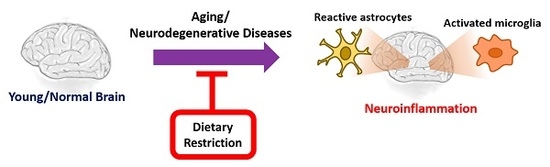
Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link
Abstract
1. Introduction
2. Neuroinflammation in the Aged Brain
2.1. Evidence of Increased Neuroinflammation with Age
2.2. Microglia in the Aged Brain
2.3. Astrocytes in the Aged Brain
3. The Effects of Dietary Restriction on Neuroinflammation
3.1. The Effects of Dietary Restriction on Neuroinflammation in Normal Aging
3.2. The Effects of Dietary Restriction in Age-Related Neurodegenerative Diseases
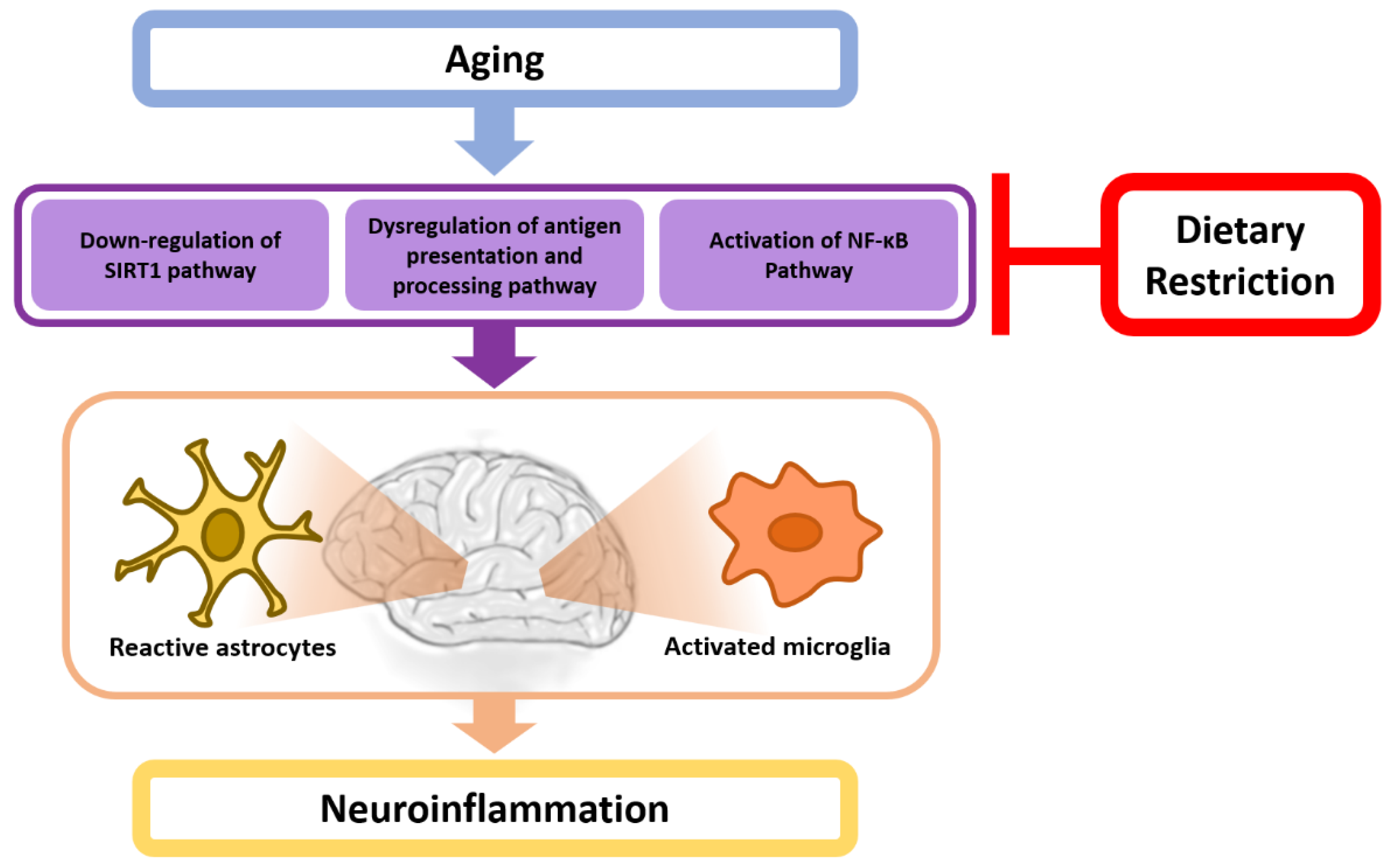
4. A Potential Mechanistic Link between Dietary Restriction and Neuroinflammation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DR | Dietary restriction |
| CALERIE | Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy |
| BBB | Blood–brain barrier |
| IL-1β | Interleukin-1 beta |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin-6 |
| GFAP | Glial fibrillary acidic protein |
| MHC II | Major histocompatibility complex II |
| TLRs | Toll-like receptors |
| Iba-1 | Ionized calcium-binding adapter molecule 1 |
| LPS | Lipopolysaccharide |
| TBI | Traumatic brain injury |
| CCL4 | C-C chemokine ligand 4 |
| DEG | Differentially expressed genes |
| ORM2 | Orosomucoid-2 |
| BDNF | Brain-derived neurotrophic factor |
| SIRT1 | Sirtuin 1 |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALS | Amyotrophic lateral sclerosis |
| NTFs | Neurofibrillary tangles |
| Aβ | Amyloid beta |
| MPTP | Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MS | Multiple sclerosis |
| HD | Huntington’s disease |
| AL | Ad libitum |
| EAE | Experimental allergic encephalomyelitis |
| NOX2 | NADPH oxidase 2 |
References
- Hadem, I.K.H.; Majaw, T.; Kharbuli, B.; Sharma, R. Beneficial effects of dietary restriction in aging brain. J. Chem. Neuroanat. 2019, 95, 123–133. [Google Scholar] [CrossRef]
- Prolla, T.A.; Mattson, M.P. Molecular mechanisms of brain aging and neurodegenerative disorders: Lessons from dietary restriction. Trends Neurosci. 2001, 24, S21–S31. [Google Scholar] [CrossRef]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 1989, 5, 155–171. [Google Scholar] [PubMed]
- Speakman, J.R.; Mitchell, S.E.; Mazidi, M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp. Gerontol. 2016, 86, 28–38. [Google Scholar] [CrossRef]
- Sridharan, A.; Pehar, M.; Salamat, M.S.; Pugh, T.D.; Bendlin, B.B.; Willette, A.A.; Anderson, R.M.; Kemnitz, J.W.; Colman, R.J.; Weindruch, R.H.; et al. Calorie restriction attenuates astrogliosis but not amyloid plaque load in aged rhesus macaques: A preliminary quantitative imaging study. Brain Res. 2013, 1508, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kastman, E.K.; Willette, A.A.; Coe, C.L.; Bendlin, B.B.; Kosmatka, K.J.; McLaren, D.G.; Xu, G.; Canu, E.; Field, A.S.; Alexander, A.L.; et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J. Neurosci. 2012, 32, 11897–11904. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A.; Bendlin, B.B.; Colman, R.J.; Kastman, E.K.; Field, A.S.; Alexander, A.L.; Sridharan, A.; Allison, D.B.; Anderson, R.; Voytko, M.L.; et al. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes 2012, 61, 1036–1042. [Google Scholar] [CrossRef]
