A Prediction Model for Preoperative Risk Assessment in Endometrial Cancer Utilizing Clinical and Molecular Variables
Abstract
1. Introduction
2. Results
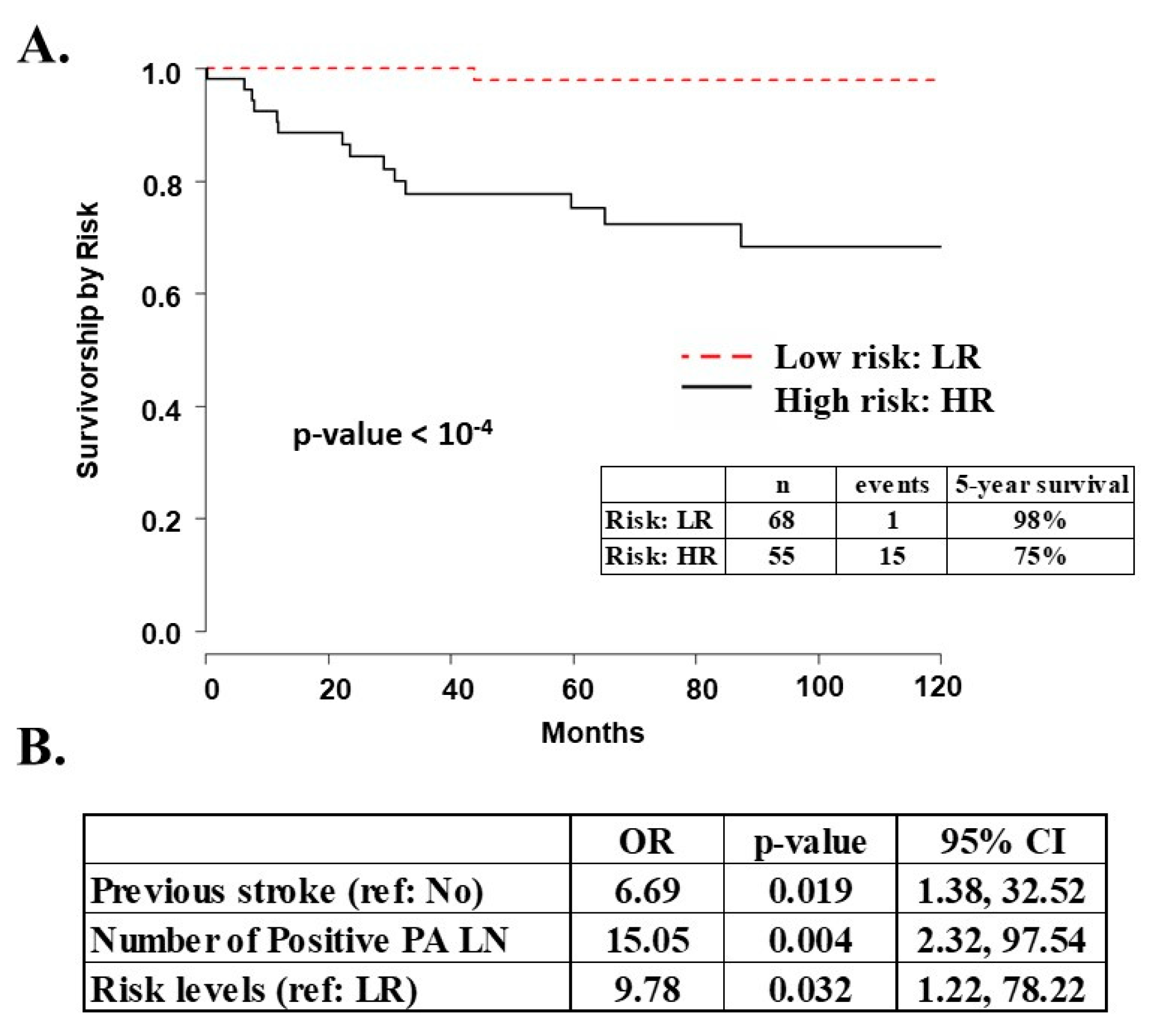
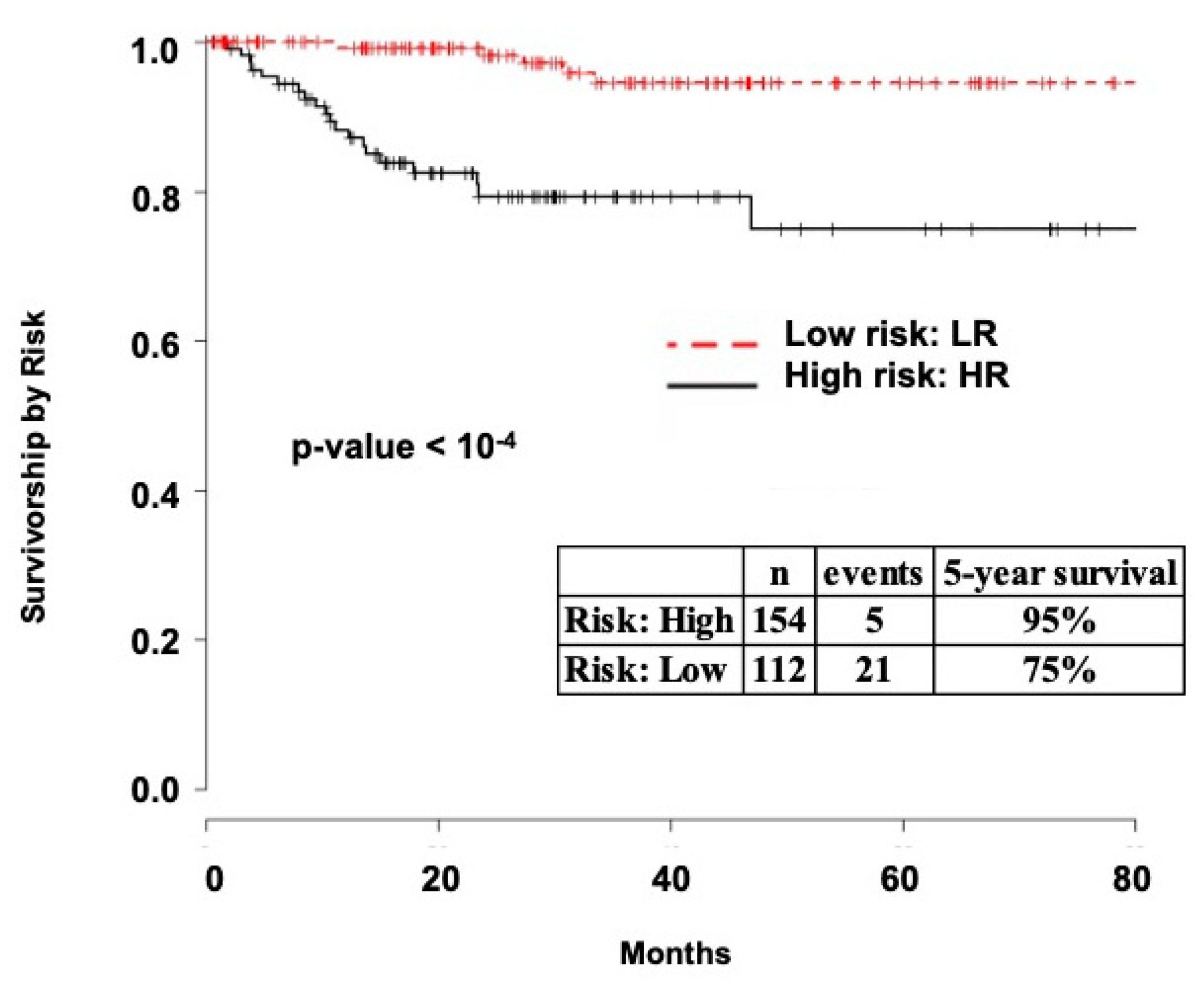
2.1. Survival Analysis
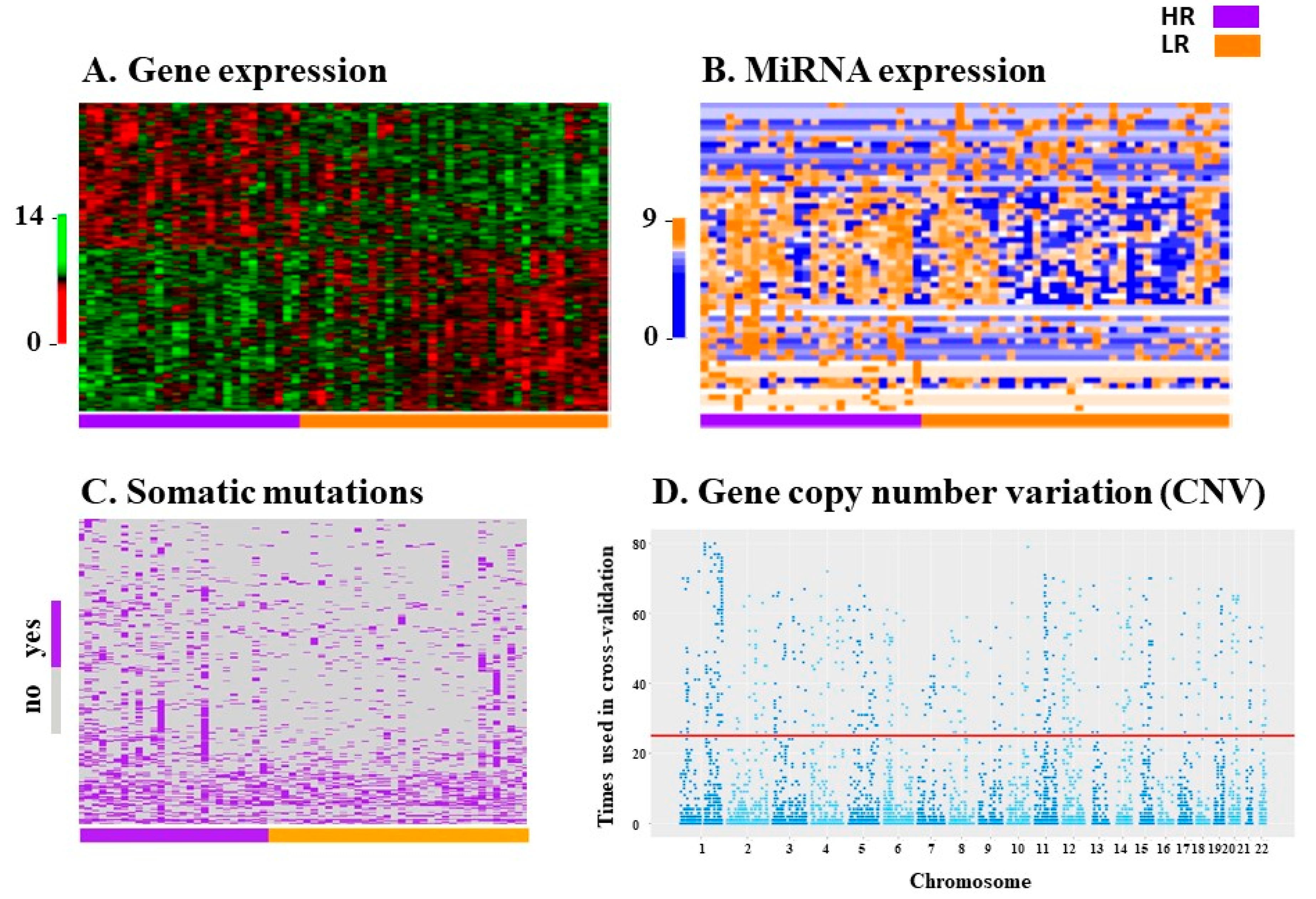
2.2. Variable Selection for Prediction Modeling
2.3. Prediction Models
2.3.1. UI Prediction Models
2.3.2. TCGA/GEO Replication
2.3.3. TCGA Validation
3. Discussion
4. Materials and Methods
4.1. Classification of EEC Risk
4.2. Patients and Clinical Data Collection
4.2.1. University of Iowa (UI)
4.2.2. The Cancer Genome Atlas (TCGA)
4.2.3. Gene Expression Omnibus (GEO)
4.3. Biological Data
University of Iowa (UI)
4.4. Statistical Analysis
4.4.1. Survival Analysis
4.4.2. Variable Selection for Prediction Modeling
4.4.3. Prediction Model Construction
4.4.4. The Cancer Genome Atlas Replication and Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EEC | Endometrioid endometrial cancer |
| AUC | Area under the (receiver operating characteristic) curve |
| SLN | Sentinel lymph nodes |
| LN | Lymph nodes |
| TCGA | The Cancer Genome Atlas |
| GEO | Gene Expression Omnibus |
| UI | University of Iowa |
| RNA-seq | RNA sequencing |
| CNV | Gene copy number variation |
| miRNA | Micro RNA |
| NPV | Negative predictive value |
| PCR | Polymerase chain reaction |
| GOG | Gynecologic Oncology Group |
Appendix A
| Weights for Each miRNA Included in the Model | ||
|---|---|---|
| miRNA | M2-B | M3-D |
| MIR125B1 | 3.01 | 1.07 |
| MIR181A1 | 1.67 | 1.27 |
| MIR181A2HG | 1.53 | 1.07 |
| MIR188 | 1.86 | 1.31 |
| MIR301B | 1.98 | - |
| MIR30B | 0.35 | 0.61 |
| MIR3142 | 12.08 | - |
| MIR345 | 0.38 | - |
| MIR3690 | 1.36 | 1.15 |
| MIR4269 | 2.09 | 1.58 |
| MIR4307 | 14.25 | 1.19 |
| MIR4463 | 8.68 | - |
| MIR492 | 0.95 | - |
| MIR5692A1 | 0.41 | - |
| MIR578 | 2.01 | - |
| MIR601 | 2.59 | - |
| MIR633 | 14.71 | 1.20 |
| MIR6503 | 0.21 | - |
| MIR6769A | 1.51 | - |
| MIR6820 | 0.29 | - |
| MIR876 | 0.22 | 0.97 |
| Weights for Each Somatic Mutation Included in the Model | |||
|---|---|---|---|
| M2-C | M3-C | M3-D | |
| AARS2 | 1.55 | 1.1 | |
| ABCD1 | 83.96 | 35.65 | |
| ADAMTS13 | 3.9 | 4.57 | 3.2 |
| ATL1 | 2.58 | 2.87 | |
| C14orf37 | 13.02 | 1.05 | 10.85 |
| CEP350 | 0.98 | 0.88 | |
| CGNL1 | 0.94 | 0.93 | |
| COL9A3 | 4.23 | 6.64 | |
| CR2 | 3.22 | ||
| CTAGE8 | 0.71 | 0.61 | 0.74 |
| DAGLA | 12.51 | 4.28 | |
| ENTPD1 | 1.01 | ||
| FAM111A | 1.42 | 1.34 | |
| HIP1R | 4.12 | 4.29 | 2.81 |
| HSD17B8 | 1.56 | ||
| KIF20B | 1.13 | ||
| KIZ | 1.34 | 1.04 | |
| LCORL | 3.63 | 4.41 | |
| MAP3K12 | 1.33 | 2.06 | |
| MAPKBP1 | 0.93 | 0.94 | |
| MPHOSPH8 | 0.9 | 0.86 | |
| NOTCH4 | 2.38 | 1.95 | |
| NR2C2 | 17.57 | 5.83 | 12.5 |
| PANK2 | 0.86 | ||
