Gold Nanoparticle-Based Fluorescent Theranostics for Real-Time Image-Guided Assessment of DNA Damage and Repair
Abstract
1. Introduction
2. Results
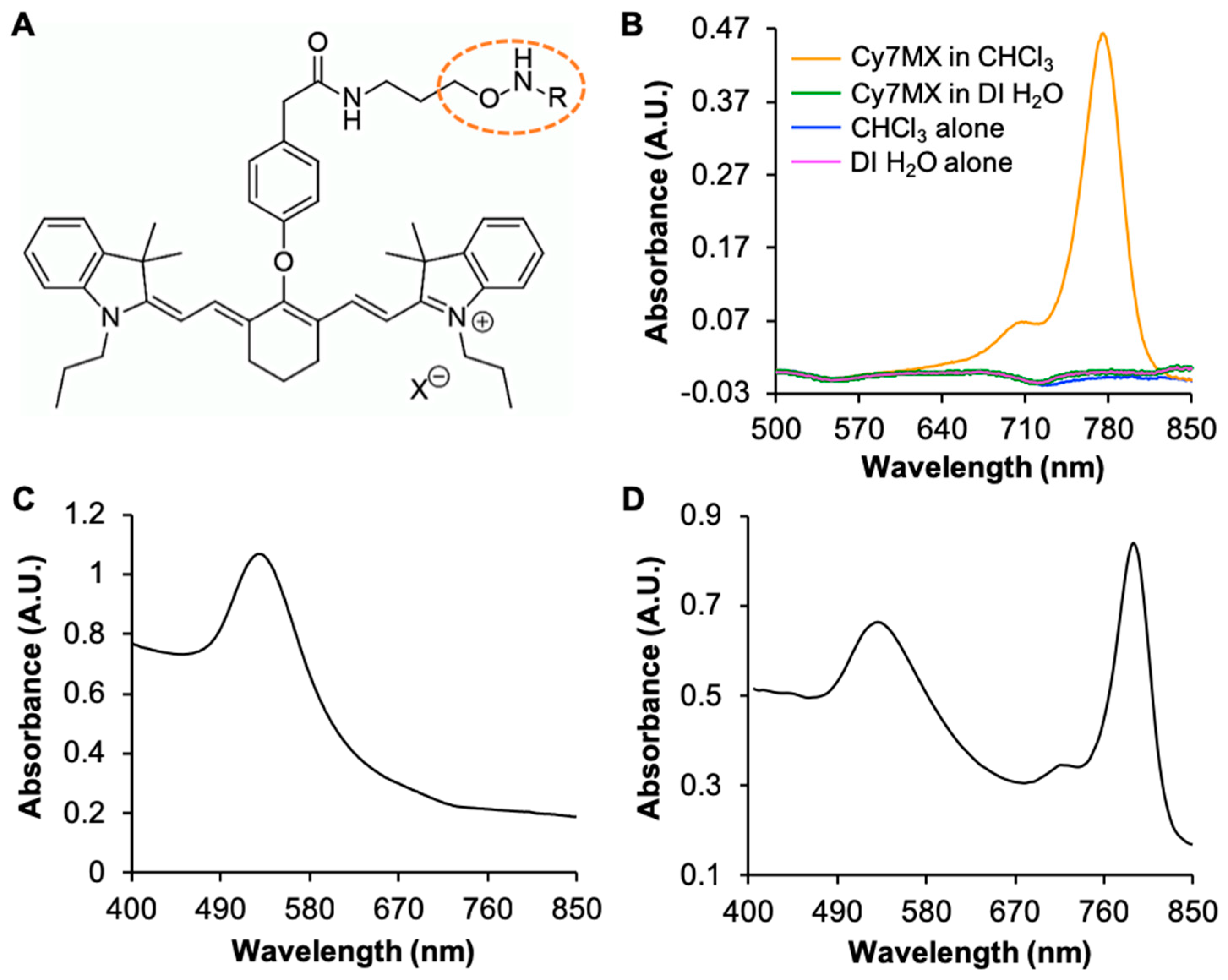
2.1. UV-Vis Characterization of Molecular Probe and AuNPs
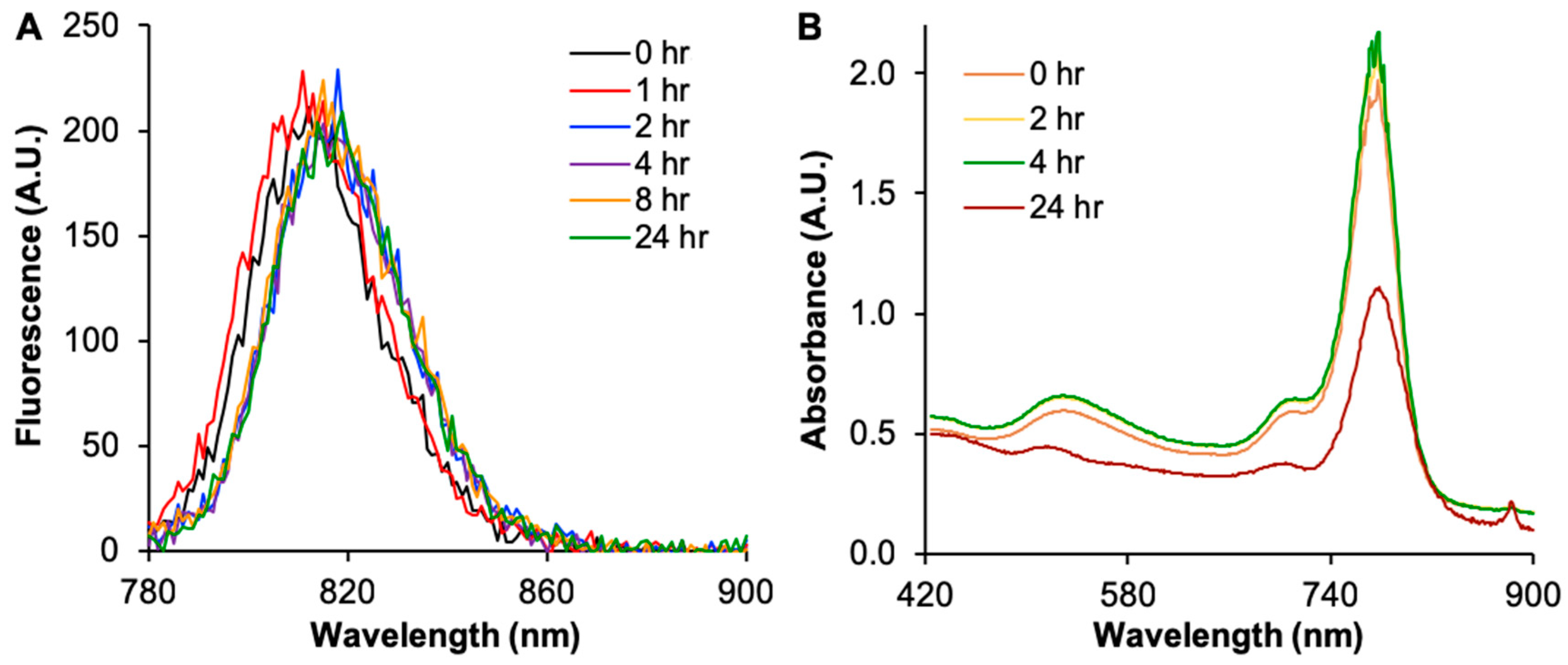
2.2. Cy7MX-AuNPs Loading Profile
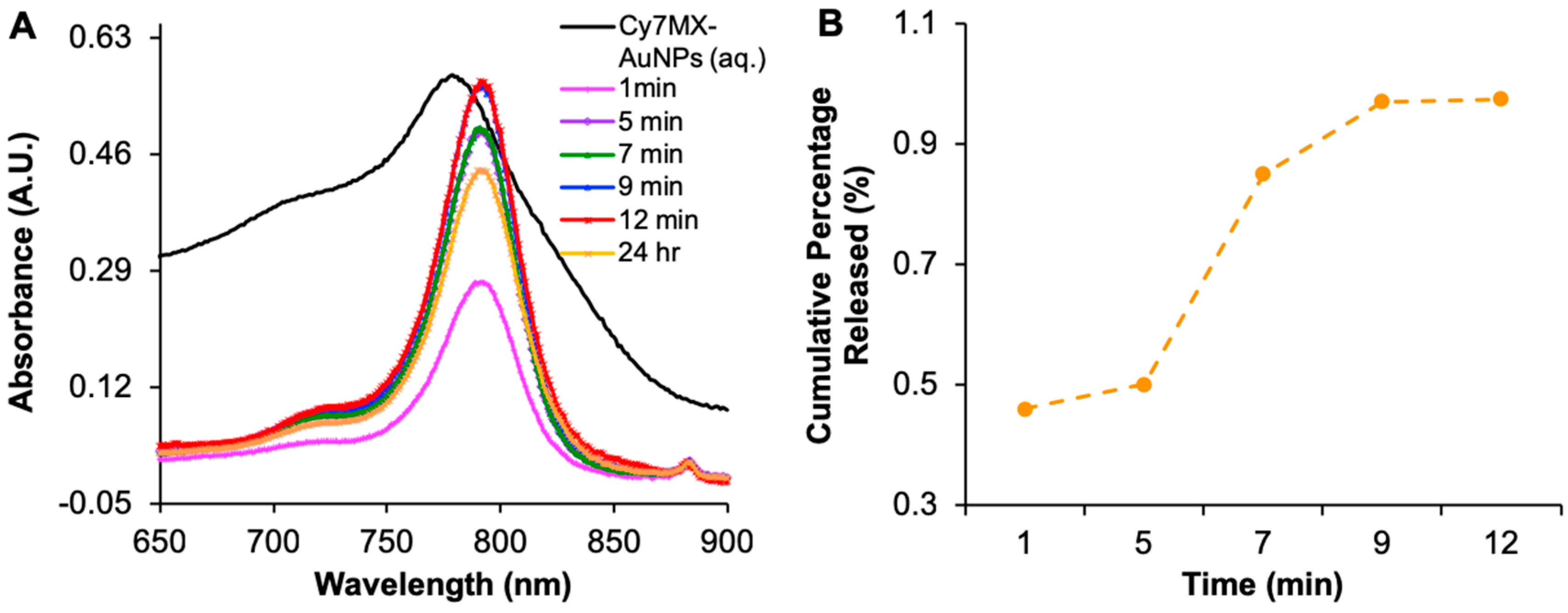
2.3. Biphasic Release Profile
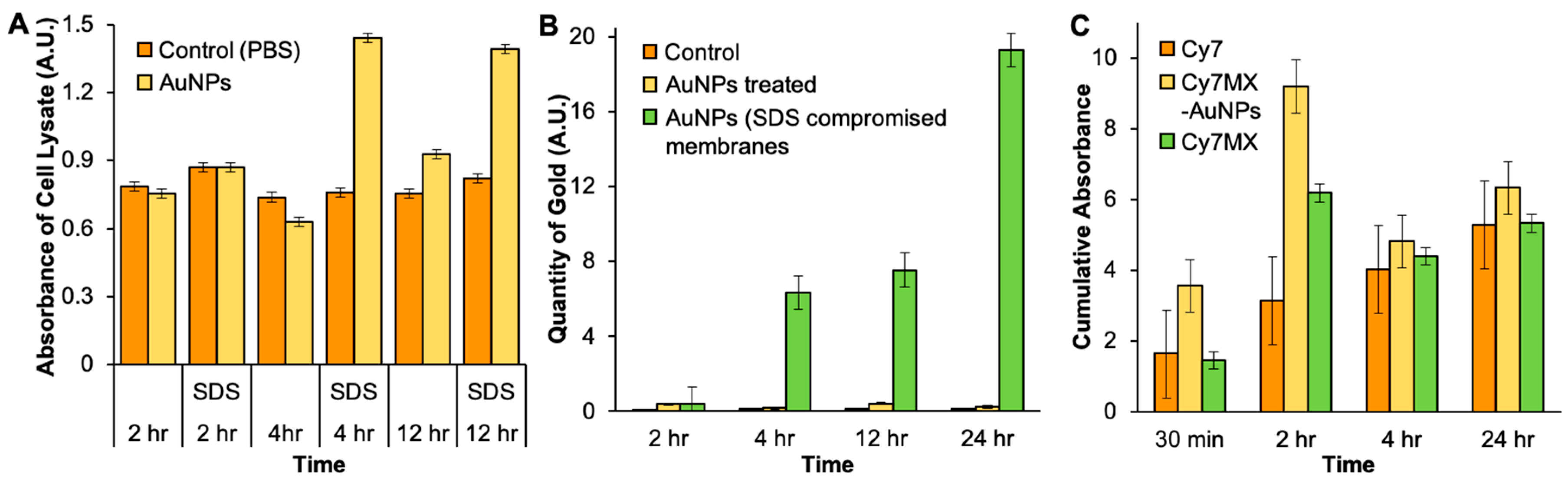
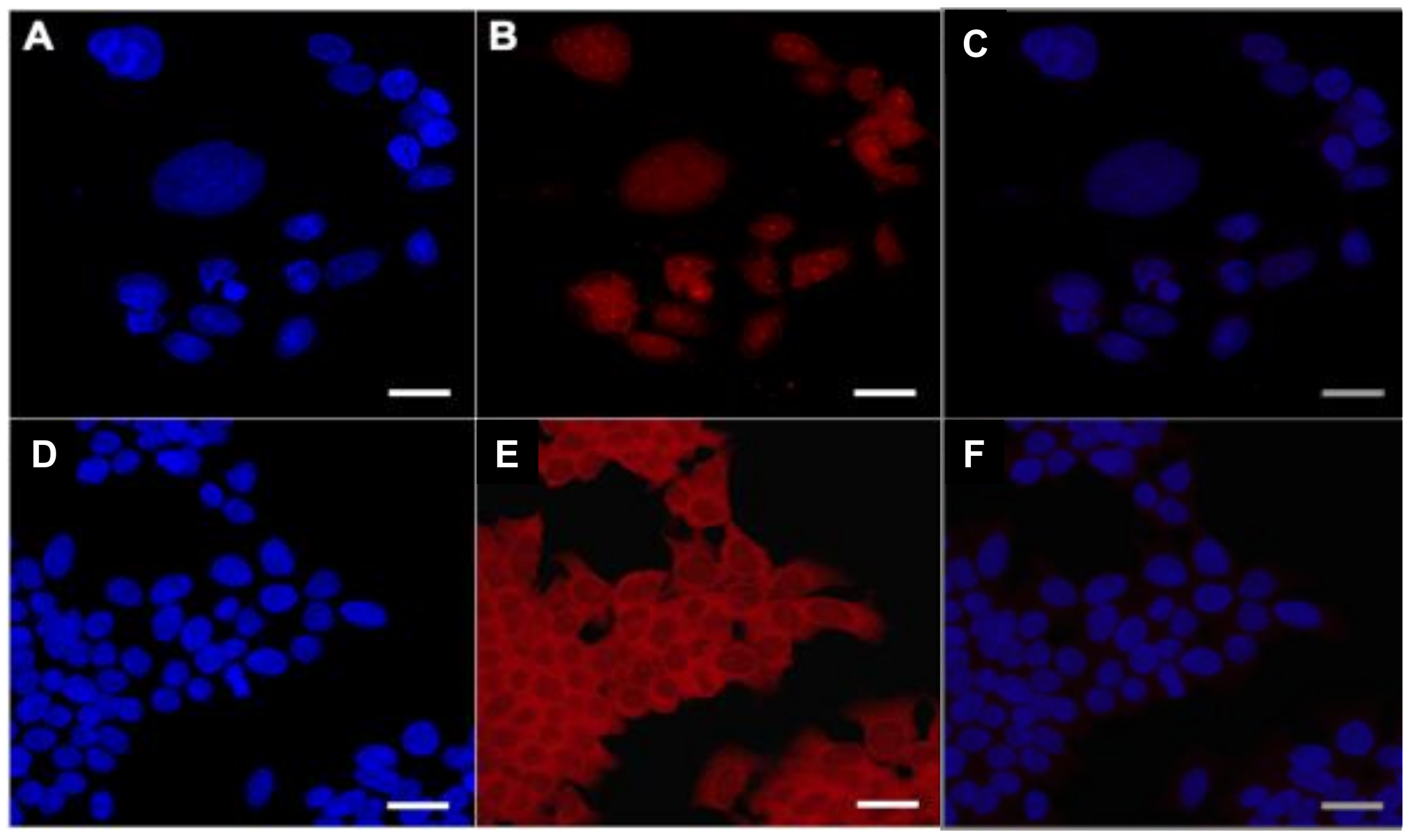
2.4. In Vitro Uptake of AuNPs and Cy7MX
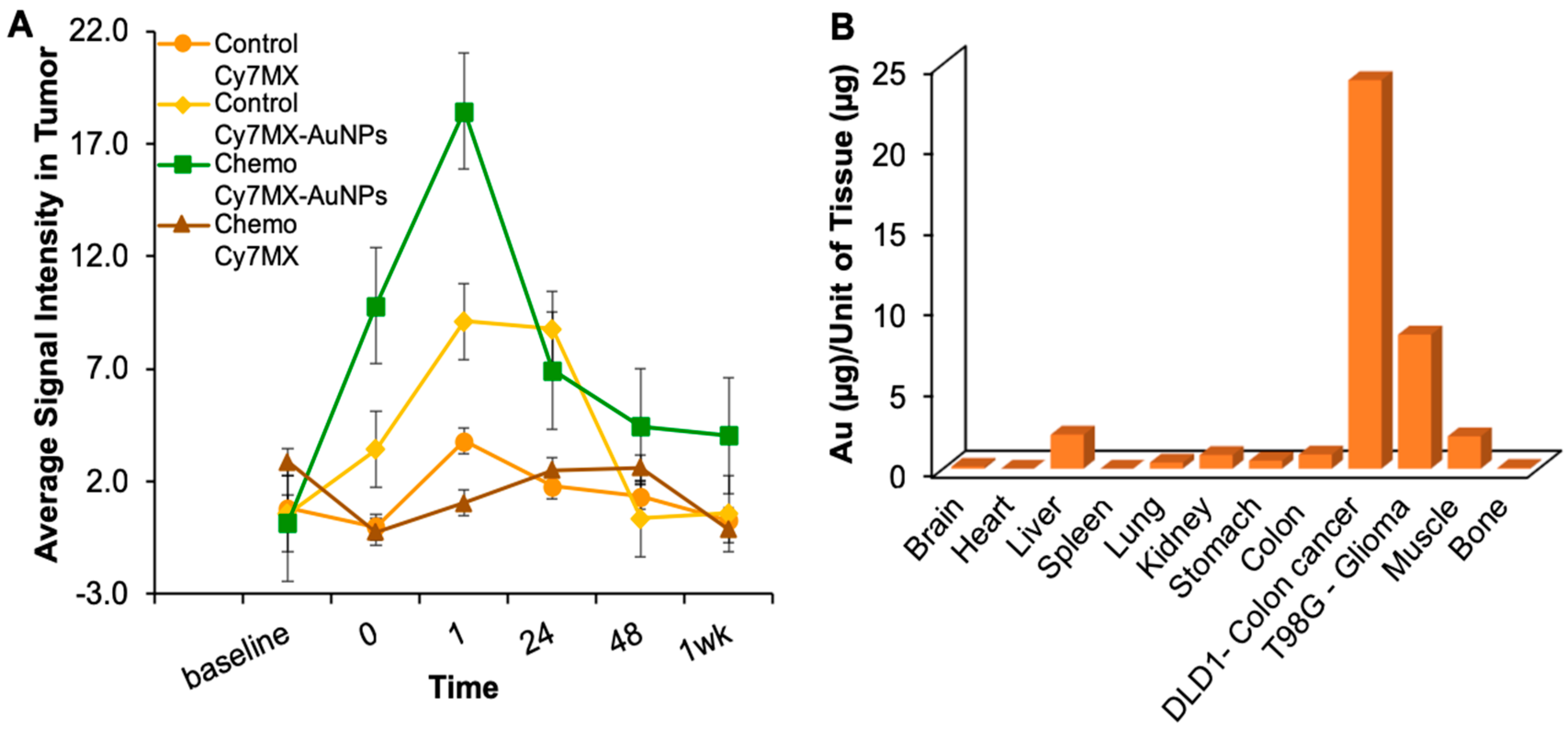
2.5. Xenografted Tumors Treated with Cy7MX-AuNPs
2.6. Biodistribution Following Systemic Administration of Cy7MX-AuNPs
3. Discussion
4. Materials and Methods
4.1. Synthesis of DDA-Coated AuNPs
4.2. PEGylation of DDA Coated AuNPs
4.3. Cy7MX Dye Loading on PEGylated AuNPs
4.4. DLS Characterization
4.5. Loading and Stability of Cy7MX
4.6. Biphasic Release Study
4.7. AuNPs Uptake
4.8. Cy7MX Uptake
4.9. Cy7MX Localization
4.10. In Vivo Testing of Cy7MX Uptake
4.11. Biodistribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cy7MX | cyanine-7 methoxyamine |
| AuNP | gold nanoparticle |
| PEG | polyethylene glycol |
| AP | apurinic/apyrimidinic |
| PET | positron emission tomography |
| NIR | near infrared |
| BER | base excision repair |