- Willette, A.A.; Coe, C.L.; Birdsill, A.C.; Bendlin, B.B.; Colman, R.J.; Alexander, A.L.; Allison, D.B.; Weindruch, R.H.; Johnson, S.C. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age 2013, 35, 2215–2227. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef]
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun 2014, 5, 3557. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il'yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Pani, G. Brain response to calorie restriction. Cell. Mol. Life Sci. 2013, 70, 3157–3170. [Google Scholar] [CrossRef] [PubMed]
- Pani, G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin. Cell Dev. Biol. 2015, 40, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R.E. Caloric restriction: Beneficial effects on brain aging and Alzheimer’s disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.; Muller, L.; Wenger, E.; Duzel, S.; Pawelec, G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017, 75, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Okuma, Y.; Nomura, Y. The mechanisms of immune-to-brain communication in inflammation as a drug target. Curr. Drug Targets Inflamm. Allergy 2002, 1, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Mader, S.; Valdes-Ferrer, S.I. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef] [PubMed]
- Cribbs, D.H.; Berchtold, N.C.; Perreau, V.; Coleman, P.D.; Rogers, J.; Tenner, A.J.; Cotman, C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflamm. 2012, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Soreq, L.; Consortium, U.K.B.E.; North American Brain Expression, C.; Rose, J.; Soreq, E.; Hardy, J.; Trabzuni, D.; Cookson, M.R.; Smith, C.; Ryten, M.; et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017, 18, 557–570. [Google Scholar] [CrossRef]
- Abraham, J.; Johnson, R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef]
- Swanson, K.S.; Vester, B.M.; Apanavicius, C.J.; Kirby, N.A.; Schook, L.B. Implications of age and diet on canine cerebral cortex transcription. Neurobiol. Aging 2009, 30, 1314–1326. [Google Scholar] [CrossRef]
- Chen, J.; Buchanan, J.B.; Sparkman, N.L.; Godbout, J.P.; Freund, G.G.; Johnson, R.W. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav. Immun. 2008, 22, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Horrillo, D.; Sierra, J.; Arribas, C.; Garcia-San Frutos, M.; Carrascosa, J.M.; Lauzurica, N.; Fernandez-Agullo, T.; Ros, M. Age-associated development of inflammation in Wistar rats: Effects of caloric restriction. Arch. Physiol. Biochem. 2011, 117, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, O.; Castillo-Ruiz, M.M.; Acarin, L.; Castellano, B.; Gonzalez, B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J. Neurosci. Res. 2009, 87, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.M.; Johnson, R.W. Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 1999, 93, 139–148. [Google Scholar] [CrossRef]
- Maher, F.O.; Nolan, Y.; Lynch, M.A. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol. Aging 2005, 26, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.M.; Johnson, R.W. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 2001, 9, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Huang, Y.; Wynne, A.M.; Godbout, J.P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009, 23, 309–317. [Google Scholar] [CrossRef]
