The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products
Abstract
1. Introduction
2. Genetics, Structure and Physicochemical Properties of Lactoperoxidase
2.1. Genetics
2.2. Structure and Physicochemical Properties
3. Secretion in Physiological and Pathological Conditions
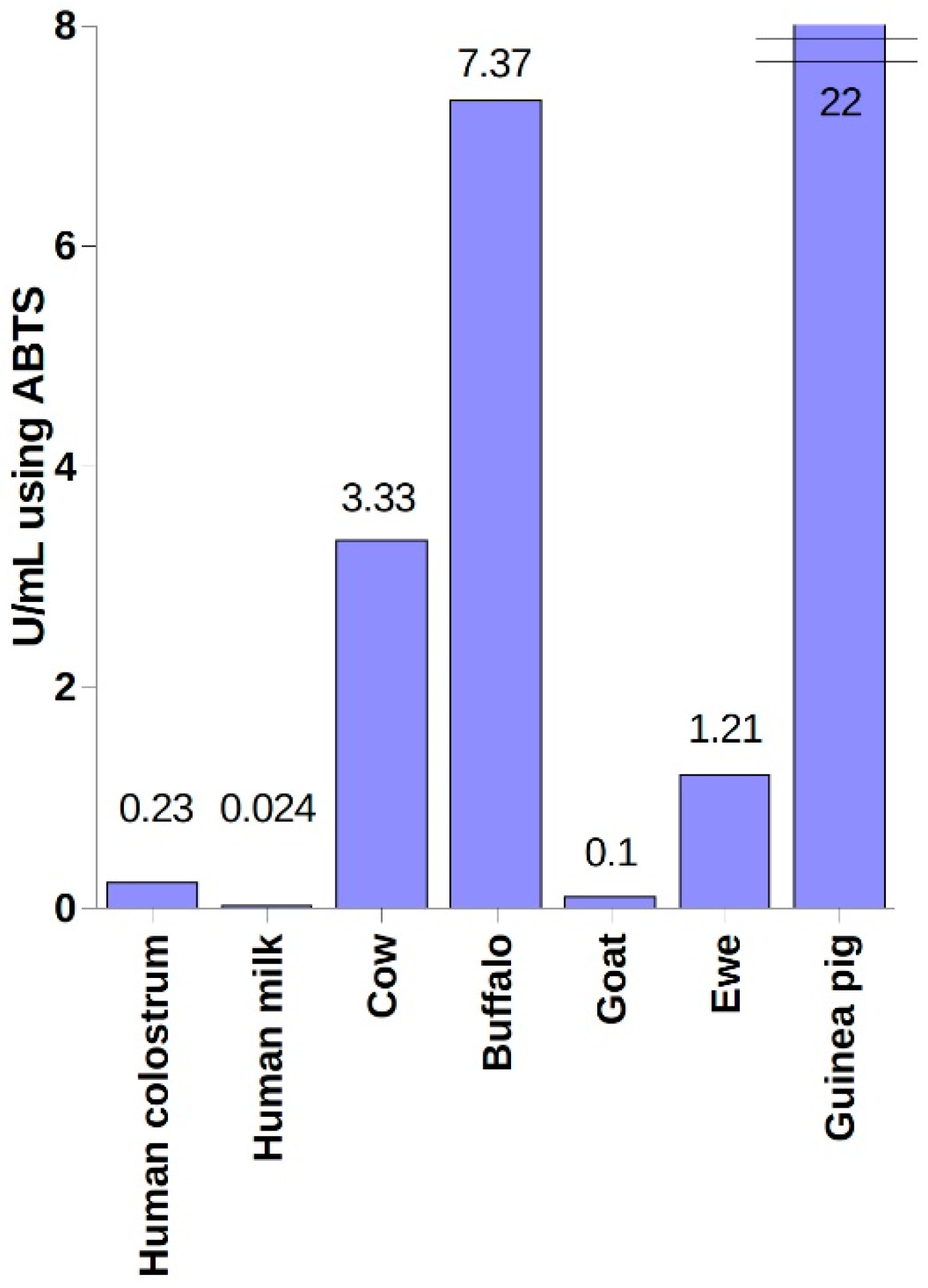
4. Industrial Sources of LPO and Methods of Its Purification
5. Substrates—Availability and Importance
5.1. Hydrogen Peroxide
5.2. Thiocyanates, Iodides, Bromides
6. Chemical Mechanism of Action
6.1. Halogenation Cycle
6.2. Peroxidation Cycle
- LPO (Compound I) + AH → LPO (Compound II) + A•
- LPO (Compound II) + AH → LPO (native form) + A•
6.3. Inactive Forms
6.4. Reactivators
6.5. Inhibitors
7. Biological Role of the LPO System
7.1. Mechanism of Biocidal Activity
7.2. The Effect of the LPO System on Dental Plaque
7.3. Inhibition of Organic Acids Production
7.4. Defense against Oxidative Stress
7.5. Effect on Carcinogens
8. Clinical Application
8.1. Review of Clinical Trials and In vitro Tests
8.1.1. Caries Prevention
8.1.2. Periodontal Disease
8.1.3. Other Applications
8.2. Dentifrices with the LPO System
9. Stability Extension
Application of Nanotechnology
10. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LPO | Lactoperoxidases |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| GOx | Glucose oxidase |
| LYS | Lysozyme |
| LF | Lactoferrin |
References
- Ihalin, R.; Loimaranta, V.; Tenovuo, J. Origin, structure, and biological activities of peroxidases in human saliva. Arch. Biochem. Biophys. 2006, 445, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzym. Res. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gudipaneni, R.K.; Kumar, R.V.; Jesudass, G.; Peddengatagari, S.; Duddu, Y. Short term comparative evaluation of antimicrobial efficacy of tooth paste containing lactoferrin, lysozyme, lactoperoxidase in children with severe early childhood caries: A clinical study. J. Clin. Diagn. Res. 2014, 8, ZC18–ZC20. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Kobayashi, T.; Wakabayashi, H.; Yamauchi, K.; Iwatsuki, K.; Yoshie, H. Effects of Orally Administered Lactoferrin and Lactoperoxidase-Containing Tablets on Clinical and Bacteriological Profiles in Chronic Periodontitis Patients. Int. J. Dent. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borzouee, F.; Mofid, M.R.; Varshosaz, J.; Samsam Shariat, S.Z.A. Purification of lactoperoxidase from bovine whey and investigation of kinetic parameters. Adv. Biomed. Res. 2016, 5, 189. [Google Scholar] [PubMed]
- Zámocký, M.; Hofbauer, S.; Schaffner, I.; Gasselhuber, B.; Nicolussi, A.; Soudi, M.; Pirker, K.F.; Furtmüller, P.G.; Obinger, C. Independent evolution of four heme peroxidase superfamilies. Arch. Biochem. Biophys. 2015, 574, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Sakamaki, K.; Kuroki, T.; Yano, I.; Nagata, S. Molecular cloning and characterization of the chromosomal gene for human lactoperoxidase. Eur. J. Biochem. 1997, 243, 32–41. [Google Scholar] [CrossRef]
- Sakamaki, K.; Kanda, N.; Ueda, T.; Aikawa, E.; Nagata, S. The eosinophil peroxidase gene forms a cluster with the genes for myeloperoxidase and lactoperoxidase on human chromosome 17. Cytogenet. Cell Genet. 2000, 88, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, A.K.; Kaushik, S.; Sinha, M.; Singh, R.P.; Sharma, P.; Sirohi, H.; Kaur, P.; Singh, T.P. Lactoperoxidase: Structural insights into the function, ligand binding and inhibition. Int. J. Biochem. Mol. Biol. 2013, 4, 108–128. [Google Scholar] [PubMed]
- Shin, K.; Tomita, M.; Lönnerdal, B. Identification of lactoperoxidase in mature human milk. J. Nutr. Biochem. 2000, 11, 94–102. [Google Scholar] [CrossRef]
- Kiser, C.; Caterina, C.K.; Engler, J.A.; Rahemtulla, B.; Rahemtulla, F. Cloning and sequence analysis of the human salivary peroxidase-encoding cDNA. Gene 1996, 173, 261–264. [Google Scholar] [CrossRef]
- Dull, T.J.; Uyeda, C.; Strosberg, A.D.; Nedwin, G.; Seilhamer, J.J. Molecular cloning of cDNAs encoding bovine and human lactoperoxidase. DNA Cell Biol. 1990, 9, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.A.; Torbati, A.; Fregien, N.; Conner, G.E. Molecular heterogeneity and alternative splicing of human lactoperoxidase. Arch. Biochem. Biophys. 2009, 482, 52–57. [Google Scholar] [CrossRef]
- Mohan Reddy, P.; Kottekad, S. Comparative Site-Specific N -Glycosylation Analysis of Lactoperoxidase from Buffalo and Goat Milk Using RP-UHPLC–MS/MS Reveals a Distinct Glycan Pattern. J. Agric. Food Chem. 2018, 66, 11492–11499. [Google Scholar]
- Banerjee, S.; Furtmüller, P.G.; Obinger, C. Bovine lactoperoxidase—A versatile one- and two-electron catalyst of high structural and thermal stability. Biotechnol. J. 2011, 6, 231–243. [Google Scholar] [CrossRef]
- Shin, K.; Hayasawa, H.; Lönnerdal, B. Purification and quantification of lactoperoxidase in human milk with use of immunoadsorbents with antibodies against recombinant human lactoperoxidase. Am. J. Clin. Nutr. 2001, 73, 984–989. [Google Scholar] [CrossRef]
- Booth, K.S.; Kimura, S.; Lee, H.C.; Ikeda-Saito, M.; Caughey, W.S. Bovine myeloperoxidase and lactoperoxidase each contain a high affinity site for calcium. Biochem. Biophys. Res. Commun. 1989, 160, 897–902. [Google Scholar] [CrossRef]
- Kussendrager, K.D.; van Hooijdonk, A.C. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br. J. Nutr. 2000, 84 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef]
- Mansson-Rahemtulla, B.; Rahemtulla, F.; Baldone, D.C.; Pruitt, K.M.; Hjerpe, A. Purification and characterization of human salivary peroxidase. Biochemistry 1988, 27, 233–239. [Google Scholar] [CrossRef]
- Hayes, H.C.; Popescu, P.; Dutrillaux, B. Comparative gene mapping of lactoperoxidase, retinoblastoma, and ?-lactalbumin genes in cattle, sheep, and goats. Mamm. Genome 1993, 4, 593–597. [Google Scholar] [CrossRef]
- Morrison, M.; Allen, P.Z. Lactoperoxidase: Identification and isolation from Harderian and lacrimal glands. Science 1966, 152, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.A.; Ahluwalia, B.S.; Westney, L.S.; Burnett, C.C.; Rüchel, R. Cervical mucus peroxidase is a reliable indicator for ovulation in humans. Fertil. Steril. 1984, 41, 697–702. [Google Scholar] [CrossRef]
- Gerson, C.; Sabater, J.; Scuri, M.; Torbati, A.; Coffey, R.; Abraham, J.W.; Lauredo, I.; Forteza, R.; Wanner, A.; Salathe, M.; et al. The lactoperoxidase system functions in bacterial clearance of airways. Am. J. Respir. Cell Mol. Biol. 2000, 22, 665–671. [Google Scholar] [CrossRef]
- Riva, A.; Puxeddu, P.; del Fiacco, M.; Testa-Riva, F. Ultrastructural localization of endogenous peroxidase in human parotid and submandibular glands. J. Anat. 1978, 127, 181–191. [Google Scholar] [PubMed]
- Tenovuo, J.; Pruitt, K.M. Relationship of the human salivary peroxidase system to oral health. J. Oral Pathol. 1984, 13, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, J.; Grahn, E.; Lehtonen, O.-P.; Hyyppa, T.; Karhuvaara, L.; Vilja, P. Antimicrobial Factors in Saliva: Ontogeny and Relation to Oral Health. J. Dent. Res. 1987, 66, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Klangprapan, S.; Chaiyarit, P.; Hormdee, D.; Kampichai, A.; Khampitak, T.; Daduang, J.; Tavichakorntrakool, R.; Panijpan, B.; Boonsiri, P. Salivary Myeloperoxidase, Assessed by 3,3′-Diaminobenzidine Colorimetry, Can Differentiate Periodontal Patients from Nonperiodontal Subjects. Enzym. Res. 2016, 2016, 7517928. [Google Scholar] [CrossRef]