| RES | reticuloendothelial system |
| EPR | enhanced permeability and retention |
| DLS | dynamic laser scattering |
| AAS | atomic absorption spectroscopy |
| GFAAS | graphite furnace atomic absorption spectroscopy |
| SDS | sodium dodecyl sulfate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| gold | Au |
| CHCl3 | chloroform |
| DI H2O | deionized water |
| PBS | phosphate buffer saline |
| HAuCl4·3H2O | gold(III) chloride trihydrate |
| DDA | dodecylamine |
| Meo-PEG-SH | alpha-methoxy-omega-mercapto poly(ethylene glycol) |
| 5-Fdu | 5-fluorodeoxyuridine |
| UDG | uracil DNA glycosylase |
| HNO3 | nitic acid |
References
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-Damaging Agents in Cancer Chemotherapy: Serendipity and Chemical Biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. DNA Damage and Repair. Nature 2003, 421, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sadahiro, S.; Suzuki, T.; Okada, K.; Saito, G.; Miyakita, H. Retrospective Study of Regorafenib and Trifluridine/Tipiracil Efficacy as a Third-Line or Later Chemotherapy Regimen for Refractory Metastatic Colorectal Cancer. Oncol. Lett. 2018, 16, 6589–6597. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H. Impact of Chemotherapy Dose Intensity on Cancer Patient Outcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- Fridborg, H.; Nygren, P.; Larsson, R. Relationship between Pharmacokinetic Parameters in Patients and Cytotoxicity in Vitro of Standard and Investigational Anticancer Drugs. Anti-Cancer Drugs 1995, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Gurney, H. I Don’t Underdose My Patients…do I? Lancet Oncol. 2005, 6, 637–638. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Prognostic Value of Baseline ALBI Core Among Patients with Colorectal Liver Metastases: A Pooled Analysis of Two Randomized Trials. Clin. Colorectal Cancer 2018. [Google Scholar] [CrossRef]
- Tan, W.; Yang, M.; Yang, H.; Zhou, F.; Shen, W. Predicting the Response to Neoadjuvant Therapy for Early-Stage Breast Cancer: Tumor-, Blood-, and Imaging-Related Biomarkers. Cancer Manag. Res. 2018, 10, 4333–4347. [Google Scholar] [CrossRef]
- Machado, M.C.; da Costa-Neto, J.M.; Portela, R.D.; D’Assis, M.J.M.H.; Martins-Filho, O.A.; Barrouin-Melo, S.M.; Borges, N.F.; Silva, F.L.; Estrela-Lima, A. The Effect of Naltrexone as a Carboplatin Chemotherapy-Associated Drug on the Immune Response, Quality of Life and Survival of Dogs with Mammary Carcinoma. PLoS ONE 2018, 13, e0204830. [Google Scholar] [CrossRef]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.-H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.