- Morgan, T.E.; Rozovsky, I.; Goldsmith, S.K.; Stone, D.J.; Yoshida, T.; Finch, C.E. Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: Implications for the role of oxidative stress. Free Radic. Biol. Med. 1997, 23, 524–528. [Google Scholar] [CrossRef]
- Morgan, T.E.; Wong, A.M.; Finch, C.E. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip. Top. Gerontol. 2007, 35, 83–97. [Google Scholar]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Morgan, T.E.; Xie, Z.; Goldsmith, S.; Yoshida, T.; Lanzrein, A.S.; Stone, D.; Rozovsky, I.; Perry, G.; Smith, M.A.; Finch, C.E. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 1999, 89, 687–699. [Google Scholar] [CrossRef]
- Sheng, J.G.; Mrak, R.E.; Griffin, W.S. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998, 95, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.R.; Finch, C.E.; Morgan, D.G. GFAP RNA increases during a wasting state in old mice. Exp. Neurol. 1990, 108, 266–268. [Google Scholar] [CrossRef]
- O’Callaghan, J.P.; Miller, D.B. The concentration of glial fibrillary acidic protein increases with age in the mouse and rat brain. Neurobiol. Aging 1991, 12, 171–174. [Google Scholar] [CrossRef]
- Kohama, S.G.; Goss, J.R.; Finch, C.E.; McNeill, T.H. Increases of glial fibrillary acidic protein in the aging female mouse brain. Neurobiol. Aging 1995, 16, 59–67. [Google Scholar] [CrossRef]
- Haley, G.E.; Kohama, S.G.; Urbanski, H.F.; Raber, J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age 2010, 32, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Nally, R.; Nolan, Y.; McCartney, Y.; Linden, J.; Lynch, M.A. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006, 99, 1263–1272. [Google Scholar] [CrossRef]
- Sloane, J.A.; Hollander, W.; Moss, M.B.; Rosene, D.L.; Abraham, C.R. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol. Aging 1999, 20, 395–405. [Google Scholar] [CrossRef]
- Hinman, J.D.; Duce, J.A.; Siman, R.A.; Hollander, W.; Abraham, C.R. Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J. Neurochem. 2004, 89, 430–441. [Google Scholar] [CrossRef]
- Perry, V.H.; Matyszak, M.K.; Fearn, S. Altered antigen expression of microglia in the aged rodent CNS. Glia 1993, 7, 60–67. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Holscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Fuger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.C.; Schlosser, C.; Obermuller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S.; et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Reu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.M.; Kitt, M.M.; Watkins, L.R.; Maier, S.F. Neuroinflammation in the normal aging hippocampus. Neuroscience 2015, 309, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J. Neuroimmunol. 1998, 90, 113–121. [Google Scholar] [CrossRef]
- Facci, L.; Barbierato, M.; Marinelli, C.; Argentini, C.; Skaper, S.D.; Giusti, P. Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1beta release. Sci. Rep. 2014, 4, 6824. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Xue, Q.S. The Brain’s Aging Immune System. Aging Dis. 2010, 1, 254–261. [Google Scholar] [PubMed]
- Hwang, I.K.; Lee, C.H.; Li, H.; Yoo, K.Y.; Choi, J.H.; Kim, D.W.; Kim, D.W.; Suh, H.W.; Won, M.H. Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochem. Res. 2008, 33, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, C.H.; Hwang, I.K.; Won, M.H.; Seong, J.K.; Yoon, Y.S.; Lee, H.S.; Lee, I.S. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J. Vet. Med. Sci. 2007, 69, 1131–1136. [Google Scholar] [CrossRef]