- Sakamoto, W.; Fujii, Y.; Kanehira, T.; Asano, K.; Izumi, H. A novel assay system for myeloperoxidase activity in whole saliva. Clin. Biochem. 2008, 41, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Gau, J.; Arnhold, J.; Flemmig, J. Reactivation of peroxidase activity in human saliva samples by polyphenols. Arch. Oral Biol. 2018, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.M.; Adamson, M. Enzyme activity of salivary lactoperoxidase adsorbed to human enamel. Infect. Immun. 1977, 17, 112–116. [Google Scholar]
- Tenovuo, J.; Valtakoski, J.; Knuuttila, M.L.E. Antibacterial Activity of Lactoperoxidase Adsorbed by Human Salivary Sediment and Hydroxyapatite. Caries Res. 1977, 11, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Göcke, R.; Gerath, F.; Schwanewede, H. von Quantitative determination of salivary components in the pellicle on PMMA denture base material. Clin. Oral Investig. 2002, 6, 227–235. [Google Scholar] [PubMed]
- Haberska, K.; Svensson, O.; Shleev, S.; Lindh, L.; Arnebrant, T.; Ruzgas, T. Activity of lactoperoxidase when adsorbed on protein layers. Talanta 2008, 76, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Halthur, T.J.; Arnebrant, T.; Macakova, L.; Feiler, A. Sequential Adsorption of Bovine Mucin and Lactoperoxidase to Various Substrates Studied with Quartz Crystal Microbalance with Dissipation. Langmuir 2010, 26, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Gothefors, L.; Marklund, S. Lactoperoxidase activity in human milk and in saliva of newborn infants. Infect. Immun. 1975, 11, 1210–1215. [Google Scholar] [PubMed]
- Salvolini, E.; Martarelli, D.; Di Giorgio, R.; Mazzanti, L.; Procaccini, M.; Curatola, G. Age-related modifications in human unstimulated whole saliva: A biochemical study. Aging Clin. Exp. Res. 2000, 12, 445–448. [Google Scholar] [CrossRef]
- Memarzadeh Zahedani, M.; Schwahn, C.; Baguhl, R.; Kocher, T.; Below, H.; Welk, A. Association of salivary peroxidase activity and concentration with periodontal health: A validity study. J. Clin. Periodontol. 2017, 44, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Damirchi, A.; Kiani, M.; Jafarian, V.; Sariri, R. Response of salivary peroxidase to exercise intensity. Eur. J. Appl. Physiol. 2010, 108, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Arazi, H.; Simaei, E.; Taati, B. Comparison of responses of salivary antioxidant markers to exhaustive aerobic exercise in smoker and non-smoker young girls. J. Sports Med. Phys. Fit. 2016, 56, 1132–1138. [Google Scholar]
- Cockle, S.M.; Harkeness, R.A. Changes in salivary peroxidase and polymorphonuclear neutrophil leucocyte enzyme activities during the menstrual cycle. BJOG Int. J. Obstet. Gynaecol. 1978, 85, 776–782. [Google Scholar] [CrossRef]
- Tenovuo, J.; Laine, M.; Söderling, E.; Irjala, K. Evaluation of salivary markers during the menstrual cycle: Peroxidase, protein, and electrolytes. Biochem. Med. 1981, 25, 337–345. [Google Scholar] [CrossRef]
- Laine, M.; Tenovuo, J.; Lehtonen, O.-P.; Ojanotko-Harri, A.; Vilja, P.; Tuohimaa, P. Pregnancy-related changes in human whole saliva. Arch. Oral Biol. 1988, 33, 913–917. [Google Scholar] [CrossRef]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjänen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.-A.; Laine, M. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef]
- Goi, N.; Hirai, Y.; Harada, H.; Ikari, A.; Ono, T.; Kinae, N.; Hiramatsu, M.; Nakamura, K.; Takagi, K. Comparison of peroxidase response to mental arithmetic stress in saliva of smokers and non-smokers. J. Toxicol. Sci. 2007, 32, 121–127. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Klein, I.; Eiserich, J.P.; Cross, C.E.; Nagler, R.M. Inhibition of oral peroxidase activity by cigarette smoke: In vivo and in vitro studies. Free Radic. Biol. Med. 2003, 34, 377–384. [Google Scholar] [CrossRef]
- Klein, I.; Nagler, R.M.; Toffler, R.; van Der Vliet, A.; Reznick, A.Z. Effect of cigarette smoke on oral peroxidase activity in human saliva: Role of hydrogen cyanide. Free Radic. Biol. Med. 2003, 35, 1448–1452. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Zalewska, A.; Szajda, S.D.; Szulc, A.; Kępka, A.; Minarowska, A.; Wojewódzka-Żelezniakowicz, M.; Konarzewska, B.; Chojnowska, S.; Supronowicz, Z.B.; et al. The effect of chronic alcohol intoxication and smoking on the activity of oral peroxidase. Folia Histochem. Cytobiol. 2012, 50, 450–455. [Google Scholar] [CrossRef]
- Jin, M.; Ande, A.; Kumar, A.; Kumar, S. Regulation of cytochrome P450 2e1 expression by ethanol: Role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013, 4, e554. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Szajda, S.D.; Jankowska, A.; Zwierz, P.; Czernikiewicz, A.; Szulc, A.; Zwierz, K. The Effect of Acute Ethanol Intoxication on Salivary Proteins of Innate and Adaptive Immunity. Alcohol. Clin. Exp. Res. 2008, 32, 652–656. [Google Scholar] [CrossRef]
- Nayak, P.A.; Nayak, U.A.; Khandelwal, V. The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 2014, 6, 89–94. [Google Scholar] [CrossRef]
- Mäkinen, K.K.; Tenovuo, J.; Scheinin, A. Xylitol-induced increase of lactoperoxidase activity. J. Dent. Res. 1976, 55, 652–660. [Google Scholar] [CrossRef]
- Kim, B.-S.; Chang, J.-Y.; Kim, Y.-Y.; Kho, H.-S. The effects of xylitol and sorbitol on lysozyme- and peroxidase-related enzymatic and candidacidal activities. Arch. Oral Biol. 2015, 60, 998–1006. [Google Scholar] [CrossRef]
- Saxén, L.; Tenovuo, J.; Vilja, P. Salivary defense mechanisms in juvenile periodontitis. Acta Odontol. Scand. 1990, 48, 399–407. [Google Scholar] [CrossRef]
- Güven, Y.; Satman, I.; Dinççağ, N.; Alptekin, S. Salivary peroxidase activity in whole saliva of patients with insulin-dependent (type-1) diabetes mellitus. J. Clin. Periodontol. 1996, 23, 879–881. [Google Scholar] [CrossRef]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef]
- Rosin, M.; Hanschke, M.; Splieth, C.; Kramer, A. Activities of lysozyme and salivary peroxidase in unstimulated whole saliva in relation to plaque and gingivitis scores in healthy young males. Clin. Oral Investig. 1999, 3, 133–137. [Google Scholar] [CrossRef]
- Rudney, J.D.; Krig, M.A.; Neuvar, E.K.; Soberay, A.H.; Iverson, L. Antimicrobial proteins in human unstimulated whole saliva in relation to each other, and to measures of health status, dental plaque accumulation and composition. Arch. Oral Biol. 1991, 36, 497–506. [Google Scholar] [CrossRef]
- Bielawski, K. The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014, 20, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, B.L.; Pruitt, K.M.; Pederson, E.D.; Golding, M.P. Comparison of Salivary Peroxidase System Components in Caries-Free and Caries-Active Naval Recruits. Caries Res. 1984, 18, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.C.; Reginald, B.A. Aphthous ulcers, salivary peroxidase and stress: Are they related? J Oral Maxillofac Pathol 2015, 19, 37–41. [Google Scholar] [PubMed]
- Uguz, M.T.; Ozdemir, H. Purification of Bovine Milk Lactoperoxidase and Investigation of Antibacterial Properties at Different Thiocyanate Mediated. Appl. Biochem. Microbiol. 2005, 41, 349–353. [Google Scholar] [CrossRef]
- Kumar, R.; Bhatia, K.L. Standardization of method for lactoperoxidase assay in milk. Le Lait 1999, 79, 269–274. [Google Scholar] [CrossRef]
- Fonteh, F.A.; Grandison, A.S.; Lewis, M.J. Variations of lactoperoxidase activity and thiocyanate content in cows’ and goats’ milk throughout lactation. J. Dairy Res. 2002, 69, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Gaya, P.; Nuñez, M. The lactoperoxidase system in ewes’ milk: Levels of lactoperoxidase and thiocyanate. Lett. Appl. Microbiol. 1989, 8, 147–149. [Google Scholar] [CrossRef]
- Tayefi-Nasrabadi, H.; Hoseinpour-fayzi, M.A.; Mohasseli, M. Effect of heat treatment on lactoperoxidase activity in camel milk: A comparison with bovine lactoperoxidase. Small Rumin. Res. 2011, 99, 187–190. [Google Scholar] [CrossRef]
