Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits
Abstract
1. Introduction
2. Results
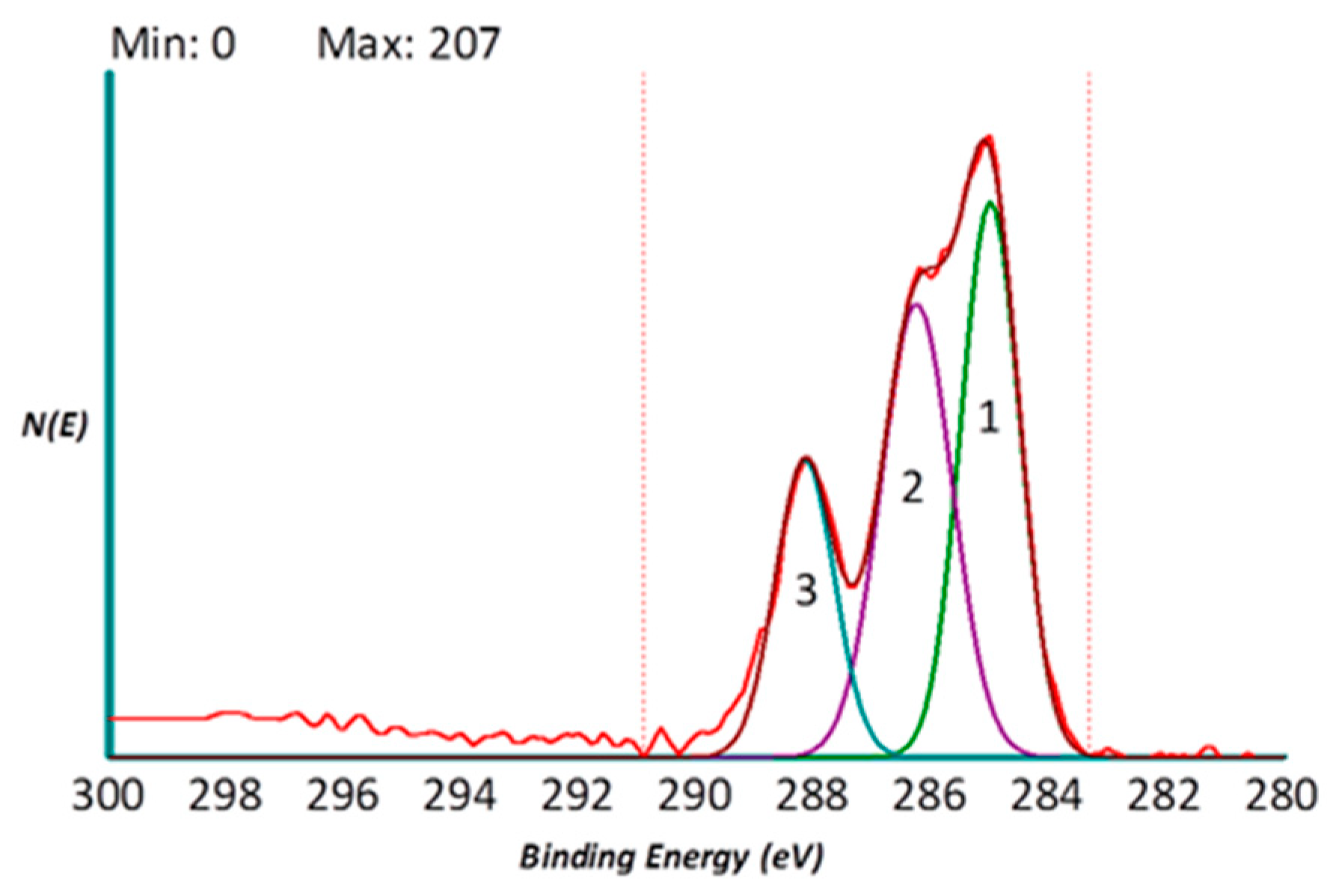
2.1. Surface Analysis by X-Ray Photoelectron Spectroscopy (XPS)
2.1.1. Surface (Uncoated Implant)
2.1.2. Surface (Coated Collagen Type I Implant)
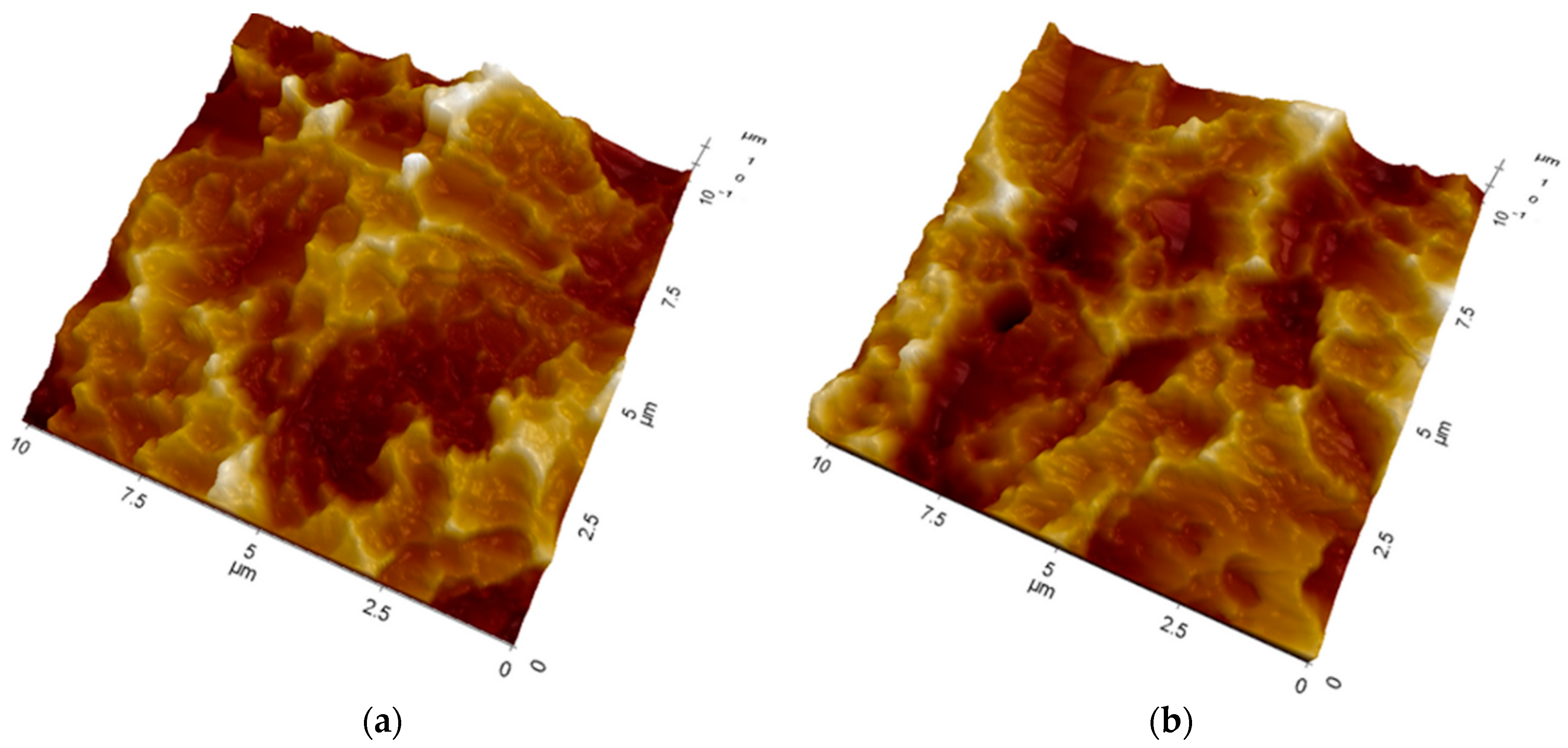
2.2. Atomic Force Microscopy (AFM)
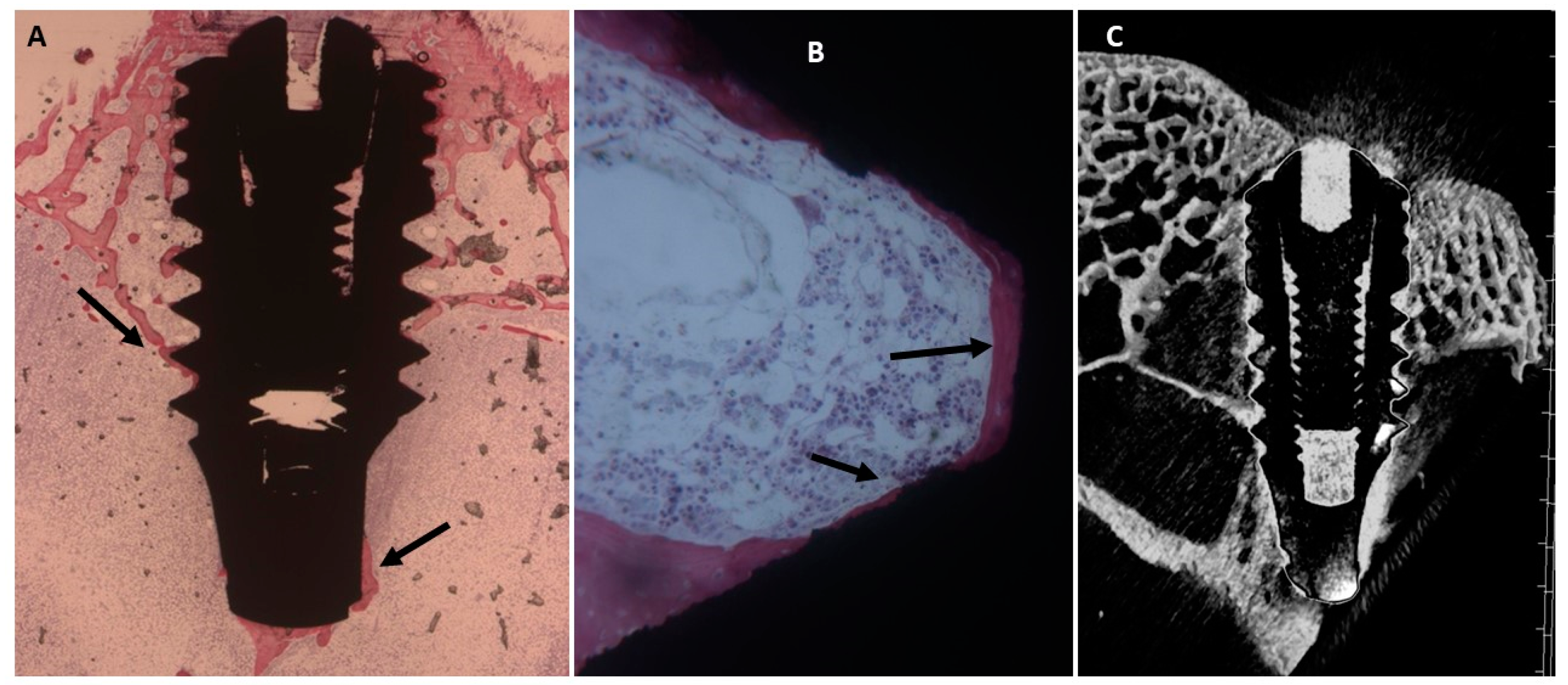
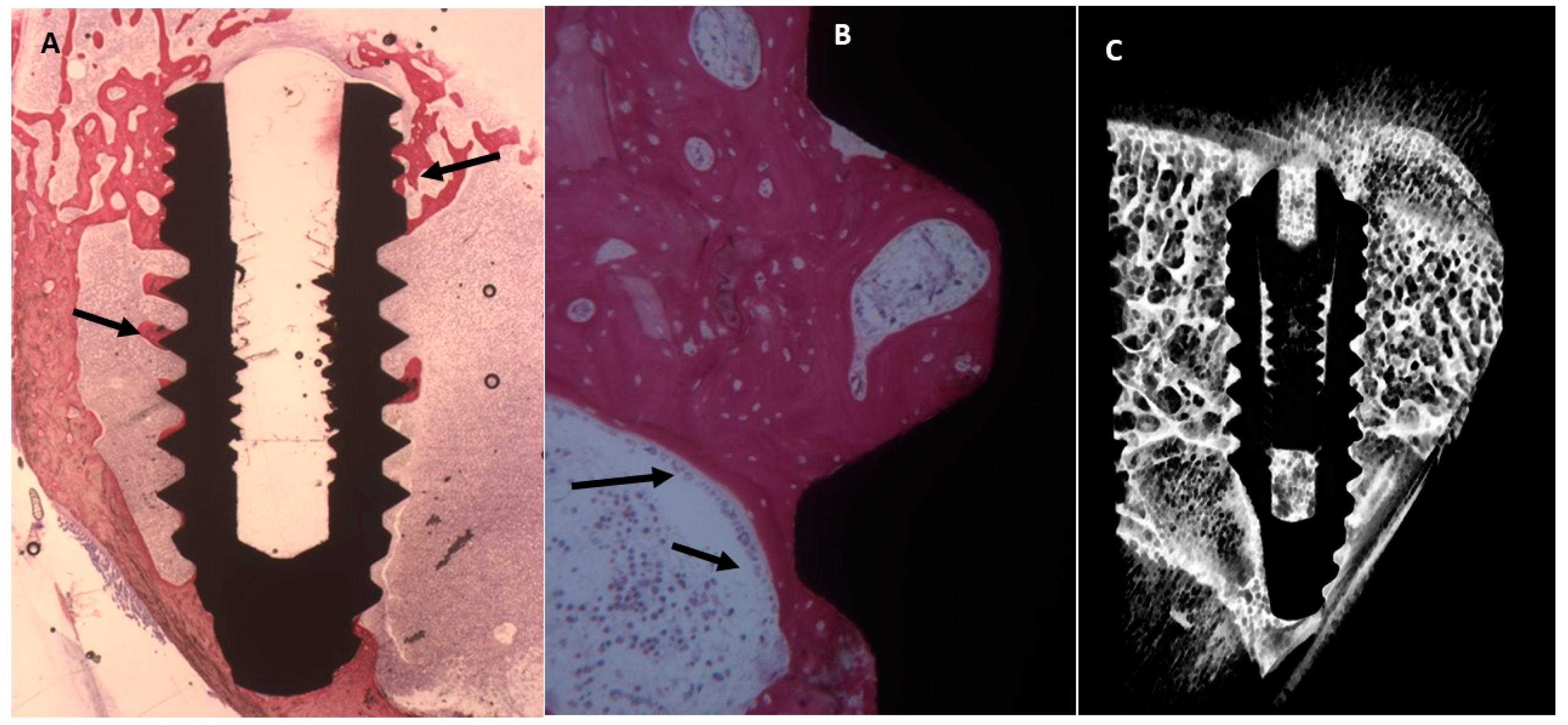
2.3. Micro-CT Evaluation
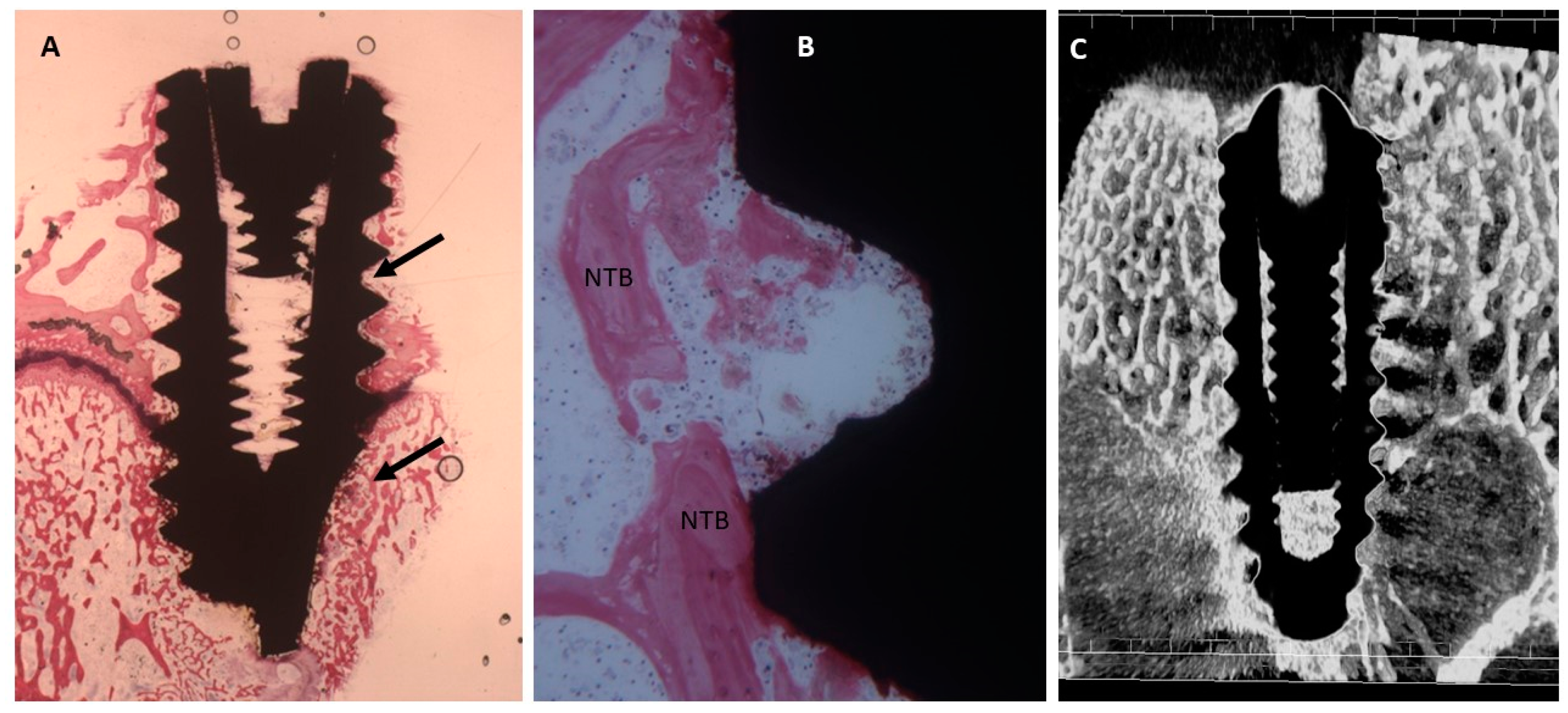
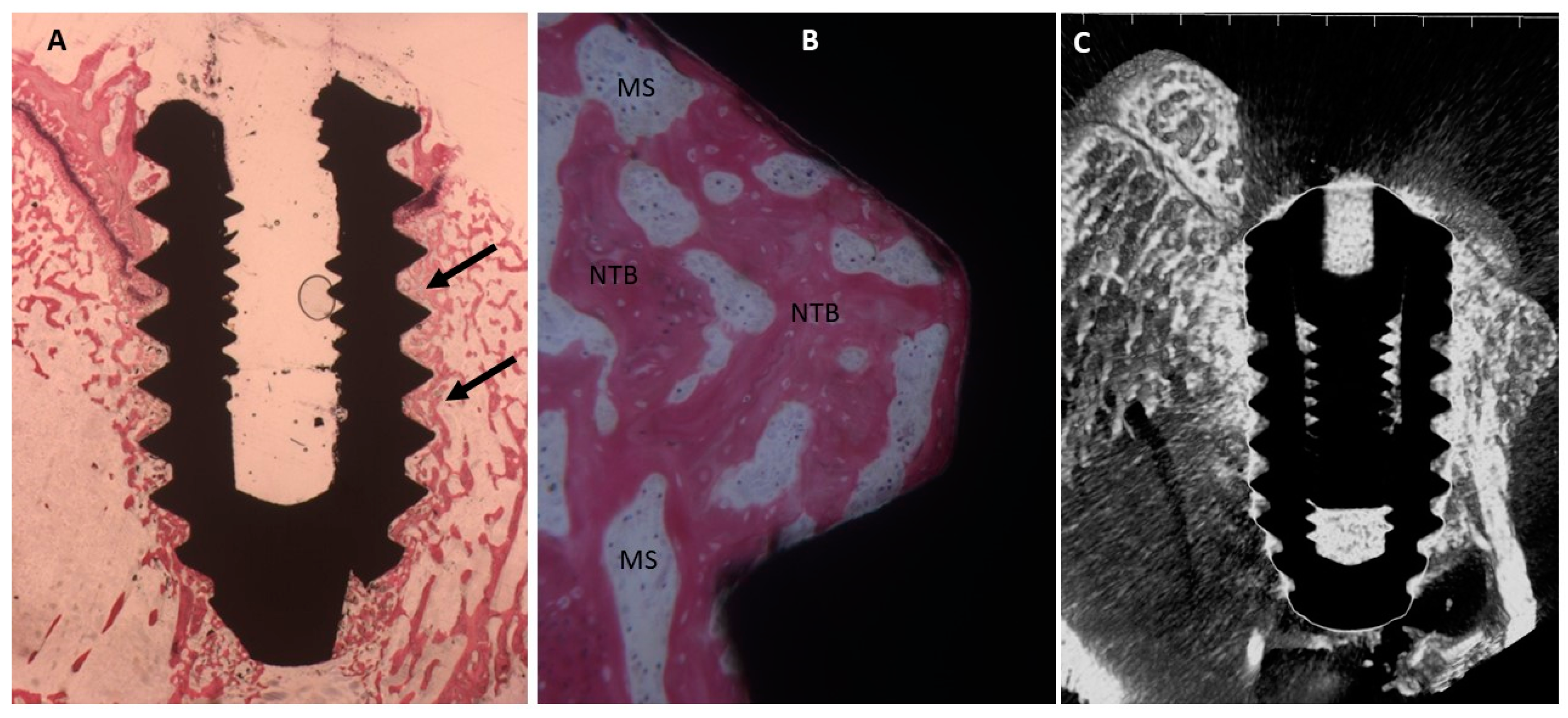
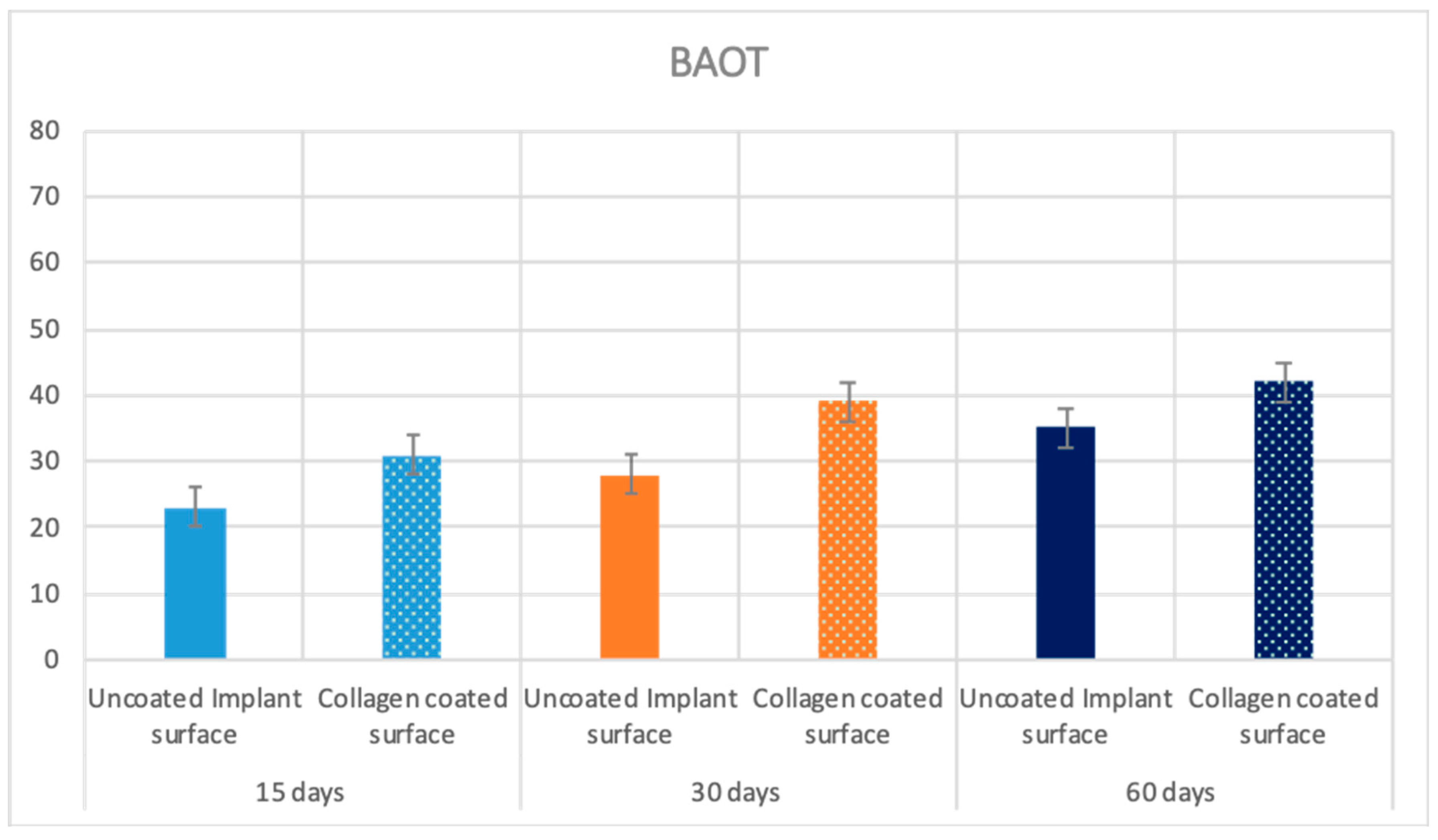
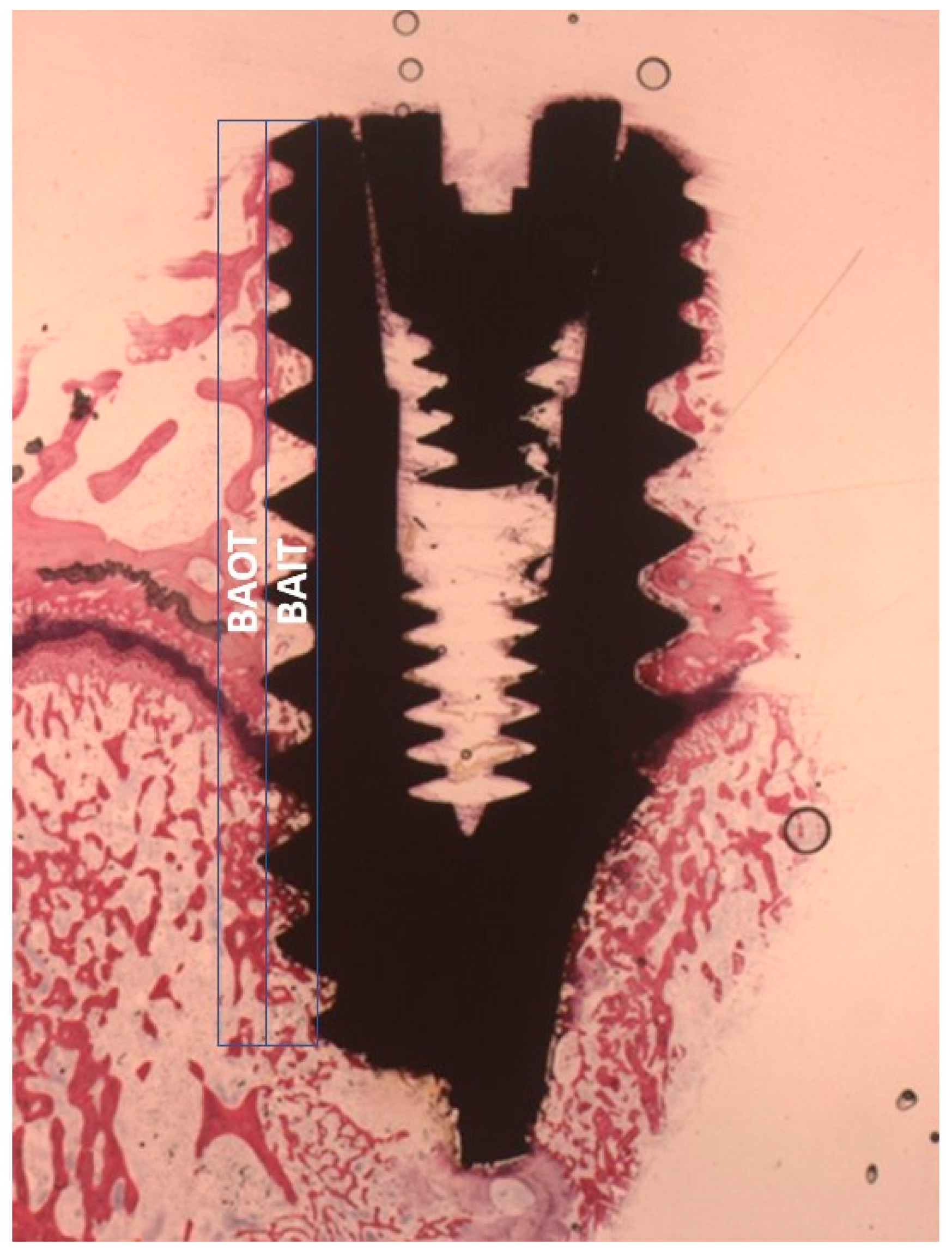
2.4. Histological Evaluation
2.4.1. Fifteen Days
Uncoated Implant Surface
Collagen-Coated Surface
2.4.2. Thirty Days
Uncoated Implant Surface
Collagen-Coated Surface
2.4.3. Sixty Days
Uncoated Implant Surface
Collagen-Coated Surface
2.5. Statistical Evaluation
3. Discussion
4. Materials and Methods
4.1. Surface Preparation by the Manufacturer
4.2. Surface Analysis by X-Ray Photoelectron Spectroscopy (XPS)
4.3. Atomic Force Microscopy (AFM)
4.4. In Vivo Experiment
4.5. Micro-CT Analysis
4.6. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodriguez, G.M.; Bowen, J.; Grossin, D.; Ben-Nissan, B.; Stamboulis, A. Functionalisation of Ti6Al4V and hydroxyapatite surfaces with combined peptides based on KKLPDA and EEEEEEEE peptides. Colloids Surf. B 2017, 160, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piatelli, A.; Quaranta, A.; Lorusso, F. Bone response to two dental implants with different sandblasted/acid-etched implant surfaces: A histological and histomorphometrical study in rabbits. Biomed Res. Int. 2017, 2017, 8724951. [Google Scholar] [CrossRef] [PubMed]
- Cordioli, G.; Majzoub, Z.; Piattelli, A.; Scarano, A. Removal torque and histomorphometric investigation of 4 different titanium surfaces: An experimental study in the rabbit tibia. Int. J. Oral Maxillofac. Implants 2000, 15, 668–674. [Google Scholar] [PubMed]
- Scarano, A.; Perrotti, V.; Artese, L.; Degidi, M.; Degidi, D.; Piattelli, A.; Iezzi, G. Blood vessels are concentrated within the implant surface concavities: A histologic study in rabbit tibia. Odontology 2014, 102, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Maghaireh, H.; Calvo-Guirado, J. Engineering the Bone-Implant Interface. Biomed Res. Int. 2018, 2018, 4956491. [Google Scholar] [CrossRef]
- Sinjari, B.; Traini, T.; Caputi, S.; Mortellaro, C.; Scarano, A. Evaluation of Fibrin Clot Attachment on Titanium Laser-Conditioned Surface Using Scanning Electron Microscopy. J. Craniofac. Surg. 2018, 29, 2277–2281. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Ribeiro da Silva, J.; Castellano, A.; Malta Barbosa, J.P.; Gil, L.F.; Marin, C.; Granato, R.; Bonfante, E.A.; Tovar, N.; Janal, M.N.; Coelho, P.G. Histomorphological