| PCSK5 | 4.34 | 2.33 | |
| PIGN | 1.81 | 1.45 | 1.62 |
| PVR | 2.07 | 3.15 | |
| RPAP1 | 1.17 | ||
| RSF1 | 2.67 | 2.55 | 2.41 |
| SHROOM2 | 5.45 | 4.91 | 6.33 |
| TMEM41B | 1.37 | ||
| VDR | 1.41 | 1.32 | 1.52 |
| ZDHHC24 | 1.74 | ||
| ZNF780B | 3.65 | 3.47 | 2.78 |
| Weights for Each Somatic Mutation Included in the Model | |
|---|---|
| M3-C | |
| AQP2 | 1.04 |
| C1QL4 | 1.19 |
| C5orf17 | 1.18 |
| CDH19 | 1.33 |
| COLCA2 | 0.98 |
| FAIM2 | 1.12 |
| FGF18 | 1.83 |
| HAS3 | 1.07 |
| IGFL2 | 1.27 |
| IGFL4 | 1.05 |
| IL23R | 0.82 |
| LINC01128 | 1.19 |
| LOC101927701 | 1.28 |
| LOC101929529 | 1.71 |
| LONP2 | 0.55 |
| MAN2A2 | 2.01 |
| MRPS28 | 0.58 |
| P4HA2 | 0.57 |
| SCARNA4 | 1.33 |
| SLC25A21 | 0.65 |
| SPATA4 | 0.95 |
| TAC1 | 1.21 |
| TBATA | 1.45 |
| TFAP2A.AS1 | 1.16 |
| TGFA-IT1 | 1.41 |
| TUBAL3 | 1.48 |
| VAX2 | 1.18 |
| ZNF398 | 0.66 |
Appendix B
Appendix B.1. Clinical Data Available in Databases Used to Validate the UI Prediction Models
Appendix B.1.1. University of Iowa (UI)
Appendix B.1.2. The Cancer Genome Atlas (TCGA)
| Low Risk (N = 206) | High Risk (N = 194) | p-Value | ||
|---|---|---|---|---|
| Preoperative characteristics | Age (mean) | 60 | 62 | <0.001 |
| BMI (mean) | 36.1 | 33.6 | 0.064 | |
| Grade | <0.001 | |||
| 1 | 80 | 17 | ||
| 2 | 57 | 59 | ||
| 3 | 69 | 118 | ||
| Postoperative characteristics | Myometrial invasion | 0.984 | ||
| <50% | 204 | 54 | ||
| >50% | 0 | 17 | ||
| 2009 FIGO Stage | 0.984 | |||
| I | 204 | 71 | ||
| II | 0 | 33 | ||
| III | 0 | 70 | ||
| IV | 0 | 13 | ||
| Lymph nodes (positive) | 0 (0%) | 40 (27%) | <0.001 | |
| Peritoneal Cytology (positive) | 3 (2%) | 24 (16%) | <0.001 |
Appendix B.1.3. Gene Expression Omnibus (GEO)
| Low Risk (N = 49) | High Risk (N = 22) | p-Value | ||
|---|---|---|---|---|
| Preoperative characteristics | Age (mean) | 58 | 67 | 0.002 |
| Grade | <0.001 | |||
| 1 | 26 | 0 | ||
| 2 | 17 | 13 | ||
| 3 | 6 | 9 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S105–S143. [Google Scholar] [CrossRef]
- Astec Study Group; Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [PubMed]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60 (Suppl. 8), 2035–2041. [Google Scholar] [CrossRef]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef]
- Convery, P.A.; Cantrell, L.A.; Di Santo, N.; Broadwater, G.; Modesitt, S.C.; Secord, A.A.; Havrilesky, L.J. Retrospective review of an intraoperative algorithm to predict lymph node metastasis in low-grade endometrial adenocarcinoma. Gynecol. Oncol. 2011, 123, 65–70. [Google Scholar] [CrossRef]