- Streit, W.J.; Sammons, N.W.; Kuhns, A.J.; Sparks, D.L. Dystrophic microglia in the aging human brain. Glia 2004, 45, 208–212. [Google Scholar] [CrossRef]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Hein, A.M.; Higgins, E.A.; Watkins, L.R.; Rudy, J.W.; Maier, S.F. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 2009, 23, 46–54. [Google Scholar] [CrossRef]
- Onyszchuk, G.; He, Y.Y.; Berman, N.E.; Brooks, W.M. Detrimental effects of aging on outcome from traumatic brain injury: A behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma 2008, 25, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stoica, B.A.; Sabirzhanov, B.; Burns, M.P.; Faden, A.I.; Loane, D.J. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 2013, 34, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Pelinka, L.E.; Kroepfl, A.; Leixnering, M.; Buchinger, W.; Raabe, A.; Redl, H. GFAP versus S100B in serum after traumatic brain injury: Relationship to brain damage and outcome. J. Neurotrauma 2004, 21, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Holtman, I.R.; Raj, D.D.; Miller, J.A.; Schaafsma, W.; Yin, Z.; Brouwer, N.; Wes, P.D.; Moller, T.; Orre, M.; Kamphuis, W.; et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: A co-expression meta-analysis. Acta Neuropathol. Commun. 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhauser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, H.J.; Vidyadhara, D.J.; Mahadevan, A.; Philip, M.; Parmar, S.K.; Manohari, S.G.; Shankar, S.K.; Raju, T.R.; Alladi, P.A. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol. Aging 2015, 36, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Jo, M.; Kim, J.H.; Song, G.J.; Seo, M.; Hwang, E.M.; Suk, K. Astrocytic Orosomucoid-2 Modulates Microglial Activation and Neuroinflammation. J. Neurosci. 2017, 37, 2878–2894. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Hirotsu, I.; Davis, P.J.; Carpenter, D.O. Long-term potentiation is lost in aged rats but preserved by calorie restriction. Neuroreport 1992, 3, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Weindruch, R.; Spangler, E.L.; Freeman, J.R.; Walford, R.L. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 1987, 42, 78–81. [Google Scholar] [CrossRef]
- Joseph, J.A.; Whitaker, J.; Roth, G.S.; Ingram, D.K. Life-long dietary restriction affects striatally-mediated behavioral responses in aged rats. Neurobiol. Aging 1983, 4, 191–196. [Google Scholar] [CrossRef]
- Pitsikas, N.; Algeri, S. Deterioration of spatial and nonspatial reference and working memory in aged rats: Protective effect of life-long calorie restriction. Neurobiol. Aging 1992, 13, 369–373. [Google Scholar] [CrossRef]
- Roth, G.S.; Ingram, D.K.; Joseph, J.A. Delayed loss of striatal dopamine receptors during aging of dietarily restricted rats. Brain Res. 1984, 300, 27–32. [Google Scholar] [CrossRef]
- Beatty, W.W.; Clouse, B.A.; Bierley, R.A. Effects of long-term restricted feeding on radial maze performance by aged rats. Neurobiol. Aging 1987, 8, 325–327. [Google Scholar] [CrossRef]
- Bond, N.W.; Everitt, A.V.; Walton, J. Effects of dietary restriction on radial-arm maze performance and flavor memory in aged rats. Neurobiol. Aging 1989, 10, 27–30. [Google Scholar] [CrossRef]