and Histomorphometric Analyses of Grade IV Commercially Pure Titanium and Grade V Ti-6Al-4V Titanium Alloy Implant Substrates: An In Vivo Study in Dogs. Implant Dent. 2016, 25, 650–655. [Google Scholar] [CrossRef]
- Orsini, G.; Piattelli, M.; Scarano, A.; Petrone, G.; Kenealy, J.; Piattelli, A.; Caputi, S. Randomized, controlled histologic and histomorphometric evaluation of implants with nanometer-scale calcium phosphate added to the dual acid-etched surface in the human posterior maxilla. J. Periodontol. 2007, 78, 209–218. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Iezzi, G.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Mangano, C. Early bone formation around immediately loaded implants with nanostructured calcium-incorporated and machined surface: A randomized, controlled histologic and histomorphometric study in the human posterior maxilla. Clin. Oral Investig. 2017, 21, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the Thermal Treatment to Address a Better Osseointegration of Ti6Al4V Dental Implants: Histological and Histomorphometrical Study in a Rabbit Model. Biomed. Res. Int. 2018, 2018, 2349698. [Google Scholar] [PubMed]
- Geissler, U.; Hempel, U.; Wolf, C.; Scharnweber, D.; Worch, H.; Wenzel, K. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J. Biomed. Mater. Res. 2000, 51, 752–760. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nakayama, K.; Matsumoto, T. Differentiation and cell surface expression of transforming growth factor-beta receptors are regulated by interaction with matrix collagen in murine osteoblastic cells. J. Biol. Chem. 1996, 271, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Staiti, G.; Sinjari, B.; Tampieri, A.; Mortellaro, C. Sinus Augmentation with Biomimetic Nanostructured Matrix: Tomographic, Radiological, Histological and Histomorphometrical Results after 6 Months in Humans. Front. Physiol. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.-Y.; Xie, Y.-T.; Yang, S.-B.; Wu, X.-D.; Li, K.; Zheng, X.-B.; Tang, T.-T. Covalently immobilised type I collagen facilitates osteoconduction and osseointegration of titanium coated implants. J. Orthop. Translat. 2016, 5, 16–25. [Google Scholar] [CrossRef]
- Maghdouri-White, Y.; Bowlin, G.L.; Lemmon, C.A.; Dréau, D. Mammary epithelial cell adhesion, viability, and infiltration on blended or coated silk fibroin-collagen type I electrospun scaffolds. Mater. Sci. Eng. C 2014, 43, 37–44. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Bollati, D. Collagen I-coated titanium surfaces for bone implantation. In Biological Interactions on Materials Surfaces; Springer: Heidelberg, Germany, 2009; pp. 373–396. [Google Scholar]
- Kasemo, B.; Lausmaa, J. Biomaterial and implant surfaces: On the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res. 1988, 22, 145–158. [Google Scholar] [CrossRef]