- Mitamura, T.; Watari, H.; Todo, Y.; Kato, T.; Konno, Y.; Hosaka, M.; Sakuragi, N. Lymphadenectomy can be omitted for low-risk endometrial cancer based on preoperative assessments. J. Gynecol. Oncol. 2014, 25, 301–305. [Google Scholar] [CrossRef]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Orr, J.W., Jr.; Holimon, J.L.; Orr, P.F. Stage I corpus cancer: Is teletherapy necessary? Am. J. Obstet. Gynecol. 1997, 176, 777–788. [Google Scholar] [CrossRef]
- Homesley, H.D.; Kadar, N.; Barrett, R.J.; Lentz, S.S. Selective pelvic and periaortic lymphadenectomy does not increase morbidity in surgical staging of endometrial carcinoma. Am. J. Obstet. Gynecol. 1992, 167, 1225–1230. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Soliman, P.T.; Westin, S.N.; Dioun, S.; Sun, C.C.; Euscher, E.; Munsell, M.F.; Fleming, N.D.; Levenback, C.; Frumovitz, M.; Ramirez, P.T.; et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol. Oncol. 2017, 146, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology, G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Haddock, M.G.; Calori, G.; Podratz, K.C. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am. J. Obstet. Gynecol. 2000, 182, 1506–1519. [Google Scholar] [CrossRef]
- Dai, D.; Thiel, K.W.; Salinas, E.A.; Goodheart, M.J.; Leslie, K.K.; Gonzalez Bosquet, J. Stratification of endometrioid endometrial cancer patients into risk levels using somatic mutations. Gynecol. Oncol. 2016, 142, 150–157. [Google Scholar] [CrossRef]
- Hedenfalk, I.; Duggan, D.; Chen, Y.; Radmacher, M.; Bittner, M.; Simon, R.; Meltzer, P.; Gusterson, B.; Esteller, M.; Kallioniemi, O.P.; et al. Gene-expression profiles in hereditary breast cancer. N. Engl. J. Med. 2001, 344, 539–548. [Google Scholar] [CrossRef]
- West, M.; Blanchette, C.; Dressman, H.; Huang, E.; Ishida, S.; Spang, R.; Zuzan, H.; Olson, J.A., Jr.; Marks, J.R.; Nevins, J.R. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc. Natl. Acad. Sci. USA 2001, 98, 11462–11467. [Google Scholar] [CrossRef] [PubMed]
- Berchuck, A.; Iversen, E.S.; Lancaster, J.M.; Dressman, H.K.; West, M.; Nevins, J.R.; Marks, J.R. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. Am. J. Obstet. Gynecol. 2004, 190, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Devor, E.J.; Salinas, E.A.; Newtson, A.M.; Goodheart, M.J.; Leslie, K.K.; Gonzalez-Bosquet, J. Population substructure has implications in validating next-generation cancer genomics studies with TCGA. Int. J. Mol. Sci. 2019, 20, 1192. [Google Scholar] [CrossRef]
- Simon, R.; Radmacher, M.D.; Dobbin, K.; McShane, L.M. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J. Natl. Cancer Inst. 2003, 95, 14–18. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Naves, L.C.; Ter Haar, N.T.; Creutzberg, C.L.; Smit, V.T.; Bosse, T. High concordance of molecular tumor alterations between pre-operative curettage and hysterectomy specimens in patients with endometrial carcinoma. Gynecol. Oncol. 2014, 133, 197–204. [Google Scholar] [CrossRef]