- May, P.C.; Telford, N.; Salo, D.; Anderson, C.; Kohama, S.G.; Finch, C.E.; Walford, R.L.; Weindruch, R. Failure of dietary restriction to retard age-related neurochemical changes in mice. Neurobiol. Aging 1992, 13, 787–791. [Google Scholar] [CrossRef]
- Shi, L.; Poe, B.H.; Constance Linville, M.; Sonntag, W.E.; Brunso-Bechtold, J.K. Caloric restricted male rats demonstrate fewer synapses in layer 2 of sensorimotor cortex. Brain Res. 2002, 931, 32–40. [Google Scholar] [CrossRef]
- Minor, R.K.; Villarreal, J.; McGraw, M.; Percival, S.S.; Ingram, D.K.; de Cabo, R. Calorie restriction alters physical performance but not cognition in two models of altered neuroendocrine signaling. Behav. Brain Res. 2008, 189, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Banati, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.R.; Finch, C.E.; Nelson, J.F. Food restriction delays the age-related increase in GFAP mRNA in rat hypothalamus. Neurobiol. Aging 1995, 16, 105–110. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Kaur, G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 2008, 9, 441–454. [Google Scholar] [CrossRef]
- Major, D.E.; Kesslak, J.P.; Cotman, C.W.; Finch, C.E.; Day, J.R. Life-long dietary restriction attenuates age-related increases in hippocampal glial fibrillary acidic protein mRNA. Neurobiol. Aging 1997, 18, 523–526. [Google Scholar] [CrossRef]
- Rozovsky, I.; Wei, M.; Morgan, T.E.; Finch, C.E. Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol. Aging 2005, 26, 705–715. [Google Scholar] [CrossRef]
- Radler, M.E.; Wright, B.J.; Walker, F.R.; Hale, M.W.; Kent, S. Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience 2015, 285, 236–247. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Kuwamura, M.; Yamaji, R.; Inui, H.; Nakano, Y. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J. Nutr. 2001, 131, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.C.; Walford, R.L.; Effros, R.B. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech. Ageing Dev. 1997, 93, 87–94. [Google Scholar] [CrossRef]
- Quintas, A.; de Solis, A.J.; Diez-Guerra, F.J.; Carrascosa, J.M.; Bogonez, E. Age-associated decrease of SIRT1 expression in rat hippocampus: Prevention by late onset caloric restriction. Exp. Gerontol. 2012, 47, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 2000, 15, 99–108. [Google Scholar] [CrossRef]
- Duan, W.; Lee, J.; Guo, Z.; Mattson, M.P. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J. Mol. Neurosci. 2001, 16, 1–12. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ju, W.K.; Neufeld, A.H. Neuronal susceptibility to damage: Comparison of the retinas of young, old and old/caloric restricted rats before and after transient ischemia. Neurobiol. Aging 2004, 25, 491–500. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, G. Dietary restriction enhances kainate-induced increase in NCAM while blocking the glial activation in adult rat brain. Neurochem. Res. 2008, 33, 1178–1188. [Google Scholar] [CrossRef]
- Wahl, D.; Solon-Biet, S.M.; Wang, Q.P.; Wali, J.A.; Pulpitel, T.; Clark, X.; Raubenheimer, D.; Senior, A.M.; Sinclair, D.A.; Cooney, G.J.; et al. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Rep. 2018, 25, 2234–2243. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, C.; Shen, Q.; Hu, Y. Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech. Ageing Dev. 2009, 130, 602–610. [Google Scholar] [CrossRef]