- Wieland, M. Measurement and evaluation of the chemical composition and topography of titanium implant surfaces. Bone engineering 2000. [Google Scholar]
- Morra, M.; Cassinelli, C.; Bruzzone, G.; Carpi, A.; Di Santi, G.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int. J. Oral Maxillofac. Implants 2003, 18, 40–45. [Google Scholar] [PubMed]
- Scharnweber, D.; Born, R.; Flade, K.; Roessler, S.; Stoelzel, M.; Worch, H. Mineralization behaviour of collagen type I immobilized on different substrates. Biomaterials 2004, 25, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Bollati, D.; Baena, R.R.Y. Gene expression of markers of osteogenic differentiation of human mesenchymal cells on collagen I-modified microrough titanium surfaces. J. Biomed. Mater. Res. A 2011, 96, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.; Devlin, H.; Fu, E.; Ho, K.-Y.; Liang, S.-Y.; Hsieh, Y.-D. The osteoinductive effect of chitosan-collagen composites around pure titanium implant surfaces in rats. J. Periodont. Res. 2011, 46, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Schulz, M.C.; Hintze, V.; Range, U.; Mai, R.; Eckelt, U.; Schnabelrauch, M.; Möller, S.; Becher, J.; Scharnweber, D.; et al. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. J. Biomed. Mater. Res. A 2014, 102, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Mazzucco, L.; Borzini, P.; Fini, M.; Giavaresi, G.; Giardino, R. Collagen I-coated titanium surfaces: Mesenchymal cell adhesion and in vivo evaluation in trabecular bone implants. J. Biomed. Mater. Res. A 2006, 78, 449–458. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Roesseler, S.; Dard, M.; Sewing, A.; Aref, A. Biomimetic calcium phosphate composite coating of dental implants. Int. J. Oral Maxillofac. Implants 2006, 21, 738–746. [Google Scholar]
- Bae, E.-B.; Yoo, J.-H.; Jeong, S.-I.; Kim, M.-S.; Lim, Y.-M.; Ahn, J.-J.; Lee, J.-J.; Lee, S.-H.; Kim, H.-J.; Huh, J.-B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials 2018, 11, 2520. [Google Scholar] [CrossRef]
- Rotenberg, S.A.; Steiner, R.; Tatakis, D.N. Collagen-Coated Bovine Bone in Peri-implantitis Defects: A Pilot Study on a Novel Approach. Int. J. Oral Maxillofac. Implants 2016, 31, 701–707. [Google Scholar] [CrossRef]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Scarano, A.; Iezzi, G.; Petrone, G.; Orsini, G.; Degidi, M.; Strocchi, R.; Piattelli, A. Cortical bone regeneration with a synthetic cell-binding peptide: A histologic and histomorphometric pilot study. Implant Dent. 2003, 12, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative bone anatomy of commonly used laboratory animals: Implications for drug discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Zarb, G.A. Tissue-integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Co. Inc.: Batavia, IL, USA, 1985. [Google Scholar]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M. Real Opportunity for the Present and a Forward Step for the Future of Bone Tissue Engineering. J. Craniofac. Surg. 2017, 28, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef]


| Sample | O | C | N | Ti |
|---|---|---|---|---|
| Ti | 49.2 | 31.6 | 0.4 | 18.8 |
| ColTi | 23.8 | 59.3 | 14.3 | 2.7 |
| BIC | p Value | BAIT | p Value | BAOT | p Value | ||
|---|---|---|---|---|---|---|---|
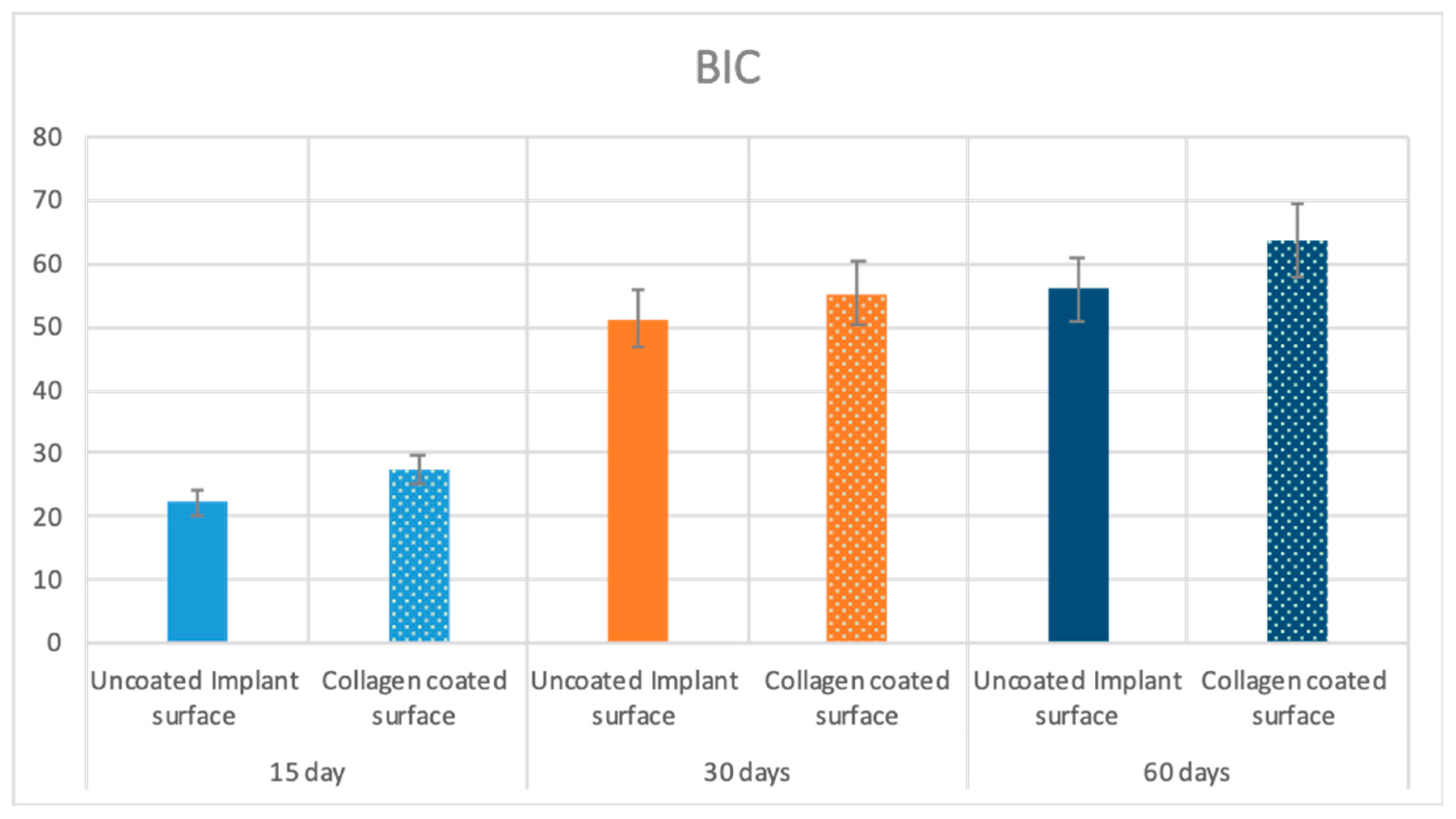
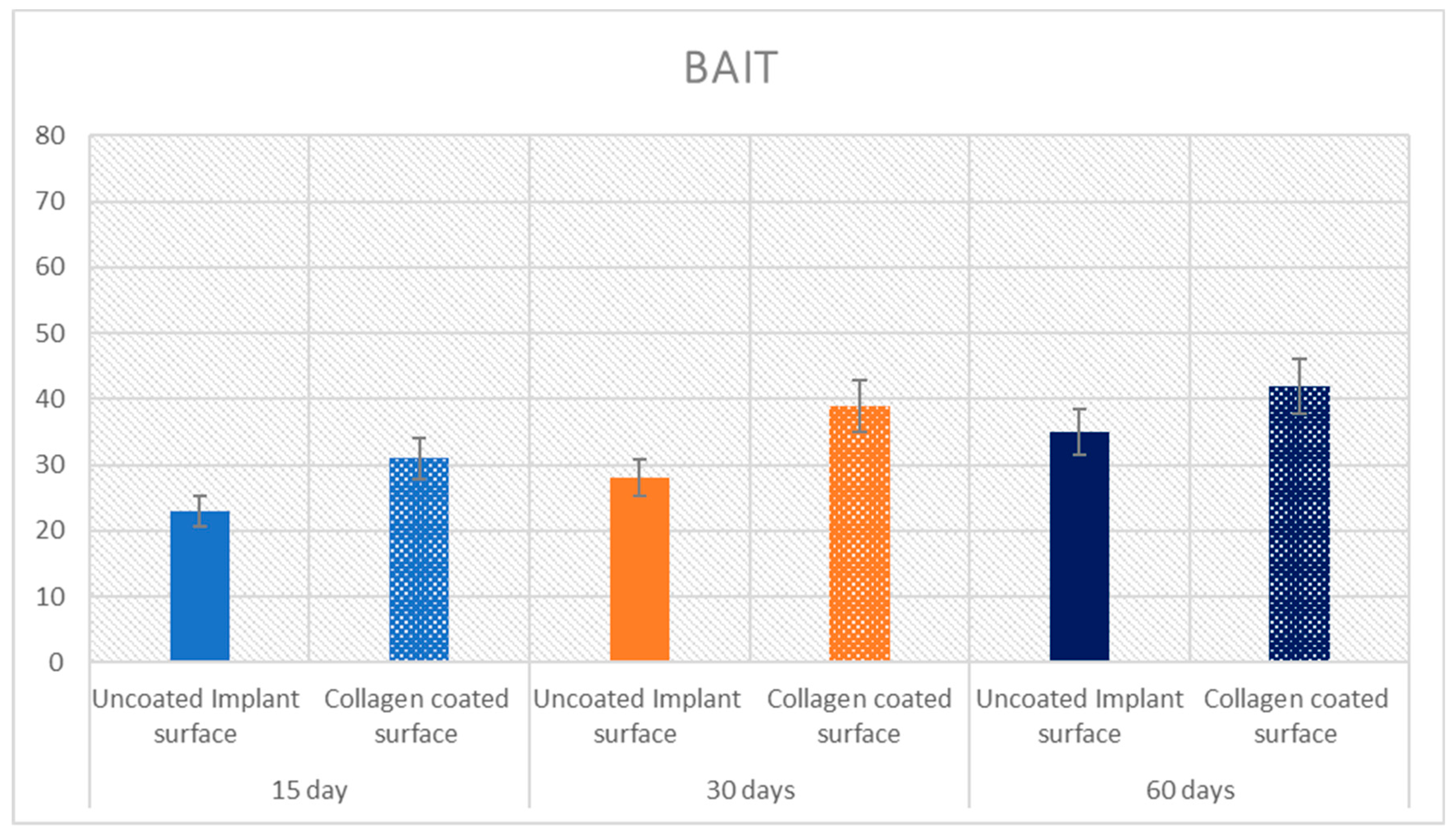
| 15 Days | Uncoated surface | 22.4 ± 4.5 | p = 0.0005 | 23 ± 0.8 | p = 0.00004 | 19 ± 0.8 | p = 0.00003 |
| Collagen coated surface | 27.5 ± 3.1 | 31 ± 0.8 | 21.8 ± 1 | ||||
| 30 Days | Uncoated surface | 51.2 ± 3.9 | p = 0.0021 | 28 ± 0.8 | p = 0.00001 | 36 ± 0.8 | p = 0.0008 |
| Collagen coated surface | 55.3 ± 3.2 | 39 ± 2.2 | 38 ± 2.2 | ||||
| 60 Days | Uncoated surface | 56 ± 4.2 | p = 0.00001 | 35 ± 2.3 | p = 0.00006 | 36 ± 2.3 | p = 0.0196 |
| Collagen coated surface | 63.6 ± 2.9 | 42 ± 2.3 | 44 ± 2.3 |
| BIC | BAIT | BOAT | |||
|---|---|---|---|---|---|
| 15 Days | Uncoated surface | BIC | - | 0.620 * | −0.547 * |
| BAIT | 0.620 * | - | −0.951 ** | ||
| BOAT | −0.547 * | −0.951 ** | - | ||
| Collagen coated surface | BIC | - | 0.576* | 0.578 * | |
| BAIT | 0.576 * | - | 0.935 ** | ||
| BOAT | 0.578 * | 0.935** | - | ||