-X.; Qiu, J.; et al. Imaging Tumor-Stroma Interactions during Chemotherapy Reveals Contributions of the Microenvironment to Resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar] [CrossRef]
- Gangadaran, P.; Ahn, B.-C. Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies. Front. Immunol. 2017, 8, 1090. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Okigami, M.; Toiyama, Y.; Morimoto, Y.; Matsushita, K.; Kawamura, M.; Hashimoto, K.; Saigusa, S.; Okugawa, Y.; Inoue, Y.; et al. In Vivo Real-Time Imaging of Chemotherapy Response on the Liver Metastatic Tumor Microenvironment Using Multiphoton Microscopy. Oncol. Rep. 2012, 28, 1822–1830. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the Era of Molecular Oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.; Wood, R.D. Quality Control by DNA Repair. Science 1999, 286, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Wu, C.; Bulgar, A.; Somoza, E.; Zhu, W.; Gerson, S.L. Direct Detection and Quantification of Abasic Sites for In Vivo Studies of DNA Damage and Repair. Nucl. Med. Biol. 2009, 36, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, N.A.; Fokou, E.; Tsoumpas, C. Dosage Optimization in Positron Emission Tomography: State-of-the-Art Methods and Future Prospects. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 527–547. [Google Scholar] [PubMed]