- Murtaza, M.; Dawson, S.J.; Pogrebniak, K.; Rueda, O.M.; Provenzano, E.; Grant, J.; Chin, S.F.; Tsui, D.W.; Marass, F.; Gale, D.; et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat. Commun. 2015, 6, 8760. [Google Scholar] [CrossRef] [PubMed]
- Simon, R. Roadmap for developing and validating therapeutically relevant genomic classifiers. J. Clin. Oncol. 2005, 23, 7332–7341. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Creutzberg, C.L.; van Stiphout, R.G.; Nout, R.A.; Lutgens, L.C.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Smit, V.T.; Lambin, P. Nomograms for prediction of outcome with or without adjuvant radiation therapy for patients with endometrial cancer: A pooled analysis of PORTEC-1 and PORTEC-2 trials. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 530–539. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jurgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Wortman, B.G.; Bosse, T.; Nout, R.A.; Lutgens, L.; van der Steen-Banasik, E.M.; Westerveld, H.; van den Berg, H.; Slot, A.; De Winter, K.A.J.; Verhoeven-Adema, K.W.; et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol. Oncol. 2018, 151, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Devor, E.J.; Miecznikowski, J.; Schickling, B.M.; Gonzalez-Bosquet, J.; Lankes, H.A.; Thaker, P.; Argenta, P.A.; Pearl, M.L.; Zweizig, S.L.; Mannel, R.S.; et al. Dysregulation of miR-181c expression influences recurrence of endometrial endometrioid adenocarcinoma by modulating NOTCH2 expression: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 147, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Figo Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynaecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Day, R.S.; McDade, K.K.; Chandran, U.R.; Lisovich, A.; Conrads, T.P.; Hood, B.L.; Kolli, V.S.; Kirchner, D.; Litzi, T.; Maxwell, G.L. Identifier mapping performance for integrating transcriptomics and proteomics experimental results. BMC Bioinform. 2011, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Santillan, M.K.; Leslie, K.K.; Hamilton, W.S.; Boese, B.J.; Ahuja, M.; Hunter, S.K.; Santillan, D.A. Collection of a lifetime: A practical approach to developing a longitudinal collection of women’s healthcare biological samples. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Kuilman, T.; Velds, A.; Kemper, K.; Ranzani, M.; Bombardelli, L.; Hoogstraat, M.; Nevedomskaya, E.; Xu, G.; de Ruiter, J.; Lolkema, M.P.; et al. CopywriteR: DNA copy number detection from off-target sequence data. Genome Biol. 2015, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Subramanian, J.; Simon, R. Overfitting in prediction models—Is it a problem only in high dimensions? Contemp. Clin. Trials 2013, 36, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Bosquet, J.; Newtson, A.M.; Chung, R.K.; Thiel, K.W.; Ginader, T.; Goodheart, M.J.; Leslie, K.K.; Smith, B.J. Prediction of chemo-response in serous ovarian cancer. Mol. Cancer 2016, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