- Stephens, S.; Harkness, R.A.; Cockle, S.M. Lactoperoxidase activity in guinea-pig milk and saliva: Correlation in milk of lactoperoxidase with bactericidal activity against Escherichia coli. Br. J. Exp. Pathol. 1979, 60, 252–258. [Google Scholar]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals (Basel) 2016, 9, 61. [Google Scholar] [CrossRef]
- Mandal, P.K.; Mitra, M.; Acharya, S.; Ghosh, C.; Mohanty, S.; Saha, S. Salivary IgA versus HIV and Dental Caries. J. Clin. Diagn. Res. 2016, 10, ZC61–ZC64. [Google Scholar] [CrossRef]
- Andersson, J.; Mattiasson, B. Simulated moving bed technology with a simplified approach for protein purification. Separation of lactoperoxidase and lactoferrin from whey protein concentrate. J. Chromatogr. A 2006, 1107, 88–95. [Google Scholar] [CrossRef]
- Atasever, A.; Ozdemir, H.; Gulcin, I.; Irfan Kufrevioglu, O. One-step purification of lactoperoxidase from bovine milk by affinity chromatography. Food Chem. 2013, 136, 864–870. [Google Scholar] [CrossRef]
- Urtasun, N.; Baieli, M.F.; Hirsch, D.B.; Martínez-Ceron, M.C.; Cascone, O.; Wolman, F.J. Lactoperoxidase purification from whey by using dye affinity chromatography. Food Bioprod. Process. 2017, 103, 58–65. [Google Scholar] [CrossRef]
- Andersson, L.A.; Bylkas, S.A.; Wilson, A.E. Spectral Analysis of Lactoperoxidase: EVIDENCE FOR A COMMON HEME IN MAMMALIAN PEROXIDASES. J. Biol. Chem. 1996, 271, 3406–3412. [Google Scholar] [CrossRef]
- Pruitt, K.M.; Tenovuo, J.; Mansson-Rahemtulla, B.; Harrington, P.; Baldone, D.C. Is thiocyanate peroxidation at equilibrium in vivo? Biochim. Biophys. Acta 1986, 870, 385–391. [Google Scholar] [CrossRef]
- Gau, J.; Prévost, M.; Van Antwerpen, P.; Sarosi, M.-B.; Rodewald, S.; Arnhold, J.; Flemmig, J. Tannins and Tannin-Related Derivatives Enhance the (Pseudo-)Halogenating Activity of Lactoperoxidase. J. Nat. Prod. 2017, 80, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Coykendall, A.L. Classification and identification of the viridans streptococci. Clin. Microbiol. Rev. 1989, 2, 315–328. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Shi, W.; Qi, F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef]
- Carlsson, J.; Iwami, Y.; Yamada, T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect. Immun. 1983, 40, 70–80. [Google Scholar]
- Chen, L.; Ge, X.; Dou, Y.; Wang, X.; Patel, J.R.; Xu, P. Identification of hydrogen peroxide production-related genes in Streptococcus sanguinis and their functional relationship with pyruvate oxidase. Microbiology 2011, 157, 13–20. [Google Scholar] [CrossRef]
- Tong, H.; Chen, W.; Merritt, J.; Qi, F.; Shi, W.; Dong, X. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: A possible counteroffensive strategy for interspecies competition. Mol. Microbiol. 2007, 63. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Chen, W.; Shi, W.; Qi, F.; Dong, X. SO-LAAO, a novel L-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 2008, 190, 4716–4721. [Google Scholar] [CrossRef]
- Liu, L.; Tong, H.; Dong, X. Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl. Environ. Microbiol. 2012, 78, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A. Role of the NADPH oxidase systems Nox and Duox in host defense and inflammation. Expert Rev. Clin. Immunol. 2007, 3, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Witta, J.; Baffi, J.; Lekstrom, K.; Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003, 17, 1502–1504. [Google Scholar] [CrossRef] [PubMed]
- Lara-Aguilar, S.; Alcaine, S.D. Lactose oxidase: A novel activator of the lactoperoxidase system in milk for improved shelf life. J. Dairy Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sandholm, M.; Ali-Vehmas, T.; Kaartinen, L.; Junnila, M. Glucose Oxidase (GOD) as a Source of Hydrogen Peroxide for the Lactoperoxidase (LPO) System in Milk: Antibacterial Effect of the GOD-LPO System against Mastitis Pathogens. J. Vet. Med. Ser. B 1988, 35, 346–352. [Google Scholar] [CrossRef]
- Wijkstrom-Frei, C.; El-Chemaly, S.; Ali-Rachedi, R.; Gerson, C.; Cobas, M.A.; Forteza, R.; Salathe, M.; Conner, G.E. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell Mol. Biol. 2003, 29, 206–212. [Google Scholar] [CrossRef]
- Tsuge, K.; Kataoka, M.; Seto, Y. Cyanide and Thiocyanate Levels in Blood and Saliva of Healthy Adult Volunteers. J. Health Sci. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Narkowicz, S.; Jaszczak, E.; Polkowska, Ż.; Kiełbratowska, B.; Kotłowska, A.; Namieśnik, J. Determination of thiocyanate as a biomarker of tobacco smoke constituents in selected biological materials of human origin. Biomed. Chromatogr. 2018, 32, e4111. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.P.; Ahmed, M.K.; Dawes, C.; Mantsch, H.H. Thiocyanate levels in human saliva: Quantitation by Fourier transform infrared spectroscopy. Anal. Biochem. 1996, 240, 7–12. [Google Scholar] [CrossRef]
- Paul, B.D.; Smith, M.L. Cyanide and thiocyanate in human saliva by gas chromatography-mass spectrometry. J. Anal. Toxicol. 2006, 30, 511–515. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Darvell, B.W.; Leung, V.W.-H. Human salivary anionic analysis using ion chromatography. Arch. Oral Biol. 2004, 49, 863–869. [Google Scholar] [CrossRef]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Conner, G.E.; Wijkstrom-Frei, C.; Randell, S.H.; Fernandez, V.E.; Salathe, M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007, 581, 271–278. [Google Scholar] [CrossRef]
- Fragoso, M.A.; Fernandez, V.; Forteza, R.; Randell, S.H.; Salathe, M.; Conner, G.E. Transcellular thiocyanate transport by human airway epithelia: Transcellular thiocyanate transport by human airway epithelia. J. Physiol. 2004, 561, 183–194. [Google Scholar] [CrossRef]
- Peters, C.J.; Yu, H.; Tien, J.; Jan, Y.N.; Li, M.; Jan, L.Y. Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc. Natl. Acad. Sci. USA 2015, 112, 3547–3552. [Google Scholar] [CrossRef]
- Pedemonte, N.; Caci, E.; Sondo, E.; Caputo, A.; Rhoden, K.; Pfeffer, U.; Di Candia, M.; Bandettini, R.; Ravazzolo, R.; Zegarra-Moran, O.; et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: Role of pendrin and anion channels. J. Immunol. 2007, 178, 5144–5153. [Google Scholar] [CrossRef]
- Rosin, M.; Kramer, A.; Bradtke, D.; Richter, G.; Kocher, T. The effect of a SCN-/H2O2 toothpaste compared to a commercially available triclosan-containing toothpaste on oral hygiene and gingival health - a 6-month home-use study. J. Clin. Periodontol. 2002, 29, 1086–1091. [Google Scholar] [CrossRef]
- Kirstilä, V.; Lenander-Lumikari, M.; Tenovuo, J. Effects of a lactoperoxidase-system-containing toothpaste on dental plaque and whole saliva in vivo. Acta Odontol. Scand. 1994, 52, 346–353. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Jantschko, W.; Regelsberger, G.; Obinger, C. Two-electron reduction and one-electron oxidation of organic hydroperoxides by human myeloperoxidase. FEBS Lett. 2000, 484, 139–143. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Obinger, C. Reaction of Myeloperoxidase Compound I with Chloride, Bromide, Iodide, and Thiocyanate. Biochemistry 1998, 37, 17923–17930. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Jantschko, W.; Regelsberger, G.; Jakopitsch, C.; Arnhold, J.; Obinger, C. Reaction of Lactoperoxidase Compound I with Halides and Thiocyanate †. Biochemistry 2002, 41, 11895–11900. [Google Scholar] [CrossRef]
- Harden, R.M.; Alexander, W.D.; Shimmins, J.; Chisholm, D. A comparison between the gastric and salivary concentration of iodide, pertechnetate, and bromide in man. Gut 1969, 10, 928–930. [Google Scholar] [CrossRef]
- Rodríguez-López, J.N.; Gilabert, M.A.; Tudela, J.; Thorneley, R.N.F.; García-Cánovas, F. Reactivity of Horseradish Peroxidase Compound II toward Substrates: Kinetic Evidence for a Two-Step Mechanism †. Biochemistry 2000, 39, 13201–13209. [Google Scholar] [CrossRef]
