestration by using recombinant carbonic anhydrase. Process Biochem. 2018, 73, 38–46. [Google Scholar] [CrossRef]
- Brinkman, R.; Margaria, R.; Roughton, F.J.W. The kinetics of the carbon dioxide-carbonic acid reaction. Philos. Trans. R. Soc. Ser. A 1933, 232, 65–97. [Google Scholar] [CrossRef]
- Ho, C.; Sturtevant, J. The kinetics of the hydration of carbon dioxide at 25 °C. J. Biol. Chem. 1963, 238, 3499–3501. [Google Scholar] [PubMed]
- Roughton, F.; Booth, V. The catalytic effect of buffers on the reaction CO2 + H2O ⇌ H2CO3. Biochem. J. 1938, 32, 2049–2069. [Google Scholar] [CrossRef]
- Johnson, K.S. Carbon dioxide hydration and dehydration in sea water. Limnol. Ocean. 1982, 27, 849–855. [Google Scholar] [CrossRef]
- Jo, B.H.; Kim, I.G.; Seo, J.H.; Kang, D.G.; Cha, H.J. Engineered Escherichia coli with periplasmic carbonic anhydrase as a biocatalyst for CO2 sequestration. Appl. Environ. Microbiol. 2013, 79, 6697–6705. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, A.; Shrivastava, A. Biomimetic CO2 sequestration using purified carbonic anhydrase from indigenous bacterial strains immobilized on biopolymeric materials. Enzyme Microb. Technol. 2011, 48, 416–426. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzyme Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef]
- Ozensoy Guler, O.; Capasso, C.; Supuran, C.T. A magnificent enzyme superfamily: Carbonic anhydrases, their purification and characterization. J. Enzyme Inhib. Med. Chem. 2016, 31, 689–694. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Bacterial carbonic anhydrases as drug targets: Toward novel antibiotics? Front. Pharmacol. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Stefanucci, A.; Angeli, A.; Dimmito, M.P.; Luisi, G.; Prete, D.; Capasso, C.; Donald, W.A.; Mollica, A.; Supuran, C.T. Activation of β—And γ -carbonic anhydrases from pathogenic bacteria with tripeptides. J. Enzyme Inhib. Med. Chem. 2018, 33, 945–950. [Google Scholar] [CrossRef]
- Peirce, S.; Perfetto, R.; Russo, M.E.; Capasso, C.; Rossi, M.; Salatino, P.; Marzocchella, A. Characterization of technical grade carbonic anhydrase as biocatalyst for CO2 capture in potassium carbonate solutions. Greenh. Gases Sci. Technol. 2018, 8, 279–291. [Google Scholar] [CrossRef]
- Leimbrink, M.; Sandkämper, S.; Wardhaugh, L.; Maher, D.; Green, P.; Puxty, G.; Conway, W.; Bennett, R.; Botma, H.; Feron, P.; et al. Energy-efficient solvent regeneration in enzymatic reactive absorption for carbon dioxide capture. Appl. Energy 2017, 208, 263–276. [Google Scholar] [CrossRef]
- Leimbrink, M.; Tlatlik, S.; Salmon, S.; Kunze, A.K.; Limberg, T.; Spitzer, R.; Gottschalk, A.; Górak, A.; Skiborowski, M. Pilot scale testing and modeling of enzymatic reactive absorption in packed columns for CO2 capture. Int. J. Greenh. Gas Control 2017, 62, 100–112. [Google Scholar] [CrossRef]
- Abdelrahim, M.Y.; Martins, C.F.; Neves, L.A.; Capasso, C.; Supuran, C.T.; Coelhoso, I.M.; Crespo, J.G.; Barboiu, M. Supported ionic liquid membranes immobilized with carbonic anhydrases for CO2 transport at high temperatures. J. Membr. Sci. 2017, 528, 225–230. [Google Scholar] [CrossRef]
- Bhagat, C.; Dudhagara, P.; Tank, S. Trends, application and future prospectives of microbial carbonic anhydrase mediated carbonation process for CCUS. J. Appl. Microbiol. 2018, 124, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Chern, J.T.; Chao, Y.P. Chitin-binding domain based immobilization of d-hydantoinase. J. Biotechnol. 2005, 117, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ikegami, T.; Seino, S.; Ohuchi, N.; Fukada, H.; Sugiyama, J.; Shirakawa, M.; Watanabe, T. Expression and characterization of the chitin-binding domain of chitinase A1 from Bacillus circulans WL-12. J. Bacteriol. 2000, 182, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, Y.; Wu, X. Characterization of a novel thermostable chitin-binding domain and its application in immobilization of a multifunctional hemicellulase. J. Agric. Food Chem. 2013, 61, 3074–3081. [Google Scholar] [CrossRef]
