Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway
Abstract
1. Introduction
2. Results
2.1. Kaempferol Protects Striatum Neuron Injuried by LPS in Mice
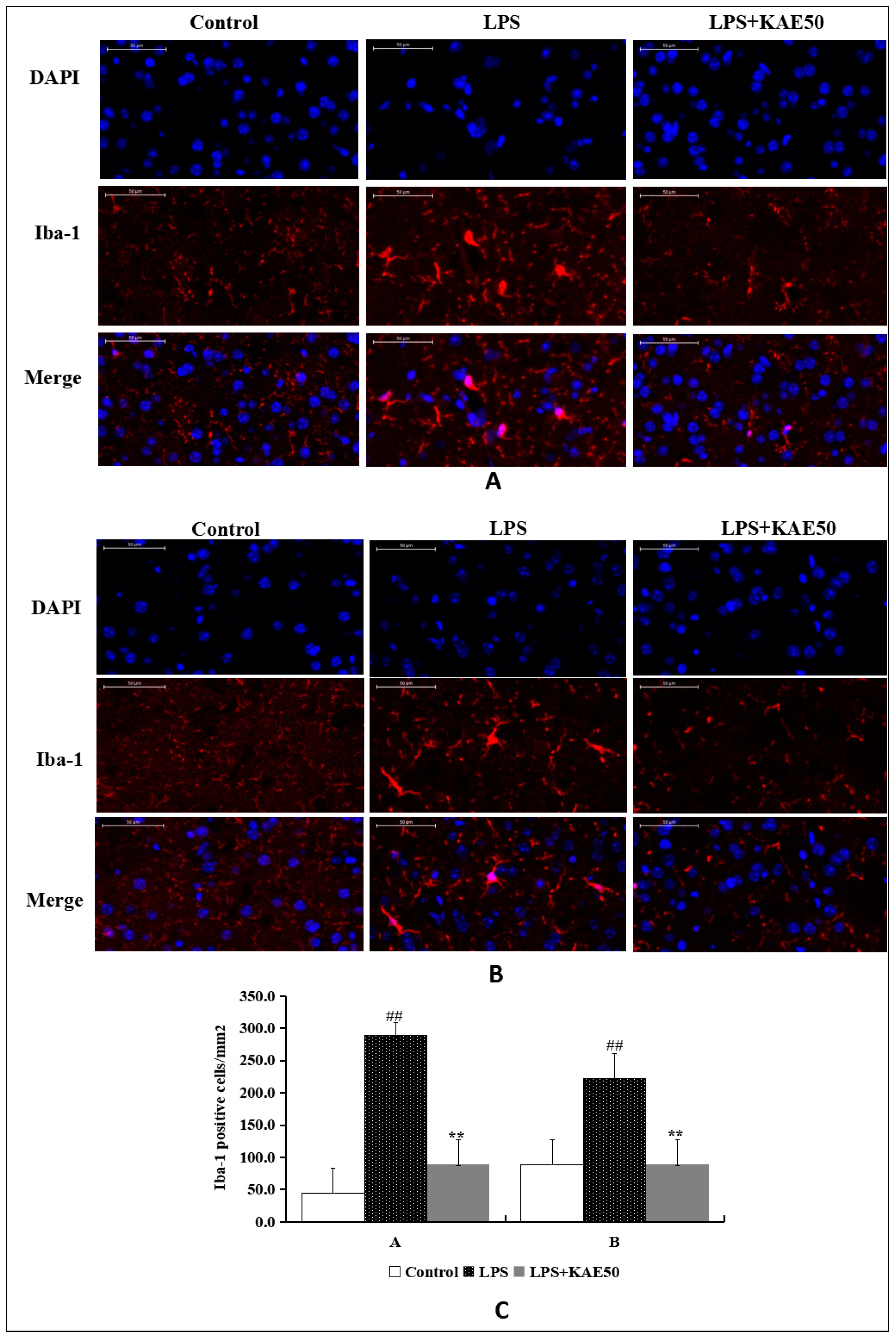
2.2. Kaempferol Inhibits Microglia Activation in Striatum of Mice Injured by LPS
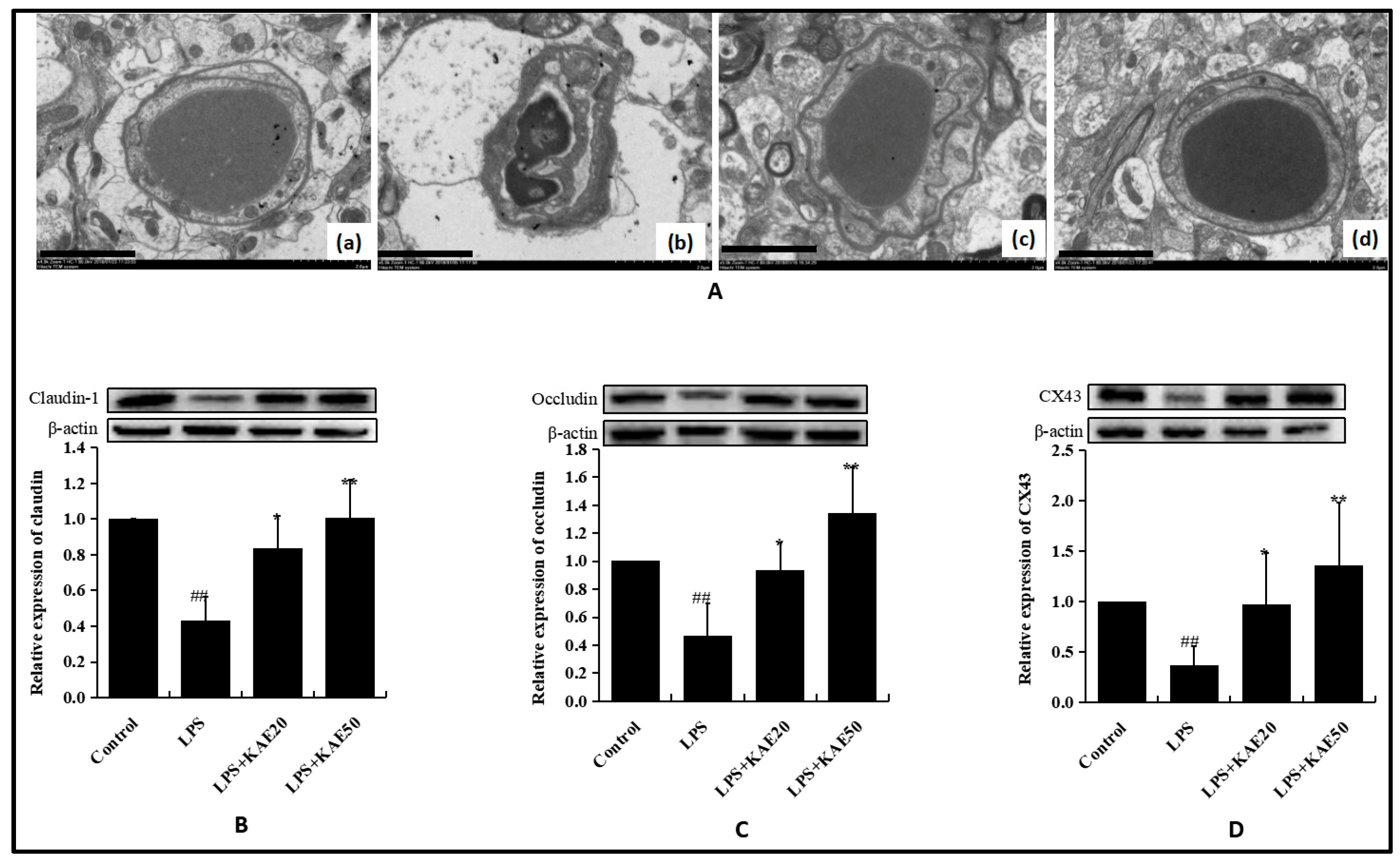
2.3. Kaempferol Blocked BBB Dysfunction Injured by LPS in the Striatum of Mice
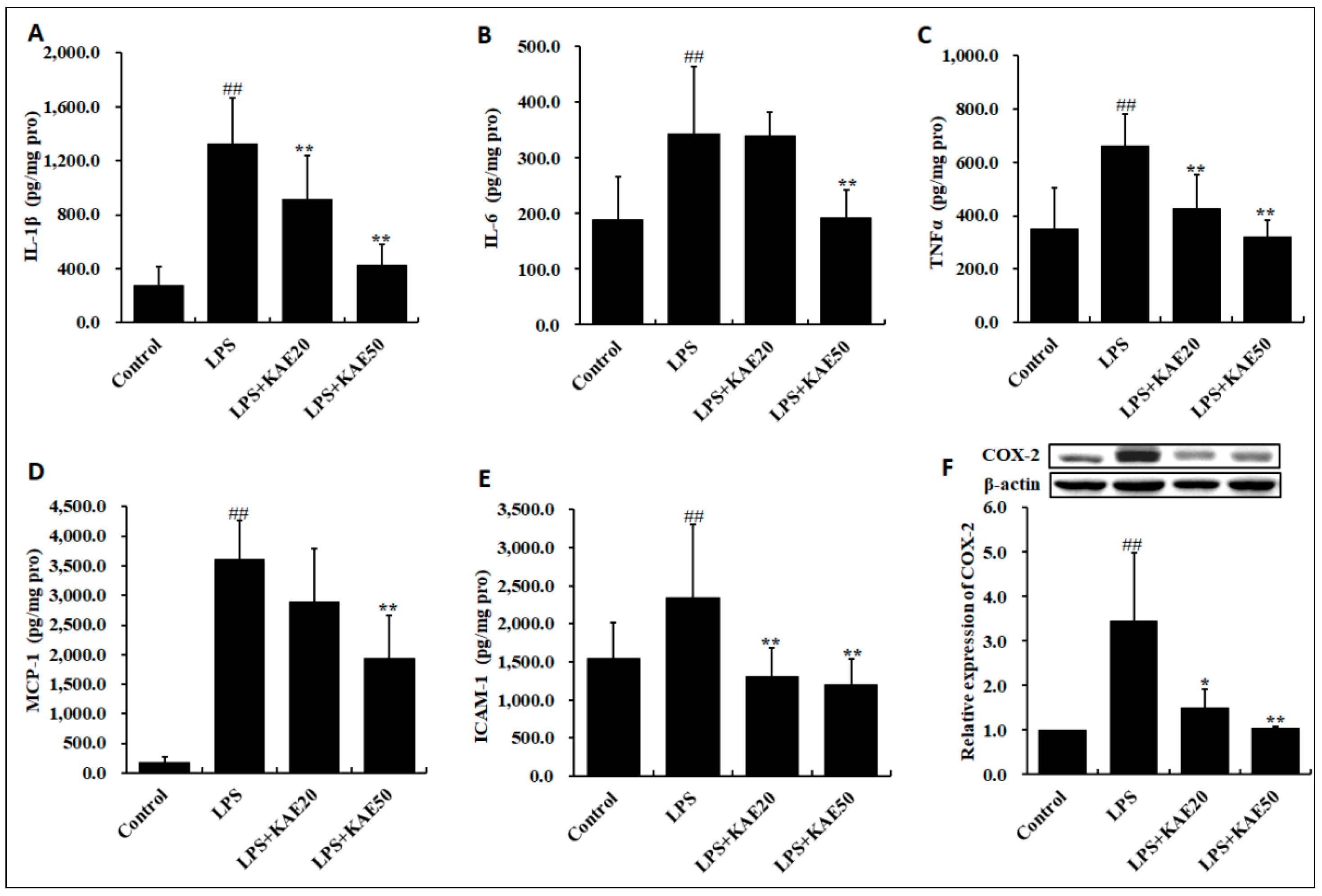
2.4. Kaempferol Inhibits Inflammatory Cytokines Release in Striatum of Mice Injured by LPS
2.5. Kaempferol Blocked the Expression of Inflammatory Proteins in Striatum of Mice Injured by LPS
3. Discussion
4. Material and Methods
4.1. Reagents and Antibody
4.2. Animals and Experimental Procedure
4.3. Immunofluorescence Staining
4.4. Electron Microscopy Analysis
4.5. ELISA Assay
4.6. Western Blot Analysis
4.7. Real-Time PCR Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kortekaas, R.; Leenders, K.L.; van Oostrom, J.C.; Vaalburg, W.; Bart, J.; Willemsen, A.T.; Hendrikse, N.H. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005, 57, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Teismann, P.; Vila, M.; Choi, D.K.; Tieu, K.; Wu, D.C.; Jackson-Lewis, V.; Przedborski, S. COX-2 and neurodegeneration in Parkinson’s disease. Ann. N. Y. Acad. Sci. 2003, 991, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Tieu, K.; Choi, D.K.; Wu, D.C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Herrera, A.J.; Venero, J.L.; Santiago, M.; de Pablos, R.M.; Villarán, R.F.; Espinosa-Oliva, A.M.; Argüelles, S.; Sarmiento, M.; Delgado-Cortés, M.J.; et al. Inflammatory animal model for Parkinson’s disease: The intranigral injection of LPS induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Sasaki, T.; Liu, K.; Agari, T.; Yasuhara, T.; Morimoto, J.; Okazaki, M.; Takeuchi, H.; Toyoshima, A.; Sasada, S.; Shinko, A.; et al. Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp. Neurol. 2016, 275 Pt 1, 220–231. [Google Scholar] [CrossRef]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef]
- Park, J.S.; Gamboni-Robertson, F.; He, Q.; Svetkauskaite, D.; Kim, J.Y.; Strassheim, D.; Sohn, J.W.; Yamada, S.; Maruyama, I.; Banerjee, A.; et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006, 290, C917–C924. [Google Scholar] [CrossRef]
- Hreggvidsdóttir, H.S.; Lundberg, A.M.; Aveberger, A.C.; Klevenvall, L.; Andersson, U.; Harris, H.E. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol. Med. 2012, 18, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tsirka, S.E. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell. Mol. Life Sci. 2014, 71, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.B. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J. Neuroimmunol. 2002, 128, 58–68. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, C.; Bustin, M.; Talwar, S.; Tropea, M.; Gerstenberger, E.; Shelhamer, J.H.; Suffredini, A.F. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003, 101, 2560–2652. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, Y.L.; Yang, H.; Wang, Y.H.; Du, G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Gangadharan, S.; Padmakumar, C.P. Parkinson’s disease in the older patient. Clin. Med. 2016, 16, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Guo, C.; Tang, W.; Liu, W.; Liu, Y. Astragalus polysaccharide protects neurons and stabilizes mitochondrial in a mouse model of Parkinson disease. Med. Sci. Monit. 2018, 24, 5192–5199. [Google Scholar] [CrossRef]