- Che, H.; Tian, B.; Bai, L.; Cheng, L.; Liu, L.; Zhang, X.; Jiang, Z.; Xu, X. Development of a test strip for rapid detection of lactoperoxidase in raw milk. J. Zhejiang Univ. Sci. B 2015, 16, 672–679. [Google Scholar] [CrossRef]
- Bach, C.E.; Warnock, D.D.; Van Horn, D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D.; German, D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef]
- Huwiler, M.; Jenzer, H.; Kohler, H. The role of compound III in reversible and irreversible inactivation of lactoperoxidase. Eur. J. Biochem. 1986, 158, 609–614. [Google Scholar] [CrossRef]
- George, P. The third intermediate compound of horseradish peroxidase and hydrogen peroxide. J. Biol. Chem. 1953, 201, 427–434. [Google Scholar]
- Gau, J.; Furtmüller, P.G.; Obinger, C.; Prévost, M.; Van Antwerpen, P.; Arnhold, J.; Flemmig, J. Flavonoids as promoters of the (pseudo-)halogenating activity of lactoperoxidase and myeloperoxidase. Free Radic. Biol. Med. 2016, 97, 307–319. [Google Scholar] [CrossRef]
- Gau, J.; Furtmüller, P.-G.; Obinger, C.; Arnhold, J.; Flemmig, J. Enhancing hypothiocyanite production by lactoperoxidase – mechanism and chemical properties of promotors. Biochem. Biophys. Rep. 2015, 4, 257–267. [Google Scholar] [CrossRef]
- Flemmig, J.; Rusch, D.; Czerwińska, M.E.; Rauwald, H.-W.; Arnhold, J. Components of a standardised olive leaf dry extract (Ph. Eur.) promote hypothiocyanite production by lactoperoxidase. Arch. Biochem. Biophys. 2014, 549, 17–25. [Google Scholar] [CrossRef]
- Flemmig, J.; Noetzel, I.; Arnhold, J.; Rauwald, H.-W. Leonurus cardiaca L. herb extracts and their constituents promote lactoperoxidase activity. J. Funct. Foods 2015, 17, 328–339. [Google Scholar] [CrossRef]
- Gomes, D.A.S.; Pires, J.R.; Zuza, E.P.; Muscara, M.N.; Herrera, B.S.; Spolidorio, L.C.; Toledo, B.E.C.; Spolidorio, D.M.P. Myeloperoxidase as inflammatory marker of periodontal disease: Experimental study in rats. Immunol. Invest. 2009, 38, 117–122. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Seidel, A.; Parker, H.; Turner, R.; Dickerhof, N.; Khalilova, I.S.; Wilbanks, S.M.; Kettle, A.J.; Jameson, G.N.L. Uric Acid and Thiocyanate as Competing Substrates of Lactoperoxidase. J. Biol. Chem. 2014, 289, 21937–21949. [Google Scholar] [CrossRef]
- Jurczak, A.; Kościelniak, D.; Skalniak, A.; Papież, M.; Vyhouskaya, P.; Krzyściak, W. The role of the saliva antioxidant barrier to reactive oxygen species with regard to caries development. Redox Rep. 2017, 22, 524–533. [Google Scholar] [CrossRef]
- Köksal, Z.; Alim, Z. Lactoperoxidase, an antimicrobial enzyme, is inhibited by some indazoles. Drug Chem. Toxicol. 2018, 1–5. [Google Scholar] [CrossRef]
- Gąsowska-Bajger, B.; Nishigaya, Y.; Hirsz-Wiktorzak, K.; Rybczyńska, A.; Yamazaki, T.; Wojtasek, H. Interference of carbidopa and other catechols with reactions catalyzed by peroxidases. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1626–1634. [Google Scholar] [CrossRef]
- Koksal, Z.; Alim, Z.; Beydemir, S.; Ozdemir, H. Potent Inhibitory Effects of Some Phenolic Acids on Lactoperoxidase. J. Biochem. Mol. Toxicol. 2016, 30, 533–538. [Google Scholar] [CrossRef]
- Sisecioglu, M.; Cankaya, M.; Gulcin, I.; Ozdemir, H. The Inhibitory Effect of Propofol on Bovine Lactoperoxidase. Protein Pept. Lett. 2009, 16, 46–49. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Jiffri, E.H.; Ashraf, G.M.; Kamal, M.A.; Beg, M.A. Structural studies on inhibitory mechanisms of antibiotic, corticosteroid and catecholamine molecules on lactoperoxidase. Life Sci. 2018, 207, 412–419. [Google Scholar] [CrossRef]
- Pruitt, K.M.; Mansson-Rahemtulla, B.; Baldone, D.C.; Rahemtulla, F. Steady-state kinetics of thiocyanate oxidation catalyzed by human salivary peroxidase. Biochemistry 1988, 27, 240–245. [Google Scholar] [CrossRef]
- Dua, S.; Maclean, M.J.; Fitzgerald, M.; McAnoy, A.M.; Bowie, J.H. Is the hypothiocyanite anion (OSCN)- the major product in the peroxidase catalyzed oxidation of the thiocyanate anion (SCN)-? A joint experimental and theoretical study. J. Phys. Chem. A 2006, 110, 4930–4936. [Google Scholar] [CrossRef]
- Thomas, E.L. Lactoperoxidase-catalyzed oxidation of thiocyanate: Equilibriums between oxidized forms of thiocyanate. Biochemistry 1981, 20, 3273–3280. [Google Scholar] [CrossRef]
- Love, D.T.; Barrett, T.J.; White, M.Y.; Cordwell, S.J.; Davies, M.J.; Hawkins, C.L. Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages. Free Radic. Biol. Med. 2016, 94, 88–98. [Google Scholar] [CrossRef]
- Thomas, E.L.; Aune, T.M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: Correlation of sulfhydryl oxidation with antimicrobial action. Infect. Immun. 1978, 20, 456–463. [Google Scholar]
- Courtois, P.H.; Pourtois, M. Purification of NADH: Hypothiocyanite oxidoreductase in Streptococcus sanguis. Biochem. Mol. Med. 1996, 57, 134–138. [Google Scholar] [CrossRef]
- Huwiler, M.; Kohler, H. Pseudo-catalytic degradation of hydrogen peroxide in the lactoperoxidase/H2O2/iodide system. Eur. J. Biochem. 1984, 141, 69–74. [Google Scholar] [CrossRef]
- Gottardi, W. Iodine and Disinfection: Theoretical Study on Mode of Action, Efficiency, Stability, and Analytical Aspects in the Aqueous System. Arch. Der Pharm. 1999, 332, 151–157. [Google Scholar] [CrossRef]
- Thomas, E.L.; Aune, T.M. Peroxidase-catalyzed oxidation of protein sulfhydryls mediated by iodine. Biochemistry 1977, 16, 3581–3586. [Google Scholar] [CrossRef]
- Vanden Abbeele, A.; De Meel, H.; Courtois, P.; Pourtois, M. Influence of a hypoiodite mouth-wash on dental plaque formation in vivo. Bull. Group Int. Rech. Sci. Stomatol. Odontol. 1996, 39, 57–61. [Google Scholar]
- Majerus, P.M.; Courtois, P.A. Susceptibility of Candida albicans to peroxidase-catalyzed oxidation products of thiocyanate, iodide and bromide. J. Biol. Buccale 1992, 20, 241–245. [Google Scholar]
- Ahariz, M.; Courtois, P. Candida albicans susceptibility to lactoperoxidase-generated hypoiodite. Clin. Cosmet. Investig. Dent. 2010, 2, 69–78. [Google Scholar]
- Bosch, E.H.; van Doorne, H.; de Vries, S. The lactoperoxidase system: The influence of iodide and the chemical and antimicrobial stability over the period of about 18 months. J. Appl. Microbiol. 2000, 89, 215–224. [Google Scholar] [CrossRef]
- Schlorke, D.; Flemmig, J.; Birkemeyer, C.; Arnhold, J. Formation of cyanogen iodide by lactoperoxidase. J. Inorg. Biochem. 2016, 154, 35–41. [Google Scholar] [CrossRef]
- Van Houte, J. Role of Micro-organisms in Caries Etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef]
- Axelsson, P.; Lindhe, J.; Nyström, B. On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. J. Clin. Periodontol. 1991, 18, 182–189. [Google Scholar] [CrossRef]
- Roger, V.; Tenovuo, J.; Lenander-Lumikari, M.; Söderling, E.; Vilja, P. Lysozyme and lactoperoxidase inhibit the adherence of Streptococcus mutans NCTC 10449 (serotype c) to saliva-treated hydroxyapatite in vitro. Caries Res. 1994, 28, 421–428. [Google Scholar] [CrossRef]
- Cawley, A.; Golding, S.; Goulsbra, A.; Hoptroff, M.; Kumaran, S.; Marriott, R. Microbiology insights into boosting salivary defences through the use of enzymes and proteins. J. Dent. 2019, 80, S19–S25. [Google Scholar] [CrossRef]
- Korpela, A.; Yu, X.; Loimaranta, V.; Lenander-Lumikari, M.; Vacca-Smith, A.; Wunder, D.; Bowen, W.H.; Tenovuo, J. Lactoperoxidase inhibits glucosyltransferases from Streptococcus mutans in vitro. Caries Res. 2002, 36, 116–121. [Google Scholar] [CrossRef]
- Tenovuo, J.; Mansson-Rahemtulla, B.; Pruitt, K.M.; Arnold, R. Inhibition of dental plaque acid production by the salivary lactoperoxidase antimicrobial system. Infect. Immun. 1981, 34, 208–214. [Google Scholar]