- Capasso, C.; De Luca, V.; Carginale, V.; Cannio, R.; Rossi, M. Biochemical properties of a novel and highly thermostable bacterial α-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J. Enzyme Inhib. Med. Chem. 2012, 27, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. The extremo-α-carbonic anhydrase from the thermophilic bacterium Sulfurihydrogenibium azorense is highly inhibited by sulfonamides. Bioorg. Med. Chem. 2013, 21, 4521–4525. [Google Scholar] [CrossRef]
- Nakagawa, S.; Shataih, Z.; Banta, A.; Beveridge, T.J.; Sako, Y.; Reysenbach, A.L. Sulfurihydrogenibium yellowstonense sp. nov., an extremely thermophilic, facultatively heterotrophic, sulfur-oxidizing bacterium from Yellowstone National Park, and emended descriptions of the genus Sulfurihydrogenibium, Sulfurihydrogenibium subterraneum. Int. J. Syst. Evol. Microbiol. 2005, 55, 2263–2268. [Google Scholar] [CrossRef]
- Roughton, F.J.W.; Booth, V.H. The effect of substrate concentration, pH and other factors upon the activity of carbonic anhydrase. Biochem. J. 1946, 40, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Hamon, V.; Dallet, S.; Legoy, M.-D. The pressure-dependence of two β-glucosidases with respect to their thermostability. Biochim. Biophys. Acta 1996, 1294, 195–203. [Google Scholar] [CrossRef]
- Varejão, N.; De-Andrade, R.A.; Almeida, R.V.; Anobom, C.D.; Foguel, D.; Reverter, D. Structural mechanism for the temperature-dependent activation of the hyperthermophilic Pf2001 esterase. Structure 2018, 26, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Kiran, B. Carbon concentration in algae: Reducing CO2 from exhaust gas. Trends Biotechnol. 2017, 35, 2–4. [Google Scholar] [CrossRef]
- Vullo, D.; De Luca, V.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. Anion inhibition studies of the fastest carbonic anhydrase (CA) known, the extremo-CA from the bacterium Sulfurihydrogenibium azorense. Bioorg. Med. Chem. Lett. 2012, 22, 7142–7145. [Google Scholar] [CrossRef] [PubMed]
- Bose, H.; Satyanarayana, T. Utility of thermo-alkali-stable γ-CA from polyextremophilic bacterium Aeribacillus pallidus TSHB1 in biomimetic sequestration of CO2 and as a virtual peroxidase. Environ. Sci. Pollut. Res. 2017, 24, 10869–10884. [Google Scholar] [CrossRef]
- Faridi, S.; Satyanarayana, T. Novel alkalistable α-carbonic anhydrase from the polyextremophilic bacterium Bacillus halodurans: Characteristics and applicability in flue gas CO2 sequestration. Environ. Sci. Pollut. Res. 2016, 23, 15236–15249. [Google Scholar] [CrossRef] [PubMed]
- Kanth, B.K.; Jun, S.Y.; Kumari, S.; Pack, S.P. Highly thermostable carbonic anhydrase from Persephonella marina EX-H1: Its expression and characterization for CO2-sequestration applications. Process. Biochem. 2014, 49, 2114–2121. [Google Scholar] [CrossRef]
- Ferrandon, S.; Sterzenbach, T.; Mersha, F.B.; Xu, M.Q. A single surface tryptophan in the chitin-binding domain from Bacillus circulans chitinase A1 plays a pivotal role in binding chitin and can be modified to create an elutable affinity tag. Biochim. Biophys. Acta 2003, 1621, 31–40. [Google Scholar] [CrossRef]