- Singh, N.; Veronese, M.; O’Doherty, J.; Sementa, T.; Bongarzone, S.; Cash, D.; Simmons, C.; Arcolin, M.; Marsden, P.K.; Gee, A.; et al. Assessing the Feasibility of Intranasal Radiotracer Administration for in Brain PET Imaging. Nucl. Med. Biol. 2018, 66, 32–39. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.J.; Taubman, K.L.; Foroudi, F.; Lee, S.T.; Scott, A.M. Molecular Imaging Using PET/CT for Radiation Therapy Planning for Adult Cancers: Current Status and Expanding Applications. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Franke, D.; Caram, J.R.; Perkinson, C.F.; Saif, M.; Askoxylakis, V.; Datta, M.; Fukumura, D.; Jain, R.K.; Bawendi, M.G.; et al. Shortwave Infrared Fluorescence Imaging with the Clinically Approved Near-Infrared Dye Indocyanine Green. Proc. Natl. Acad. Sci. USA 2018, 115, 4465–4470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bloch, S.; Akers, W.; Achilefu, S. Near-Infrared Molecular Probes for In Vivo Imaging. Curr. Protoc. Cytom. 2012. [Google Scholar] [CrossRef]
- Marshall, M.V.; Rasmussen, J.C.; Tan, I.-C.; Aldrich, M.B.; Adams, K.E.; Wang, X.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Sevick-Muraca, E.M. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg. Oncol. J. Online 2010, 2, 12–25. [Google Scholar] [CrossRef]