- Oram, J.D.; Reiter, B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem. J. 1966, 100, 373–381. [Google Scholar] [CrossRef]
- Adamson, M.; Pruitt, K.M. Lactoperoxidase-catalyzed inactivation of hexokinase. Biochim. Et Biophys. Acta (BBA) Enzymol. 1981, 658, 238–247. [Google Scholar] [CrossRef]
- Carlsson, J. Salivary peroxidase: An important part of our defense against oxygen toxicity. J. Oral Pathol. 1987, 16, 412–416. [Google Scholar] [CrossRef]
- Mickelson, M.N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J. Bacteriol. 1977, 132, 541–548. [Google Scholar]
- Hawkins, C.L. The role of hypothiocyanous acid (HOSCN) in biological systems. Free Radic. Res. 2009, 43, 1147–1158. [Google Scholar] [CrossRef]
- Reiter, B.; Härnulv, G. Lactoperoxidase Antibacterial System: Natural Occurrence, Biological Functions and Practical Applications. J. Food Prot. 1984, 47, 724–732. [Google Scholar] [CrossRef]
- Lingström, P.; van Ruyven, F.O.; van Houte, J.; Kent, R. The pH of dental plaque in its relation to early enamel caries and dental plaque flora in humans. J. Dent. Res. 2000, 79, 770–777. [Google Scholar] [CrossRef]
- Seethalakshmi, C. Correlation of Salivary pH, Incidence of Dental Caries and Periodontal Status in Diabetes Mellitus Patients: A Cross-sectional Study. J Clin Diagn Res. 2016, 10, ZC12–ZC14. [Google Scholar] [CrossRef]
- Thomas, E.L.; Pera, K.A.; Smith, K.W.; Chwang, A.K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect. Immun. 1983, 39, 767–778. [Google Scholar]
- Nakamura, J.; Purvis, E.R.; Swenberg, J.A. Micromolar concentrations of hydrogen peroxide induce oxidative DNA lesions more efficiently than millimolar concentrations in mammalian cells. Nucleic Acids Res. 2003, 31, 1790–1795. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Nishioka, H.; Nishi, K.; Kyokane, K. Human saliva inactivates mutagenicity of carcinogens. Mutat. Res./Environ. Mutagenesis Relat. Subj. 1981, 85, 323–333. [Google Scholar] [CrossRef]
- Yamada, M.; Tsuda, M.; Nagao, M.; Mori, M.; Sugimura, T. Degradation of mutagens from pyrolysates of tryptophan, glutamic acid and globulin by myeloperoxidase. Biochem. Biophys. Res. Commun. 1979, 90, 769–776. [Google Scholar] [CrossRef]
- Gorlewska-Roberts, K.M.; Teitel, C.H.; Lay, J.O.; Roberts, D.W.; Kadlubar, F.F. Lactoperoxidase-catalyzed activation of carcinogenic aromatic and heterocyclic amines. Chem. Res. Toxicol. 2004, 17, 1659–1666. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Beg, M.A.; Yasir, M. Molecular Interactions of Carcinogenic Aromatic Amines, 4-Aminobiphenyl and 4,4′-Diaminobiphenyl, with Lactoperoxidase—Insight to Breast Cancer. Anticancer Res. 2017, 37, 6245–6249. [Google Scholar]
- Nakano, M.; Shimizu, E.; Wakabayashi, H.; Yamauchi, K.; Abe, F. A randomized, double-blind, crossover, placebo-controlled clinical trial to assess effects of the single ingestion of a tablet containing lactoferrin, lactoperoxidase, and glucose oxidase on oral malodor. BMC Oral Health 2016, 16, 37. [Google Scholar] [CrossRef]
- Shin, K.; Yaegaki, K.; Murata, T.; Ii, H.; Tanaka, T.; Aoyama, I.; Yamauchi, K.; Toida, T.; Iwatsuki, K. Effects of a composition containing lactoferrin and lactoperoxidase on oral malodor and salivary bacteria: A randomized, double-blind, crossover, placebo-controlled clinical trial. Clin. Oral Investig. 2011, 15, 485–493. [Google Scholar] [CrossRef]
- Dirix, P.; Nuyts, S.; Vander Poorten, V.; Delaere, P.; Van den Bogaert, W. Efficacy of the BioXtra dry mouth care system in the treatment of radiotherapy-induced xerostomia. Support Care Cancer 2007, 15, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Wikström, M.; Kareem, K.L.; Almståhl, A.; Palmgren, E.; Lingström, P.; Wårdh, I. Effect of 12-month weekly professional oral hygiene care on the composition of the oral flora in dentate, dependent elderly residents: A prospective study. Gerodontology 2017, 34, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Ishikawa, K.; Nakano, M.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Ooka, T.; Hironaka, S. Effects of lactoferrin and lactoperoxidase-containing food on the oral hygiene status of older individuals: A randomized, double blinded, placebo-controlled clinical trial. Geriatr. Gerontol. Int. 2017, 17, 714–721. [Google Scholar] [CrossRef]
- Daly, S.; Seong, J.; Newcombe, R.; Davies, M.; Nicholson, J.; Edwards, M.; West, N. A randomised clinical trial to determine the effect of a toothpaste containing enzymes and proteins on gum health over 3 months. J. Dent. 2019, 80, S26–S32. [Google Scholar] [CrossRef]
- Stefanescu, B.M.; Hétu, C.; Slaughter, J.C.; O’Shea, T.M.; Shetty, A.K. A pilot study of Biotene OralBalance® gel for oral care in mechanically ventilated preterm neonates. Contemp. Clin. Trials 2013, 35, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Liang, J.; Shi, L.; Chu, J.; Li, B. [In vitro study of the effect of a lactoperoxidase-peroxidase-thiocyanate system with iodine on the cariogenicinity of streptococcus mutans]. Hua Xi Kou Qiang Yi Xue Za Zhi 2014, 32, 404–408. [Google Scholar] [PubMed]
- Welk, A.; Meller, C.; Schubert, R.; Schwahn, C.; Kramer, A.; Below, H. Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate hydrogen peroxide combination in a quantitative suspension test. BMC Microbiol. 2009, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Loimaranta, V.; Lenander-Lumikari, M.; Wunder, D.; Bowen, W.H.; Tenuvuo, J. Effect of lactoperoxidase system on glucosyltransferase D of Streptococcus mutans. Chin. J. Dent. Res. 2000, 3, 61–64. [Google Scholar] [PubMed]
- Tenovuo, J.; Makinen, K.K.; Sievers, G. Antibacterial effect of lactoperoxidase and myeloperoxidase against Bacillus cereus. Antimicrob. Agents Chemother. 1985, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.R.; Boon, N.; Bernaerts, K.; Slomka, V.; Verspecht, T.; Quirynen, M.; Teughels, W. Clinical concentrations of peroxidases cause dysbiosis in in vitro oral biofilms. J. Periodont. Res. 2018, 53, 457–466. [Google Scholar] [CrossRef]
- Jones, S.B.; West, N.X.; Nesmiyanov, P.P.; Krylov, S.E.; Klechkovskaya, V.V.; Arkharova, N.A.; Zakirova, S.A. The antibacterial efficacy of a foam mouthwash and its ability to remove biofilms. BDJ Open 2018, 4, 17038. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.L.; Azenha, G.R.; Araujo, G.S.A.; Puppin Rontani, R.M. Effectiveness of casein phosphopeptide-amorphous calcium phosphate and lysozyme, lactoferrin, and lactoperoxidase in reducing Streptococcus mutans counts in dentinal caries. Gen. Dent. 2017, 65, 47–50. [Google Scholar] [PubMed]
- Silva, M.P.; Chibebe Junior, J.; Jorjão, A.L.; da Silva Machado, A.K.; de Oliveira, L.D.; Junqueira, J.C.; Jorge, A.O.C. Influence of artificial saliva in biofilm formation of Candida albicans in vitro. Braz. Oral Res. 2012, 26, 24–28. [Google Scholar] [CrossRef]
- Loimaranta, V.; Tenovuo, J.; Korhonen, H. Combined inhibitory effect of bovine immune whey and peroxidase-generated hypothiocyanite against glucose uptake by Streptococcus mutans. Oral Microbiol. Immunol. 1998, 13, 378–381. [Google Scholar] [CrossRef]
- Jyoti, S.; Shashikiran, N.D.; Reddy, V.V.S. Effect of lactoperoxidase system containing toothpaste on cariogenic bacteria in children with early childhood caries. J. Clin. Pediatr. Dent. 2009, 33, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Lenander-Lumikari, M.; Tenovuo, J.; Mikola, H. Effects of a lactoperoxidase system-containing toothpaste on levels of hypothiocyanite and bacteria in saliva. Caries Res. 1993, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Tribble, G.D.; Kerr, J.E.; Wang, B.-Y. Genetic diversity in the oral pathogen Porphyromonas gingivalis: Molecular mechanisms and biological consequences. Future Microbiol. 2013, 8, 607–620. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef]