- Duan, B.; Zheng, X.; Xia, Z.; Fan, X.; Guo, L.; Liu, J.; Wang, Y.; Ye, Q.; Zhang, L. Highly biocompatible nanofibrous microspheres self-assembled from chitin in NaOH/urea aqueous solution as cell carriers. Angew. Chem. Int. Ed. 2015, 54, 5152–5156. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Suzuki, K.; Oyanagi, W.; Ohnishi, K.; Tanaka, H. Gene cloning of chitinase A1 from Bacillus circulans WL-12 revealed its evolutionary relationship to Serratia chitinase and to the type III homology units of fibronectin. J. Biol. Chem. 1990, 265, 15659–15665. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, C.; Wanjari, S.; Gawande, S.; Das, S.; Labhsetwar, N.; Kotwal, S.; Puri, A.K.; Satyanarayana, T.; Rayalu, S. Immobilization of carbonic anhydrase enriched microorganism on biopolymer based materials. J. Mol. Catal. B Enzym. 2009, 60, 13–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, K.M.; Anderson, N.G. Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 1948, 176, 147–154. [Google Scholar] [PubMed]
- Capasso, C.; De Luca, V.; Carginale, V.; Caramuscio, P.; Cavalheiro, C.; Cannio, R.; Rossi, M. Characterization and properties of a new thermoactive and thermostable carbonic anhydrase. Chem. Eng. Trans. 2012, 27, 271–276. [Google Scholar]
| No. | Purification Step | Enzyme Activity [WAU mL−1] | Total Activity [WAU] | Protein Concentration [mg L−1] | Specific Activity [WAU mgprot−1] | Purification Fold | Yield |
|---|---|---|---|---|---|---|---|
| 1 | Saz_CA Crude lysate | 312.33 | 46,849 | 598.68 | 521.69 | 1.00 | 100% |
| 2 | Saz_CA Heat treatment 60 °C 30 min | 478.28 | 71,741 | 544.39 | 878.55 | 1.68 | 153% |
| 3 | Saz_CA Heat treatment 70 °C 30 min | 505.91 | 75,887 | 311.46 | 1624.36 | 3.11 | 162% |
| 4 | Saz_CA Ni2+ affinity chromatography | 300.79 | 30,079 | 35.19 | 8546.74 | 16.38 | 64% |
| No. | Purification step | Enzyme Activity [WAU mL−1] | Total Activity [WAU] | Protein Concentration [mg L−1] | Specific Activity [WAU mgprot−1] | Purification Fold | Yield |
|---|---|---|---|---|---|---|---|
| 1 | Saz_CA-ChBD Crude lysate | 132.73 | 19,909 | 586.11 | 226.45 | 1.00 | 100% |
| 2 | Saz_CA-ChBD Heat treatment 60 °C 30 min | 263.61 | 39,542 | 553.75 | 476.04 | 2.10 | 199% |
| 3 | Saz_CA-ChBD Heat treatment 70 °C 30 min | 196.75 | 29,513 | 345.42 | 569.60 | 2.52 | 148% |
| 4 | Saz_CA-ChBD Ni2+ affinity chromatography | 152.26 | 15,227 | 45.36 | 3357.09 | 14.82 | 76% |
| Variant | Saz_CA | Saz_CA-ChBD |
|---|---|---|
| Control | 100 ± 10% | 100 ± 3% |
| Sulphonamide | 36 ± 4% | 37 ± 1% |
| Acetazolamide | 0 ± 2% | 0 ± 4% |
| HCO3− | 90 ± 1% | 96 ± 6% |
| CO32− | 99 ± 12% | 96 ± 5% |
| SO42− | 97 ± 4% | 93 ± 1% |
| Zn2+ | 49 ± 6% | 45 ± 3% |
| Mn2+ | 82 ± 4% | 81 ± 4% |
| Cu2+ | 20 ± 4% | 24 ± 3% |
| Mg2+ | 81 ± 6% | 101 ± 4% |
| Ca2+ | 98 ± 3% | 97 ± 4% |
| K+ | 93 ± 5% | 100 ± 2% |
| Na+ | 105 ± 14% | 105 ± 5% |
| Support | Untreated Chitin | Pre-Treated Chitin | Commercial Chitin Resin |
|---|---|---|---|
| Immobilisation Efficiency [%] | 82.88 | 66.64 | 80.88 |
| Activity of Immobilised Enzyme [WAU mgchitin−1] | 0.96 | 0.69 | 0.60 |
| Support | CrI020 (%) | Degree of Deacetylation |
|---|---|---|
| Untreated Chitin | 98.8 | 6.916 |
| Pre-Treated Chitin | 81.6 | 29.740 |
| Commercial Chitin Bead | 90.1 | 18.417 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Li, X.; Kaczmarek, M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. Int. J. Mol. Sci. 2019, 20, 1494. https://doi.org/10.3390/ijms20061494
Hou J, Li X, Kaczmarek MB, Chen P, Li K, Jin P, Liang Y, Daroch M. Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. International Journal of Molecular Sciences. 2019; 20(6):1494. https://doi.org/10.3390/ijms20061494
Chicago/Turabian StyleHou, Juan, Xingkang Li, Michal B. Kaczmarek, Pengyu Chen, Kai Li, Peng Jin, Yuanmei Liang, and Maurycy Daroch. 2019. "Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support" International Journal of Molecular Sciences 20, no. 6: 1494. https://doi.org/10.3390/ijms20061494
APA StyleHou, J., Li, X., Kaczmarek, M. B., Chen, P., Li, K., Jin, P., Liang, Y., & Daroch, M. (2019). Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. International Journal of Molecular Sciences, 20(6), 1494. https://doi.org/10.3390/ijms20061494