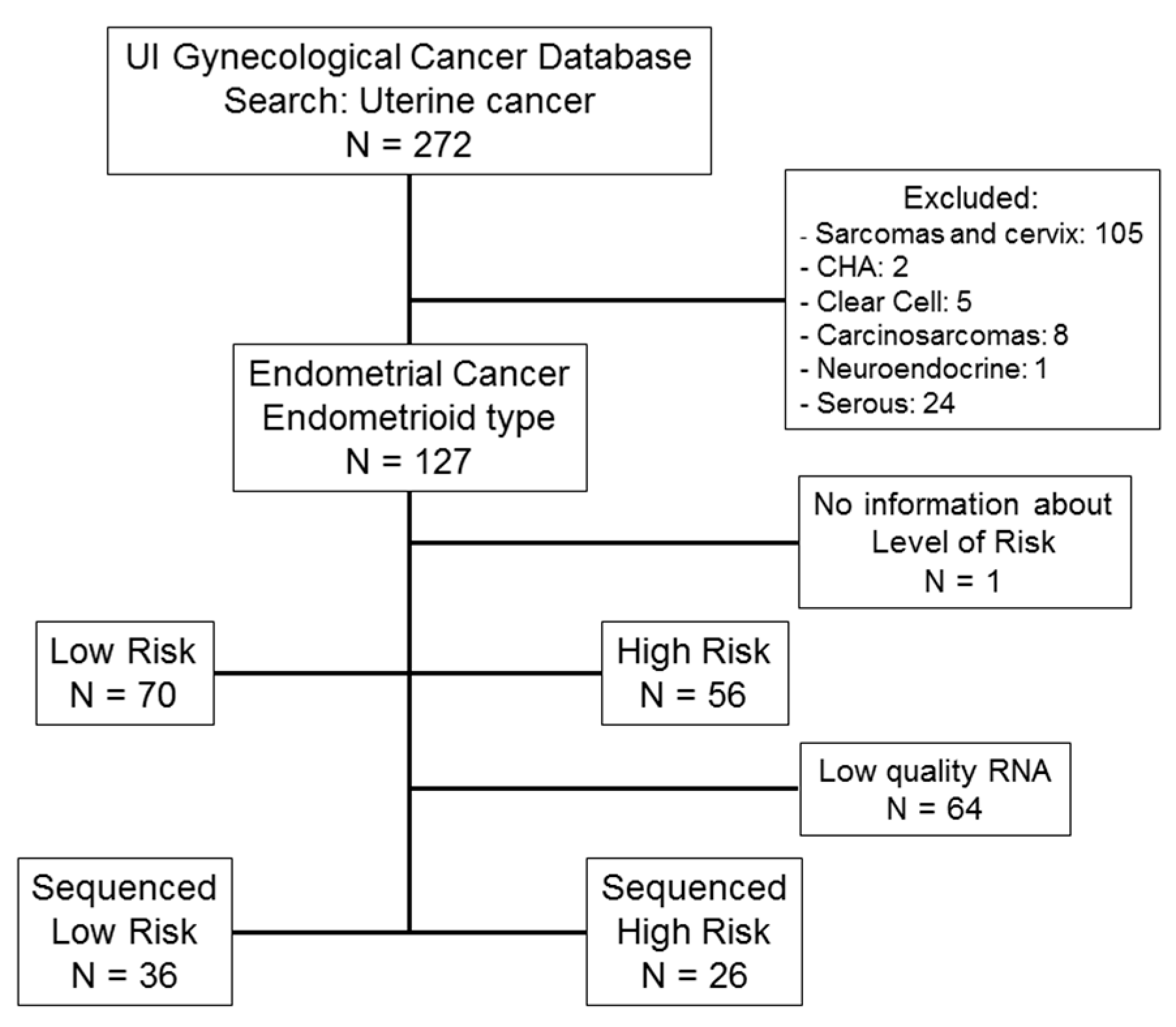
| Clinical/Pathological Variables | Low Risk (N = 70) | High Risk (N = 56) | p-Value | |
|---|---|---|---|---|
| Preoperative characteristics | Age (mean) | 58.7 | 64.8 | 0.003 * |
| BMI (mean) | 38.5 | 32.6 | <0.001 * | |
| Charlson Morbidity Index (mean) | 4.7 | 5 | 0.012 * | |
| Grade | <0.001 * | |||
| 1 | 38 | 7 | ||
| 2 | 21 | 27 | ||
| 3 | 8 | 22 | ||
| Postoperative characteristics | Invasion (mean) | 19 | 62 | <0.001 * |
| 2009 FIGO Stage | 0.991 | |||
| I | 70 | 23 | ||
| II | - | 7 | ||
| III | - | 20 | ||
| IV | - | 6 | ||
| Lymph nodes (% positive) | 0 (0%) | 13 (27%) | 0.987 | |
| Peritoneal Cytology (% positive) | 2 (3%) | 31 (56%) | 0.011 * | |
| Lymphovascular involvement (% positive) | 2 (3%) | 10 (19%) | <0.001 * | |
| ER (% positive) | 38 (93%) | 31 (78%) | 0.066 | |
| PR (% positive) | 38 (93%) | 30 (75%) | 0.040 * | |
| Postoperative complications (% positive) | 12 (17%) | 17 (32%) | 0.056 | |
| LOS (mean days) | 3.3 | 6.1 | 0.002 * | |
| Adjuvant Treatment (yes) (% positive) | 8 (11%) | 39 (74%) | <0.001 * | |
| Outcomes | 5-year Survival (%) | 98% | 75% | <0.001 * |
| Recurrence (% positive) | 2 (3%) | 19 (37%) | <0.001 * | |
| Death due to disease (% positive) | 1 (1%) | 15 (30%) | 0.001 * |
| Model Number | Data Class | # Input Variables | # Resulting Variables | AUC | 95% CI |
| M1-A | Clinical | 17 | 7 | 0.88 | 0.84, 0.92 |
| M1-B | mRNAs | 255 | 38 | 0.79 | 0.73, 0.85 |
| M1-C | miRNAs | 55 | 28 | 0.84 | 0.76, 0.93 |
| M1-D | Mutations | 398 | 35 | 0.68 | 0.63, 0.73 |
| M1-E | CNVs | 846 | 65 | 0.67 | 0.56, 0.77 |
| Prediction Models Using Two Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| M2-A | mRNAs | 7 + 38 | 37 | 0.93 | 0.90, 0.96 |
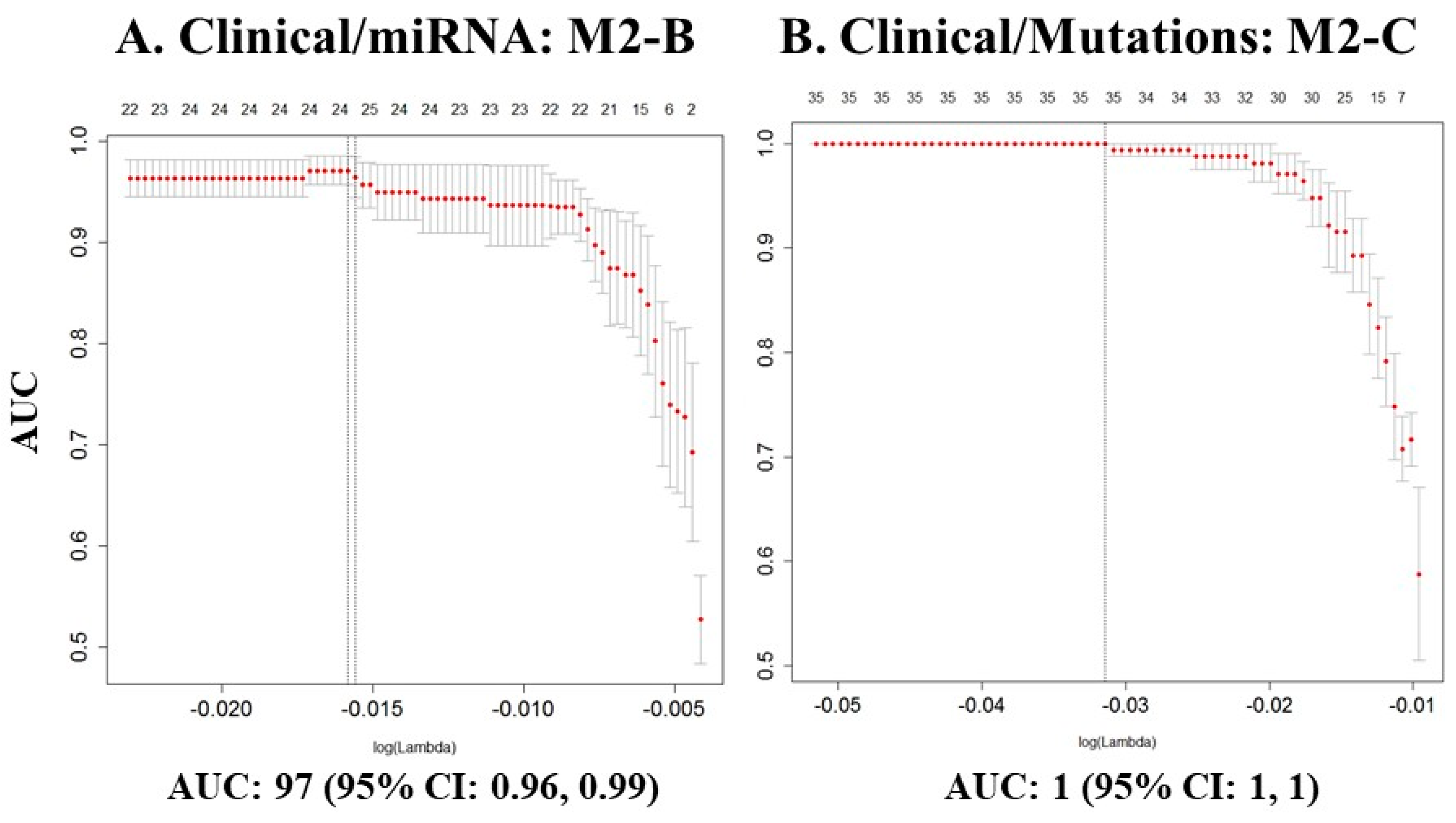
| * M2-B | miRNAs | 7 + 28 | 24 | 0.97 | 0.96, 0.99 |
| * M2-C | Mutations | 7 + 35 | 35 | 1 | 1, 1 |
| M2-D | CNVs | 7 + 65 | 61 | 0.92 | 0.89, 0.94 |
| Prediction Models Using Three Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| M3-A | mRNAs + miRNAs | 7 + 38 + 28 | 37 | 0.83 | 0.74, 0.91 |
| M3-B | Mutations + CNVs | 7 + 35 + 65 | 48 | 0.94 | 0.91, 0.97 |
| M3-C | mRNAs + Mutations | 7 + 38 + 35 | 41 | 0.95 | 0.92, 0.98 |
| M3-D | miRNAs + Mutations | 7 + 28 + 35 | 36 | 0.94 | 0.91, 0.97 |
| M3-E | miRNAs + CNVs | 7 + 28 + 65 | 46 | 0.86 | 0.81, 0.91 |
| M3-F | mRNAs + CNVs | 7 + 38 + 65 | 44 | 0.93 | 0.91, 0.95 |
| Prediction Models Using Four Data Classes | |||||