- Sharma, R.; Wendt, J.A.; Rasmussen, J.C.; Adams, K.E.; Marshall, M.V.; Sevick-Muraca, E.M. New Horizons for Imaging Lymphatic Function. Ann. N. Y. Acad. Sci. 2008, 1131, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 Dye Loaded Tumor Targeting Theranostic Nanoparticles for NIR Imaging and Photothermal Therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.K.; Kelkar, S.S.; Wojtynek, N.E.; Souchek, J.J.; Payne, W.M.; Stump, K.; Marini, F.C.; Mohs, A.M. Near Infrared Fluorescent Nanoparticles Derived from Hyaluronic Acid Improve Tumor Contrast for Image-Guided Surgery. Theranostics 2016, 6, 2314–2328. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Li, Y.; Guo, Y.; Cao, Y.; Li, P.; Wang, Z.; Gong, Y.; Ran, H. Folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green for photoacoustic/ultrasound imaging and chemo/photothermal therapy. Int. J. Nanomed. 2018, 13, 5139–5158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic Study of IR-780 Dye as a Potential Tumor Targeting and Drug Delivery Agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Talpaert-Borlé, M. Formation, Detection and Repair of AP Sites. Mutat. Res. 1987, 181, 45–56. [Google Scholar] [CrossRef]