| 30 Days | Uncoated surface | BIC | - | 0.566 * | −0.536 * |
| BAIT | 0.566 * | - | −0.766 ** | ||
| BOAT | −0.536 * | −0.766 ** | - | ||
| Collagen coated surface | BIC | - | 0.598 * | −0.603 * | |
| BAIT | 0.598 * | - | −0.894 ** | ||
| BOAT | −0.603 * | -0.894 ** | - | ||
| 60 Days | Uncoated surface | BIC | - | 0.643 * | −0.512 * |
| BAIT | 0.643 * | - | −0.857 ** | ||
| BOAT | −0.582 * | -0.857 ** | - | ||
| Collagen coated surface | BIC | - | 0.541 * | −0.611 * | |
| BAIT | 0.541 * | - | −0.863 ** | ||
| BOAT | −0.611 * | −0.863 ** | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. https://doi.org/10.3390/ijms20030724
Scarano A, Lorusso F, Orsini T, Morra M, Iviglia G, Valbonetti L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. International Journal of Molecular Sciences. 2019; 20(3):724. https://doi.org/10.3390/ijms20030724
Chicago/Turabian StyleScarano, Antonio, Felice Lorusso, Tiziana Orsini, Marco Morra, Giorgio Iviglia, and Luca Valbonetti. 2019. "Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits" International Journal of Molecular Sciences 20, no. 3: 724. https://doi.org/10.3390/ijms20030724
APA StyleScarano, A., Lorusso, F., Orsini, T., Morra, M., Iviglia, G., & Valbonetti, L. (2019). Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. International Journal of Molecular Sciences, 20(3), 724. https://doi.org/10.3390/ijms20030724