| Model Number | Data classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| M4-A | mRNAs + miRNAs + Mutations | 7 + 38 + 28 + 35 | 42 | 0.94 | 0.91, 0.96 |
| M4-B | mRNAs + miRNAs + CNVs | 7 + 38 + 28 + 65 | 40 | 0.91 | 0.88, 0.93 |
| M4-C | mRNAs + Mutations + CNVs | 7 + 38 + 35 + 65 | 42 | 0.91 | 0.88, 0.95 |
| M4-D | miRNAs + Mutations + CNVs | 7 + 28 + 35 + 65 | 53 | 0.88 | 0.84, 0.92 |
| Prediction Models Using Five Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| M5-A | mRNAs + miRNAs + Mutations + CNVs | 7 + 38 + 28 + 35 + 65 | 47 | 0.89 | 0.86, 0.92 |
| Replication of Prediction Models Using One Data Class | |||||
| Model Number | Data Class | # Input Variables | # Resulting Variables | AUC | 95% CI |
| UI model M1-A | Clinical | 17 | 7 | 0.88 | 0.84, 0.92 |
| TCGA model M1-A | Clinical | 2 * | 2 | 0.75 | 0.73, 0.78 |
| GEO model M1-A | Clinical | 2 * | 2 | 0.84 | 0.79, 0.89 |
| UI model M1-B | mRNAs | 255 | 38 | 0.79 | 0.73, 0.85 |
| TCGA model M1-B | mRNAs | 36 * | 23 | 0.60 | 0.57, 0.63 |
| GEO model M1-B | mRNAs | 14 * | 5 | 0.60 | 0.53, 0.68 |
| UI model M1-C | miRNAs | 55 | 28 | 0.84 | 0.76, 0.93 |
| TCGA model M1-C | miRNAs | 28 | 4 | 0.57 | 0.54, 0.60 |
| UI model M1-D | Mutations | 398 | 35 | 0.68 | 0.63, 0.73 |
| TCGA model M1-C | Mutations | 34 * | 18 | 0.59 | 0.57, 0.62 |
| UI model M1-C | CNVs | 846 | 65 | 0.67 | 0.56, 0.77 |
| TCGA model M1-E | CNVs | 65 | 2 | 0.63 | 0.59, 0.67 |
| Replication of Prediction Models Using Two Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| UI model M2-A | mRNAs | 7 + 38 | 37 | 0.93 | 0.90, 0.96 |
| TCGA model M2-A | mRNAs | 2 + 36 * | 15 | 0.75 | 0.72, 0.78 |
| GEO model M2-A | mRNAs | 2 + 14 * | 2 | 0.92 | 0.90, 0.95 |
| UI model M2-B | miRNAs | 7 + 28 | 24 | 0.97 | 0.96, 0.99 |
| TCGA model M2-B | miRNAs | 2 + 28 * | 3 | 0.75 | 0.72, 0.77 |
| UI model M2-C | Mutations | 7 + 35 | 35 | 1 | 1, 1 |
| TCGA model M2-C | Mutations | 2 + 34 * | 30 | 0.75 | 0.73, 0.77 |
| UI model M2-D | CNVs | 7 + 65 | 61 | 0.92 | 0.89, 0.94 |
| TCGA model M2-D | CNVs | 2 + 65 * | 3 | 0.75 | 0.71, 0.79 |
| Replication of Prediction Models Using Three Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| UI model M3-A | mRNAs + miRNAs | 7 + 38 + 28 | 37 | 0.83 | 0.74, 0.91 |
| TCGA model M3-A | mRNAs + miRNAs | 2 + 36 + 28 * | 4 | 0.75 | 0.72, 0.78 |
| UI model M3-B | Mutations + CNVs | 7 + 35 + 65 | 48 | 0.94 | 0.91, 0.97 |
| TCGA model M3-B | Mutations + CNVs | 2 + 34 + 65 * | 24 | 0.78 | 0.75, 0.80 |
| UI model M3-C | mRNAs + Mutations | 7 + 38 + 35 | 41 | 0.95 | 0.92, 0.98 |
| TCGA model M3-C | mRNAs + Mutations | 2 + 36 + 34 * | 2 | 0.74 | 0.71, 0.77 |
| UI model M3-D | miRNAs + Mutations | 7 + 28 + 35 | 36 | 0.94 | 0.91, 0.97 |
| TCGA model M3-D | miRNAs + Mutations | 2 + 28 + 34 * | 2 | 0.74 | 0.72, 0.75 |