- Wood, S.H.; van Dam, S.; Craig, T.; Tacutu, R.; O'Toole, A.; Merry, B.J.; de Magalhaes, J.P. Transcriptome analysis in calorie-restricted rats implicates epigenetic and post-translational mechanisms in neuroprotection and aging. Genome Biol. 2015, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Dolgalev, I.; Alldred, M.J.; Heguy, A.; Ginsberg, S.D. Calorie Restriction Suppresses Age-Dependent Hippocampal Transcriptional Signatures. PLoS ONE 2015, 10, e0133923. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Patrick, E.; Villani, A.C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Mangold, C.A.; Masser, D.R.; Stanford, D.R.; Bixler, G.V.; Pisupati, A.; Giles, C.B.; Wren, J.D.; Ford, M.M.; Sonntag, W.E.; Freeman, W.M. CNS-wide Sexually Dimorphic Induction of the Major Histocompatibility Complex 1 Pathway With Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 16–29. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F. Role of Neuroinflammation in Amyotrophic Lateral Sclerosis: Cellular Mechanisms and Therapeutic Implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- Patel, N.V.; Gordon, M.N.; Connor, K.E.; Good, R.A.; Engelman, R.W.; Mason, J.; Morgan, D.G.; Morgan, T.E.; Finch, C.E. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging 2005, 26, 995–1000. [Google Scholar] [CrossRef]
- Mucke, L.; Masliah, E.; Yu, G.Q.; Mallory, M.; Rockenstein, E.M.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Johnson-Wood, K.; McConlogue, L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J. Neurosci. 2000, 20, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.; Gordon, M.N.; McGowan, E.; Yu, X.; Benkovic, S.; Jantzen, P.; Wright, K.; Saad, I.; Mueller, R.; Morgan, D.; et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998, 4, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Alldred, M.J.; Lee, S.H.; Calhoun, M.E.; Petkova, E.; Mathews, P.M.; Ginsberg, S.D. Reduction of beta-amyloid and gamma-secretase by calorie restriction in female Tg2576 mice. Neurobiol. Aging 2015, 36, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Qin, W.; Rocher, A.B.; Seror, I.; Humala, N.; Maniar, K.; Dolios, G.; Wang, R.; Hof, P.R.; et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005, 19, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Mouton, P.R.; Chachich, M.E.; Quigley, C.; Spangler, E.; Ingram, D.K. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci. Lett. 2009, 464, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shen, Q.; Dong, S.; Xu, Z.; Tsien, J.Z.; Hu, Y. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol. Aging 2008, 29, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Allison, D.B.; van Groen, T.; Kadish, I. Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer’s disease pathology in a mouse model. PLoS ONE 2013, 8, e60437. [Google Scholar] [CrossRef]
- Qin, W.; Chachich, M.; Lane, M.; Roth, G.; Bryant, M.; de Cabo, R.; Ottinger, M.A.; Mattison, J.; Ingram, D.; Gandy, S.; et al. Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J. Alzheimers Dis. 2006, 10, 417–422. [Google Scholar] [CrossRef]
- Halagappa, V.K.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; Laferla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef]