- Jardim Júnior, E.G.; Bosco, J.M.D.; Lopes, A.M.; Landucci, L.F.; Jardim, E.C.G.; Carneiro, S.R.S. Occurrence of Actinobacillus actinomycetemcomitans in patients with chronic periodontitis, aggressive periodontitis, healthy subjects and children with gingivitis in two cities of the state of São Paulo, Brazil. J. Appl. Oral Sci. 2006, 14, 153–156. [Google Scholar] [CrossRef]
- Lovegrove, J.M. Dental plaque revisited: Bacteria associated with periodontal disease. J. N. Z. Soc. Periodontol. 2004, 7–21. [Google Scholar]
- Wolff, L.F.; Aeppli, D.M.; Pihlstrom, B.; Anderson, L.; Stoltenberg, J.; Osborn, J.; Hardie, N.; Shelburne, C.; Fischer, G. Natural distribution of 5 bacteria associated with periodontal disease. J. Clin. Periodontol. 1993, 20, 699–706. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, K.; Sayehmiri, F.; Tavirani, M.; Dousti, M.; Sheikhi, A. Prevalence of Anaerobic Bacteria (P. gingivalis) as Major Microbial Agent in the Incidence Periodontal Diseases by Meta-analysis. J. Dent. (Shiraz) 2018, 19, 232–242. [Google Scholar]
- Adams, S.E.; Arnold, D.; Murphy, B.; Carroll, P.; Green, A.K.; Smith, A.M.; Marsh, P.D.; Chen, T.; Marriott, R.E.; Brading, M.G. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci. Rep. 2017, 7, 43344. [Google Scholar] [CrossRef]
- Fukui, Y.; Yaegaki, K.; Murata, T.; Sato, T.; Tanaka, T.; Imai, T.; Kamoda, T.; Herai, M. Diurnal changes in oral malodour among dental-office workers. Int. Dent. J. 2008, 58, 159–166. [Google Scholar] [CrossRef]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontology 2000 2002, 28, 256–279. [Google Scholar] [CrossRef]
- Yamauchi, K.; Abe, F.; Nakano, M.; Hironaka, S.; Shin, K.; Wakabayashi, H. Inactivating effects of the lactoperoxidase system on bacterial lyases involved in oral malodour production. J. Med. Microbiol. 2015, 64, 1244–1252. [Google Scholar]
- Su, N.; Marek, C.L.; Ching, V.; Grushka, M. Caries prevention for patients with dry mouth. J. Can. Dent. Assoc. 2011, 77, b85. [Google Scholar]
- Güneri, P.; Alpöz, E.; Epstein, J.B.; Çankaya, H.; Ateş, M. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec. Care Dent. 2011, 31, 123–128. [Google Scholar] [CrossRef]
- Epstein, J.B.; Emerton, S.; Le, N.D.; Stevenson-Moore, P. A double-blind crossover trial of Oral Balance gel and Biotene toothpaste versus placebo in patients with xerostomia following radiation therapy. Oral Oncol. 1999, 35, 132–137. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [PubMed]
- Laible, N.J.; Germaine, G.R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: Inhibition by chitin oligosaccharides. Infect. Immun. 1985, 48, 720–728. [Google Scholar] [PubMed]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Primo, E.D.; Otero, L.H.; Ruiz, F.; Klinke, S.; Giordano, W. The disruptive effect of lysozyme on the bacterial cell wall explored by an in-silico structural outlook: Effect of Lysozyme on the Bacterial Cell Wall. Biochem. Mol. Biol. Educ. 2018, 46, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hukić, M.; Seljmo, D.; Ramovic, A.; Ibrišimović, M.A.; Dogan, S.; Hukic, J.; Bojic, E.F. The Effect of Lysozyme on Reducing Biofilms by Staphylococcus aureus, Pseudomonas aeruginosa, and Gardnerella vaginalis: An In vitro Examination. Microb. Drug Resist. 2018, 24, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Spitzmüller, B.; Lux, H.C.; Altenburger, M.; Al-Ahmad, A.; Hannig, M. Efficacy of enzymatic toothpastes for immobilisation of protective enzymes in the in situ pellicle. Arch. Oral Biol. 2010, 55, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kau, C.H.; Wang, J.; Palombini, A.; Abou-Kheir, N.; Christou, T. Effect of fluoride dentifrices on white spot lesions during orthodontic treatment: A randomized trial. Angle Orthod. 2019. [Google Scholar] [CrossRef]
- Topping, G.; Assaf, A. Strong evidence that daily use of fluoride toothpaste prevents caries. Evid. Based Dent. 2005, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Zajkani, E.; Norian, O.; Haghi, F.; Faghihzadeh, S.; Gholami, N. Comparison of the Effect Of 0.2% Chlorhexidine and Xylitol Plus 920 Ppm Fluoride Mouthwashes on Count of Salivary Streptococcus Mutants, a Pilot Study. J. Dent. (Shiraz) 2018, 19, 301–304. [Google Scholar]
- Lin, T.-H.; Lin, C.-H.; Pan, T.-M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018, 102, 577–586. [Google Scholar] [CrossRef]
- Aluckal, E.; Ankola, A.V. Effectiveness of xylitol and polyol chewing gum on salivary streptococcus mutans in children: A randomized controlled trial. Indian J. Dent. Res. 2018, 29, 445–449. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Sun, W.; Li, H.; Cannon, R.D.; Mei, L. Effect of non-fluoride agents on the prevention of dental caries in primary dentition: A systematic review. PLoS ONE 2017, 12, e0182221. [Google Scholar] [CrossRef]
- Oliveira, G.M.S.; Ritter, A.V.; Heymann, H.O.; Swift, E.; Donovan, T.; Brock, G.; Wright, T. Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J. Dent. 2014, 42, 1592–1602. [Google Scholar] [CrossRef]
- Horowitz, H.S. The 2001 CDC recommendations for using fluoride to prevent and control dental caries in the United States. J. Public Health Dent. 2003, 63, 3–8. [Google Scholar] [CrossRef]
- Hannuksela, S.; Tenovuo, J.; Roger, V.; Lenander-Lumikari, M.; Ekstrand, J. Fluoride inhibits the antimicrobial peroxidase systems in human whole saliva. Caries Res. 1994, 28, 429–434. [Google Scholar] [CrossRef]
- Green, A.; Crichard, S.; Ling-Mountford, N.; Milward, M.; Hubber, N.; Platten, S.; Gupta, A.K.; Chapple, I.L.C. A randomised clinical study comparing the effect of Steareth 30 and SLS containing toothpastes on oral epithelial integrity (desquamation). J. Dent. 2019, 80, S33–S39. [Google Scholar] [CrossRef]
- Herlofson, B.B.; Barkvoll, P. Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study. Acta Odontol. Scand. 1994, 52, 257–259. [Google Scholar] [CrossRef]
- Zanatta, F.B.; Antoniazzi, R.P.; Rösing, C.K. Staining and calculus formation after 0.12% chlorhexidine rinses in plaque-free and plaque covered surfaces: A randomized trial. J. Appl. Oral Sci. 2010, 18, 515–521. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J.; Davies, R.M.; Beak, A.; Lewis, A. The effect of single morning and evening rinses of chlorhexidine on the development of tooth staining and plaque accumulation. A blind cross-over trial. J. Clin. Periodontol. 1982, 9, 134–140. [Google Scholar] [CrossRef]
- Helms, J.A.; Della-Fera, M.A.; Mott, A.E.; Frank, M.E. Effects of chlorhexidine on human taste perception. Arch. Oral Biol. 1995, 40, 913–920. [Google Scholar] [CrossRef]
- Sakaue, Y.; Takenaka, S.; Ohsumi, T.; Domon, H.; Terao, Y.; Noiri, Y. The effect of chlorhexidine on dental calculus formation: An in vitro study. BMC Oral Health 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Addy, M.; Newcombe, R.; Macdonald, E.; Chapman, A.; Davies, M.; Moran, J.; Claydon, N. A randomised crossover trial to compare the potential of stannous fluoride and essential oil mouth rinses to induce tooth and tongue staining. Clin. Oral Investig. 2012, 16, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.M.; Gürsoy, U.K.; Söderling, E.M.; Ouwehand, A.C. Effects of Xylitol and Sucrose Mint Products on Streptococcus mutans Colonization in a Dental Simulator Model. Curr. Microbiol. 2017, 74, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Trahan, L. Xylitol: A review of its action on mutans streptococci and dental plaque—Its clinical significance. Int. Dent. J. 1995, 45, 77–92. [Google Scholar]