- Liu, H.; Doane, T.L.; Cheng, Y.; Lu, F.; Srinivasan, S.; Zhu, J.J.; Burda, C. Control of Surface Ligand Density on PEGylated Gold Nanoparticles for Optimized Cancer Cell Uptake. Part. Part. Syst. Charact. 2015, 32, 197–204. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Doane, T.L.; Cheng, Y.; Babar, A.; Hill, R.J.; Burda, C. Electrophoretic Mobilities of PEGylated Gold NPs. J. Am. Chem. Soc. 2010, 132, 15624–15631. [Google Scholar] [CrossRef]
- Cheng, Y.; Meyers, J.D.; Broome, A.-M.; Kenney, M.E.; Basilion, J.P.; Burda, C. Deep Penetration of a PDT Drug into Tumors by Noncovalent Drug-Gold Nanoparticle Conjugates. J. Am. Chem. Soc. 2011, 133, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Ruoslahti, E.; Meng, H. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano 2017, 11, 9567–9569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Routes, A.E. Drug Absorption, Distribution and Elimination. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-Modified Gold Nanorods with a Stealth Character for In Vivo Applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Condie, A.G.; Yan, Y.; Gerson, S.L.; Wang, Y. A Fluorescent Probe to Measure DNA Damage and Repair. PLoS ONE 2015, 10, e0131330. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, C.M.; Maly, D.J.; Ellman, J.A. Peptide Microarrays for the Determination of Protease Substrate Specificity. J. Am. Chem. Soc. 2002, 124, 14868–14870. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, S.S.; Seenivasan, R.; Condie, A.; Gerson, S.L.; Wang, Y.; Burda, C. Gold Nanoparticle-Based Fluorescent Theranostics for Real-Time Image-Guided Assessment of DNA Damage and Repair. Int. J. Mol. Sci. 2019, 20, 471. https://doi.org/10.3390/ijms20030471
Srinivasan SS, Seenivasan R, Condie A, Gerson SL, Wang Y, Burda C. Gold Nanoparticle-Based Fluorescent Theranostics for Real-Time Image-Guided Assessment of DNA Damage and Repair. International Journal of Molecular Sciences. 2019; 20(3):471. https://doi.org/10.3390/ijms20030471
Chicago/Turabian StyleSrinivasan, Shriya S., Rajesh Seenivasan, Allison Condie, Stanton L. Gerson, Yanming Wang, and Clemens Burda. 2019. "Gold Nanoparticle-Based Fluorescent Theranostics for Real-Time Image-Guided Assessment of DNA Damage and Repair" International Journal of Molecular Sciences 20, no. 3: 471. https://doi.org/10.3390/ijms20030471
APA StyleSrinivasan, S. S., Seenivasan, R., Condie, A., Gerson, S. L., Wang, Y., & Burda, C. (2019). Gold Nanoparticle-Based Fluorescent Theranostics for Real-Time Image-Guided Assessment of DNA Damage and Repair. International Journal of Molecular Sciences, 20(3), 471. https://doi.org/10.3390/ijms20030471