- Liang, H.; Wang, H.; Wang, S.; Francis, R.; Paxinos, G.; Huang, X. 3D imaging of PSD-95 in the mouse brain using the advanced CUBIC method. Mol. Brain 2018, 11, 50. [Google Scholar] [CrossRef]
- Chen, B.; Friedman, B.; Cheng, Q.; Tsai, P.; Schim, E.; Kleinfeld, D.; Lyden, P.D. Severe blood brain barrier disruption and surrounding tissue injury. Stroke 2009, 40, e666–e674. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.T.; Woulfe, J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 2015, 35, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Carvey, P.M.; Zhao, C.H.; Hendey, B.; Lum, H.; Trachtenberg, J.; Desai, B.S.; Snyder, J.; Zhu, Y.G.; Ling, Z.D. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur. J. Neurosci. 2005, 22, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000, 11, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Hill, J.; Zhang, M.; Dykstra, H.; Winfield, M.; Reichenbach, N.L.; Potula, R.; Mukherjee, A.; Ramirez, S.H.; Rom, S. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb. Blood Flow Metab. 2016, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Wahner, A.D.; Sinsheimer, J.S.; Bronstein, J.M.; Ritz, B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch. Neurol. 2007, 64, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, H.Y.; Chen, J.H.; Tseng, W.P.; Ko, P.Y.; Liu, Y.S.; Yeh, W.L.; Lu, D.Y. Effects of paeonol on anti-neuroinflammatory responses in microglial cells. Int. J. Mol. Sci. 2015, 16, 8844–8860. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Ros-Bernal, F.; Hunot, S.; Herrero, M.T.; Parnadeau, S.; Corvol, J.C.; Lu, L.; Alvarez-Fischer, D.; Carrillo-de Sauvage, M.A.; Saurini, F.; Coussieu, F.; et al. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc. Natl. Acad. Sci. USA 2011, 108, 6632–6637. [Google Scholar] [CrossRef] [PubMed]
- Knott, C.; Stern, G.; Wilkin, G.P. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 2000, 16, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M.; Earl, C.; Kaczmarczyk, E.; Ferrari, C.; Besedovsky, H.; del Rey, A.; Pitossi, F.J.; Oertel, W.H. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2003, 18, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Balliano, A.; Hao, F.; Njeri, C.; Balakrishnan, L.; Hayes, J.J. HMGB1 stimulates activity of polymerase beta on nucleosome substrates. Biochemistry 2017, 56, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Maetzler, W.; Stathakos, P.; Martin, H.L.; Hobert, M.A.; Rattay, T.W.; Gasser, T.; Forrester, J.V.; Berg, D.; Tracey, K.J.; et al. In vivo evidence that high mobility group box 1 exerts deleterious effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and Parkinson’s disease which can be attenuated by glycyrrhizin. Neurobiol. Dis. 2016, 91, 59–68. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.Y.; Choi, D.K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Piperi, C.; Papavassiliou, A.G. High-mobility group box 1 in Parkinson’s disease: From pathogenesis to therapeutic approaches. J. Neurochem. 2018, 146, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Rajeswari, M.R. Dichotomous life of DNA binding high mobility group box1 protein in human health and disease. Curr. Protein Pept. Sci. 2016, 17, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.; Fellner, L.; Reindl, M.; Masliah, E.; Poewe, W.; Wenning, G.K. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011, 179, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhao, Z.; Shang, W.; Liu, C.; Zhang, B.; Zhao, L.; Cai, H. Ginsenoside Rg1 nanoparticle penetrating the blood-brain barrier to improve the cerebral function of diabetic rats complicated with cerebral infarction. Int. J. Nanomedicine. 2017, 12, 6477–6486. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.S.; Fan, Y.Y.; Ye, G.; Li, J.; Feng, X.P.; Lin, K.; Dong, M.; Wang, Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp. Ther. Med. 2016, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-L.; Cheng, X.; Li, W.-H.; Liu, M.; Wang, Y.-H.; Du, G.-H. Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. Int. J. Mol. Sci. 2019, 20, 491. https://doi.org/10.3390/ijms20030491
Yang Y-L, Cheng X, Li W-H, Liu M, Wang Y-H, Du G-H. Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. International Journal of Molecular Sciences. 2019; 20(3):491. https://doi.org/10.3390/ijms20030491
Chicago/Turabian StyleYang, Ying-Lin, Xiao Cheng, Wei-Han Li, Man Liu, Yue-Hua Wang, and Guan-Hua Du. 2019. "Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway" International Journal of Molecular Sciences 20, no. 3: 491. https://doi.org/10.3390/ijms20030491
APA StyleYang, Y.-L., Cheng, X., Li, W.-H., Liu, M., Wang, Y.-H., & Du, G.-H. (2019). Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. International Journal of Molecular Sciences, 20(3), 491. https://doi.org/10.3390/ijms20030491