- Nimatullah, A.; Ogawa, M.; Hayakawa, S. Application of Lactoperoxidase System Using Bovine Whey and the Effect of Storage Condition on Lactoperoxidase Activity. Int. J. Dairy Sci. 2011, 6, 72–78. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef]
- Shariat, S.S.; Jafari, N.; Tavakoli, N.; Najafi, R.B. Protection of lactoperoxidase activity with sugars during lyophilization and evaluation of its antibacterial properties. Res. Pharm. Sci. 2015, 10, 152–160. [Google Scholar]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Boroujeni, M.B.; Nayeri, H. Stabilization of bovine lactoperoxidase in the presence of ectoine. Food Chem. 2018, 265, 208–215. [Google Scholar] [CrossRef]
- Graf, R.; Anzali, S.; Buenger, J.; Pfluecker, F.; Driller, H. The multifunctional role of ectoine as a natural cell protectant. Clin. Dermatol. 2008, 26, 326–333. [Google Scholar] [CrossRef]
- Jafary, F.; Kashanian, S.; Sharieat, Z.S.; Jafary, F.; Omidfar, K.; Paknejad, M. Stability improvement of immobilized lactoperoxidase using polyaniline polymer. Mol. Biol. Rep. 2012, 39, 10407–10412. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Yilmaz, I.; Koksal, Z.; Özdemir, H.; Ocsoy, I.; Özdemir, N. Preparation of lactoperoxidase incorporated hybrid nanoflower and its excellent activity and stability. Int. J. Biol. Macromol. 2016, 84, 402–409. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Yasir, M.; Khan, I.; Khan, S.B.; Azum, N.; Jiffri, E.H.; Kamal, M.A.; Ashraf, G.M.; Beg, M.A. Lactoperoxidase immobilization on silver nanoparticles enhances its antimicrobial activity. J. Dairy Res. 2018, 85, 460–464. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Junevičius, J.; Žilinskas, J.; Česaitis, K.; Česaitienė, G.; Gleiznys, D.; Maželienė, Ž. Antimicrobial activity of silver and gold in toothpastes: A comparative analysis. Stomatologija 2015, 17, 9–12. [Google Scholar]
- Shariat, S.Z.A.S.; Borzouee, F.; Mofid, M.R.; Varshosaz, J. Immobilization of lactoperoxidase on graphene oxide nanosheets with improved activity and stability. Biotechnol. Lett. 2018, 40, 1343–1353. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Chandra, K.; Dubey, A.; Atreya, H.S. An ultrastable conjugate of silver nanoparticles and protein formed through weak interactions. Nanoscale 2015, 7, 12921–12931. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar]
| Characteristic | Value | Reference |
|---|---|---|
| Mass | ~80 000 Da (hLPO); ~78 000 Da (bLPO) | [10,18] |
| Gene-containing chromosome | 17 (hLPO); 19 (bLPO) | [7,20] |
| Number of amino acid residues | 632 (hLPO); 612 (bLPO) | [18] |
| Number of glycosylation sites | 5 (hLPO); 4 (bLPO) | [14] |
| Isoelectric point | 9.6 | [18] |
| Optimal conditions | 50 °C; pH = 6 | [5] |
| Stability of the secondary structure | Start of degradation at 65 °C; Tm = 71.2 °C | [15] |
| Stability of heme | Start of degradation at 70 °C; Tm = 73 °C; Degradation at pH < 4 | [15] |
| Clinical Trials | ||||
|---|---|---|---|---|
| Preparation | Test Group | Tested Parameter | Effect | References |
| Biotène® Dry Mouth Moisturizing Spray (lysozyme, lactoferrin, LPO) | During the recruitment of people suffering from dry mouth syndrome | Time after which the participant will have a dry mouth feeling | The study is ongoing | Clinicaltrials.gov NCT03663231 |
| ZendiumTM (amyloglucosidase, GOx, LPO) | 229 healthy subjects Mean age = 32.6 years | Modified Gingival Index (MGI) Bleeding Index (BI) Plaque Index | Significant reduction vs. MGI (1.627 vs. 1.404) and PI (2.233 vs. 2.112) baseline levels in test group; significant reduction of all parameters compared to the control group | Daly et al. 2019 [164] |
| ZendiumTM (amyloglucosidase, GOx, LPO, LYS, LF) | 46 healthy subjects Mean age = 42 years | H2O2 and lysozyme levels after brushing | 64% higher H2O2 and 92% higher lysozyme concentrations vs. concentrations after brushing with a control paste | Cawley et al. 2019 [141] |
| ZendiumTM (amyloglucosidase, GOx, LPO) | 115 healthy subjects Mean age = 42 years | Composition of supra-gingival dental plaque expressed as a mean relative abundance (MRA) | In test group: significant changes in MRA of 37 taxa; the highest MRA increase in Neisseria flava (2.9%), K. denitrificans (0.7%), P. melaninogenica (0.4%); the lowest MRA decrease in R. dentocariosa (3.2%), Bacteroidales (0.2%), Treponema spp (0.08%) | Adams et al. 2017 [183] |
| ZendiumTM (amyloglucosidase, GOx, LPO) | 68 patients Age 83.7 ± 7.4 years | Composition of oral microflora collected from supra-gingival and lingual area, the presence of visible supra-gingival plaque (SP) | Significant plaque score reduction after 12 months in test group vs. baseline level (1.7 ± 0.5 vs. 0.7 ± 0.5) and vs. control (1.6 ± 0.4 vs. 1.6 ± 0.6); lack or thin layer of plaque after 12 months in 92% of test group; F. nucleatum count and ration significantly decreased in test group; significant decreased of S. sanguinis/oralis count during 12 months | Wikström et al. 2017 [162] |
| OrabarrierTM (lactoferrin, LPO, GOx) | 47 healthy subjects Mean age (test group) = 80.4 ± 6.4 years (control group) = 85.9 ± 6.7 years | Plaque control record (PCR), probing, pocket depth (PPD), bleeding on probing (BOP), tongue coating score, volatile sulfur compounds (VSCs), H2S, CH3SH, mouth dryness, Composition of oral microflora collected from supra-gingival and lingual area | Significant PPD (after 8 weeks) and BOP (after 8 weeks in test group and after 4 weeks in controls) decrease in both groups, tongue coating score decrease after 4 and 8 weeks in both groups; decrease in P. gingivalis and F. nucleatum count after 8 weeks in test group | Morita et al. 2017 [163] |
| OrabarrierTM (lactoferrin, LPO, GOx) | 40 participants with VSCs exceeding the olfactory threshold in the expired air Age 49.4 ± 15.3 years | Volatile sulfur compounds (VSCs), H2S, CH3SH in exhaled air 10 and 30 min after tablet administration | VSCs and H2S decrease (57% and 45%, respectively); no significant changes of CH3SH concentration between groups after 10 and 30 min; significantly lower VSCs (0.115 ± 0.078) and H2S (−0.085 ± 0.083) concentrations in test group after 10 min vs. controls | Nakano et al. 2016 [159] |
| Bioxtra® (GOx, LPO, lactoferrin) | 30 children with severe early childhood caries Age 3–5 years | Salivary S. mutans i L. acidophilus count (CFU) before, right after and after 7 days of toothpaste usage | Significant decrease in CFU of S. mutans (112.0 ± 13.3 vs. 8.50 ± 5.89) and L. acidophilus (109.6 ± 14.3 vs. 8.60 ± 3.98) in an enzymatic paste group during 7 days; significant decrease of S. mutans count in fluoride (123.1 ± 21.1 vs. 33.6 ± 10.3) and nonfluoride (110 ± 12.4 vs. 76.2 ± 19.6) paste groups | Gudipaneni et al. 2014 [3] |
| Biotene OralBalance® gel (LPO, lysozyme lactoferrin) | 41 mechanically ventilated newborns Age 7–10 days | Respiratory outcomes, non-respiratory short-term outcomes, time of ventilation, composition tracheal aspirate samples | No significant differences in the duration of mechanical ventilation; significantly longer hospitalization of newborns in the study group; no significant differences in the composition of the tracheal bacterial flora | Stefanescu et al. 2013 [165] |
| OrabarrierTM Tablets (lactoferrin, LPO, GOx) | 74 subjects with chronic periodontitis Age 32–73 years | Effect on human and bovine LF level, P. gingivalis count, exotoxin level, total bacterial count in saliva and gingival fluid, plaque control record (PCR), probing, depth (PD), bleeding on probing (BOP), plaque index (PI), gingival index (GI), CAL (clinical attachment level) | Significantly higher bLF concentration in saliva and CGF in study group vs. controls; non-significant effect on bacterial parameters; no significant differences in periodontal health parameters vs. control group | Shimizu et al. 2011 [4] |