| UI model M3-E | miRNAs + CNVs | 7 + 28 + 65 | 46 | 0.86 | 0.81, 0.91 |
| TCGA model M3-E | miRNAs + CNVs | 2 + 28 + 65 * | 5 | 0.76 | 0.73, 0.79 |
| UI model M3-F | mRNAs + CNVs | 7 + 38 + 65 | 44 | 0.93 | 0.91, 0.95 |
| TCGA model M3-F | mRNAs + CNVs | 2 + 36 + 65 * | 2 | 0.75 | 0.72, 0.78 |
| Replication of Prediction Models Using Four Data Classes | |||||
| Model Number | Data Classes Included Clinical + | # Input Variables | # Resulting Variables | AUC | 95% CI |
| UI model M4-A | mRNAs + miRNAs + Mutations | 7 + 38 + 28 + 35 | 42 | 0.94 | 0.91, 0.96 |
| TCGA model M4-A | mRNAs + miRNAs + Mutations | 2 + 36 + 28 + 34 * | 2 | 0.74 | 0.71, 0.77 |
| UI model M4-B | mRNAs + miRNAs + CNVs | 7 + 38 + 28 + 65 | 40 | 0.91 | 0.88, 0.93 |
| TCGA model M4-B | mRNAs + miRNAs + CNVs | 2 + 36 + 28 + 65 * | 2 | 0.76 | 0.73, 0.79 |
| UI model M4-C | mRNAs + Mutations + CNVs | 7 + 38 + 35 + 65 | 42 | 0.91 | 0.88, 0.95 |
| TCGA model M4-C | mRNAs + Mutations + CNVs | 2 + 36 + 34 + 65 * | 10 | 0.75 | 0.73, 0.78 |
| UI model M4-D | miRNAs + Mutations + CNVs | 7 + 28 + 35 + 65 | 53 | 0.88 | 0.84, 0.92 |
| TCGA model M4-D | miRNAs + Mutations + CNVs | 2 + 28 + 34 + 65 * | 9 | 0.77 | 0.74, 0.80 |
| Replication of Prediction Models using Five Data Classes | |||||
| Model Number | Data classes included Clinical + | # Input variables | # Resulting variables | AUC | 95% CI |
| UI model M5-A | mRNAs + miRNAs + Mutations + CNVs | 7 + 38 + 28 + 35 + 65 | 47 | 0.8 | 0.85, 0.91 |
| TCGA model M5-A | mRNAs + miRNAs + Mutations + CNVs | 2 + 36 + 28 + 34 + 65 * | 8 | 0.76 | 0.73, 0.78 |
| Model M2-B Clinical + miRNAs | Model M2-C Clinical + Mutations | Model M3-C Clinical + mRNAs + Mutations | Model M3-D Clinical + miRNAs + Mutations | |||||
|---|---|---|---|---|---|---|---|---|
| Recurrence probability scale * | Cut-off = 0.5004 | Cut-off = 0.4984 | Cut-off = 0.7309 | Cut-off = 0.5151 | ||||
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Sensitivity | 90% | 85%, 94% | 90% | 86%, 94% | 90% | 82%, 98% | 90% | 86%, 94% |
| Specificity | 38% | 31%, 44% | 16% | 8%, 26% | 10% | 1%, 23% | 30% | 23%, 37% |
| Positive Predictive Value (PPV) | 56% | 51%, 61% | 49% | 47%, 52% | 13% | 12%, 15% | 53% | 50%, 57% |
| Negative Predictive Value (NPV) | 79% | 70%, 84% | 64% | 47%, 74% | 87% | 45%, 94% | 76% | 66%, 81% |
| Accuracy | 62% | 54%, 68% | 51% | 47%, 56% | 20% | 13%, 32% | 58% | 52%, 63% |
| Prediction Model | M2-B | M2-C | M3-C | M3-D |
|---|---|---|---|---|
| Clinical variables | Weight of clinical variables * | |||
| Age | 1.03 | - | - | - |
| History of other cancers | 0.93 | - | - | - |
| Grade | 12.99 | 1.27 | 1.01 | 1.48 |
| BMI | - | 0.99 | - | - |
| Molecular variables | Log2 transformed and normalized miRNA expression **: | |||