| OrabarrierTM (lactoferrin, LPO, GOx) | 15 participants with VSCs exceeding the olfactory threshold in the expired air | Volatile sulfur compounds (VSCs), CH3SH in exhaled air 10 min, 1 h and 2 h after tablet administration, bacterial count in saliva | No significant differences in salivary bacterial count; significantly lower CH3SH concentration in test group vs. controls | Shin et al. 2011 [160] |
| BioXtra® (GOx, LPO, lactoferrin) | 34 patients with radiotherapy-induced xerostomia Age 63.5 ± 9.4 years | Intensity of xerostomia symptoms, effect on dysphagia, pain in oral cavity, loss of taste (0–3 scale) | Remission of symptoms severity, a significant reduction in dry mouth feeling (2.03 vs. 1.12), dysphagia improvement (1.62 vs. 0.76) after 28 days | Dirix et al. 2007 [161] |
| Biotene® (GOx, LPO) | 12 healthy subjects | SCN−, HOSCN/OSCN right after brushing | Increase in salivary HOSCN/OSCN-Level and its decomposition after 20 min | Lenander-Lumikari et al. 1993 [176] |
| Biotene® (GOx, LPO) | 26 healthy subjects | SCN−, HOSCN/OSCN, total bacterial count in saliva, streptococcal count, S. mutans, Lactobacillus spp | No significant inhibitory effect on the growth of the tested species | Lenander-Lumikari et al. 1993 [176] |
| In vitro studies | ||||
| Tested substance | Microorganism | Tested parameter | Effect | References |
| Amyloglucosidase, GOx, LPO, LF, LYS and bovine colostrum (IgG) | S. mutans, Fusobacterium nucleatum | Film integrity and polarity using fluorescent dyes | Significant increase in fluorescence connected to a film polarity (33.3% for S. mutans) and permeability (44.4% increase for S. mutans and 57.6% increase for F. nucleatum) | Cawley et al. 2019 [141] |
| Amyloglucosidase, GOx, LPO, LF, LYS and bovine colostrum (IgG) | S. mutans Fusobacterium nucleatum | Effect of toothpaste on planktonic growth | Significant reduction in the growth of both strains | Cawley et al. 2019 [141] |
| ZendiumTM | S. mutans Fusobacterium nucleatum | Effect of toothpaste on the viability of a single-species biofilm | Significant reduction in S. mutans biofilm viability (40% decrease in fluorescence); insignificant reduction of F. nucleatum biofilm viability (23% decrease in fluorescence) vs. control paste | Cawley et al. 2019 [141] |
| ZendiumTM | S. mitis S. intermedius S. oralis Actinomyces naeslundii Veillonella dispar Fusobacterium nucleatum Prevotella intermedia | Effect of toothpaste on the viability of a seven-species biofilm | 30% decrease of viability after 2 h; 27% decrease of viability after 4 h; 47% decrease of viability after 8h vs. control paste | Cawley et al. 2019 [141] |
| Splat Oral Care Foam (LF, GOx, LPO) | Staphylococcus aureus Kocuria rhizophila Micrococcus thailandicus, E. coli Chromobacterium violaceum bacteria from pooled saliva | Retention test of growing and mature biofilms made on glass, Teflon and tooth surface after 5 and 30 s of rinsing with foam | Reduction of biofilm retention on glass and Teflon after foam rinsing for 30 s (all species except C. violaceum; 86.9% retention on Teflon); significant reduction of biofilm retention from pooled saliva on enamel | Jones et al. 2018 [171] |
| MPO, CAT, LPO, HRP | S. sanguinis, S. cristatus, S. gordonii, S. parasanguinis, S. mitis, S. salivarius, S. oralis, A. viscosus, S. mutans, Actinomyces naeslundii, Veilonella parvula, S. sorbinus, Prevotella intermedia, Porphyromonas gingivalis, Fusobacterium nucleatum, Aggregatibacter, actinomycetemcomitans | Evaluation of multispecies ecology in terms of the inhibitory effect of peroxidases at the concentration of those in saliva, gingival fluid present in PD and gums, on the inhibitory effects of commensal bacteria on pathogenic strains | MPO, CAT, and HRP reduce the inhibitory effect of commensal biofilms in concentrations occurring in the gingival fluid in patients with periodontitis and gingivitis; higher P. gingivalis and P. intermedia overgrowth in a multispecies biofilm compared to the increase of CF myeloperoxidase in healthy subjects; the presence of LPO and MPO in salivary concentrations found in people with periodontitis resulted in P. gingivalis and P. intermedia overgrowth; LPO had an inhibitory effect on planktonic growth of A. naeslundii, A. viscosus and growth of S. sobrinus and S. oralis in biofilms | Herrero et al. 2018 [170] |
| (1) LPO, LF, LYS (LLL); (2) casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) | S. mutans | Bacterial count in induced carious lesions on human teeth | Significant reduction of S. mutans count after 3-fold LLL administration; CPP-ACP does not reduce S. mutans count | Pinherio et al. 2017 [172] |
| LPO system, iodide | S. mutans | Measurement of the effect on growth, glucosyltransferase activity, adhesion and synthesis of exopolysaccharides (EPS) | The inhibitory effect of the system increases with I− concentration; reduction of glucosyltransferase activity and EPS synthesis | Liu et al. 2014 [166] |
| 2 artificial salivae containing (1) GOx, LF, LYS, LPO (2) carboxymethylcellulose | Candida albicans | Comparison of the degree of inhibition of C. albicans growth on acrylic plates | Saliva containing enzymes did not show a statistically significant reduction in the amount of microorganisms vs. saliva with carboxymethylcellulose | Silva et al. 2012 [173] |
| LPO system | S. mutans S. sanguinis C. albicans | Quantitative suspension test and calculation of reduction factor (RF) after 1, 3, 5 and 15 min | Suspensions treated with only SCN/H2O2 mixtures did not show antibacterial or antifungal activity; LPO addition significantly increased RF of all microorganisms | Welk et al. 2009 [167] |
| LPO system | S. mutans glucosyltransferases B, C, and D | Glucosyltransferases activity in the liquid phase and adsorbed on hydroxyapatites | Significant inhibition of GtfC and GtfD adsorbed by the system; no effect on GtfC activity and an increase in GtfB activity in the liquid phase | Korpela et al. 2002 [142] |
| LPO system | S. mutans glucosyltransferase D | Glucosyltransferase activity | GtfD activity increased by the system; no influence of OSCN− on GtfD activity; LPO inhibits GtfD activity in low concentrations | Yu et al. 2000 [168] |
| Immune whey LPO system | S. mutans serotype c | Inhibition of glucose retention | Exposure to HOSCN/OSCN− as a product of LPO action enhances the inhibition by the immune whey glucose retention | Loimaranta et al. 1998 [174] |
| LPO LYS | S. mutans serotype c | Capacity of a strain to adhere to hydroxyapatite previously treated with saliva after administration of specific concentrations of the substance | Significant reduction in adhesion | Roger et al. 1994 [140] |
| LPO system | Bacillus cereus | Growth curves | Inhibition of bacterial growth proportional to the concentration of the produced OSCN− ion | Tenovuo et al. 1985 [169] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. https://doi.org/10.3390/ijms20061443
Magacz M, Kędziora K, Sapa J, Krzyściak W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. International Journal of Molecular Sciences. 2019; 20(6):1443. https://doi.org/10.3390/ijms20061443
Chicago/Turabian StyleMagacz, Marcin, Karolina Kędziora, Jacek Sapa, and Wirginia Krzyściak. 2019. "The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products" International Journal of Molecular Sciences 20, no. 6: 1443. https://doi.org/10.3390/ijms20061443
APA StyleMagacz, M., Kędziora, K., Sapa, J., & Krzyściak, W. (2019). The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. International Journal of Molecular Sciences, 20(6), 1443. https://doi.org/10.3390/ijms20061443