| miRNAs | MIR125B1, MIR181A1, MIR181A2HG, MIR188, MIR301B, MIR30B, MIR3142, MIR345, MIR3690, MIR4269, MIR4307, MIR4463, MIR492, MIR5692A1, MIR578, MIR601, MIR633, MIR6503, MIR6769A, MIR6820 | MIR125B1, MIR181A1, MIR181A2HG, MIR188, MIR30B, MIR3690, MIR4269, MIR4307, MIR633, MIR876 | ||
| Somatic mutations | Number of mutations per gene and person #: | |||
| AARS2, ABCD1, ADAMTS13, ATL1, C14orf37, CEP350, CGNL1, COL9A3, CR2, CTAGE8, DAGLA, ENTPD1, FAM111A, HIP1R, HSD17B8, KIF20B, KIZ, LCORL, MAP3K12, MAPKBP1, MPHOSPH8, NOTCH4, NR2C2, PANK2, PCSK5, PIGN, PVR, RPAP1, RSF1, SHROOM2, VDR, ZDHHC24, ZNF780B | ADAMTS13, C14orf37, CEP350, CTAGE8, HIP1R, MAPKBP1, NR2C2, PIGN, RSF1, SHROOM2, VDR, ZNF780B | AARS2, ABCD1, ADAMTS13, ATL1, C14orf37, CGNL1, COL9A3, CTAGE8, DAGLA, FAM111A, HIP1R, KIZ, LCORL, MAP3K12, MPHOSPH8, NOTCH4, NR2C2, PCSK5, PIGN, PVR, RSF1, SHROOM2, TMEM41B, VDR, ZNF780B | ||
| Gene expression | Log2 transformed and normalized gene expression ##: | |||
| AQP2, C1QL4, C5orf17, CDH19, COLCA2, FAIM2, FGF18, HAS3, IGFL2, IGFL4, IL23R, LINC01128, LOC101927701, LOC101929529, LONP2, MAN2A2, MRPS28, P4HA2, SCARNA4, SLC25A21, SPATA4, TAC1, TBATA, TFAP2A-AS1, TGFA.IT1, TUBAL3, VAX2, ZNF398 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, E.A.; Miller, M.D.; Newtson, A.M.; Sharma, D.; McDonald, M.E.; Keeney, M.E.; Smith, B.J.; Bender, D.P.; Goodheart, M.J.; Thiel, K.W.; et al. A Prediction Model for Preoperative Risk Assessment in Endometrial Cancer Utilizing Clinical and Molecular Variables. Int. J. Mol. Sci. 2019, 20, 1205. https://doi.org/10.3390/ijms20051205
Salinas EA, Miller MD, Newtson AM, Sharma D, McDonald ME, Keeney ME, Smith BJ, Bender DP, Goodheart MJ, Thiel KW, et al. A Prediction Model for Preoperative Risk Assessment in Endometrial Cancer Utilizing Clinical and Molecular Variables. International Journal of Molecular Sciences. 2019; 20(5):1205. https://doi.org/10.3390/ijms20051205
Chicago/Turabian StyleSalinas, Erin A., Marina D. Miller, Andreea M. Newtson, Deepti Sharma, Megan E. McDonald, Matthew E. Keeney, Brian J. Smith, David P. Bender, Michael J. Goodheart, Kristina W. Thiel, and et al. 2019. "A Prediction Model for Preoperative Risk Assessment in Endometrial Cancer Utilizing Clinical and Molecular Variables" International Journal of Molecular Sciences 20, no. 5: 1205. https://doi.org/10.3390/ijms20051205
APA StyleSalinas, E. A., Miller, M. D., Newtson, A. M., Sharma, D., McDonald, M. E., Keeney, M. E., Smith, B. J., Bender, D. P., Goodheart, M. J., Thiel, K. W., Devor, E. J., Leslie, K. K., & Gonzalez Bosquet, J. (2019). A Prediction Model for Preoperative Risk Assessment in Endometrial Cancer Utilizing Clinical and Molecular Variables. International Journal of Molecular Sciences, 20(5), 1205. https://doi.org/10.3390/ijms20051205