- Brownlow, M.L.; Joly-Amado, A.; Azam, S.; Elza, M.; Selenica, M.L.; Pappas, C.; Small, B.; Engelman, R.; Gordon, M.N.; Morgan, D. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 2014, 271, 79–88. [Google Scholar] [CrossRef]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999, 57, 195–206. [Google Scholar] [CrossRef]
- Armentero, M.T.; Levandis, G.; Bramanti, P.; Nappi, G.; Blandini, F. Dietary restriction does not prevent nigrostriatal degeneration in the 6-hydroxydopamine model of Parkinson’s disease. Exp. Neurol. 2008, 212, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Maswood, N.; Young, J.; Tilmont, E.; Zhang, Z.; Gash, D.M.; Gerhardt, G.A.; Grondin, R.; Roth, G.S.; Mattison, J.; Lane, M.A.; et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 18171–18176. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Lemus, M.B.; Stark, R.; Santos, V.V.; Thompson, A.; Rees, D.J.; Galic, S.; Elsworth, J.D.; Kemp, B.E.; Davies, J.S.; et al. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. 2016, 36, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Li, X.J.; Mattson, M.P. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA 2003, 100, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, M.J.; Rodriguez, M.C.; Kaczor, J.J.; Tarnopolsky, M.A. Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve 2005, 31, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Safdar, A.; Raha, S.; Tarnopolsky, M.A.; Hamadeh, M.J. Caloric restriction shortens lifespan through an increase in lipid peroxidation, inflammation and apoptosis in the G93A mouse, an animal model of ALS. PLoS ONE 2010, 5, e9386. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, W.A.; Mattson, M.P. No benefit of dietary restriction on disease onset or progression in amyotrophic lateral sclerosis Cu/Zn-superoxide dismutase mutant mice. Brain Res. 1999, 833, 117–120. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bokov, A.; Muller, F.L.; Jernigan, A.L.; Maslin, K.; Diaz, V.; Richardson, A.; Van Remmen, H. Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS. Neurobiol. Aging 2012, 33, 1829–1832. [Google Scholar] [CrossRef]
- Correale, J.; Gaitan, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain A J. Neurol. 2017, 140, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Esquifino, A.I.; Cano, P.; Jimenez, V.; Cutrera, R.A.; Cardinali, D.P. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J. Physiol. Biochem. 2004, 60, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Esquifino, A.I.; Cano, P.; Jimenez-Ortega, V.; Fernandez-Mateos, M.P.; Cardinali, D.P. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J. Neuroinflamm. 2007, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.L.; Goodwin, J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003, 19, 161–162. [Google Scholar] [CrossRef]
- Lee, C.K.; Weindruch, R.; Prolla, T.A. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000, 25, 294–297. [Google Scholar] [CrossRef]
- Mulrooney, T.J.; Marsh, J.; Urits, I.; Seyfried, T.N.; Mukherjee, P. Influence of caloric restriction on constitutive expression of NF-kappaB in an experimental mouse astrocytoma. PLoS ONE 2011, 6, e18085. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, H.J.; Kim, K.W.; Choi, J.S.; Yu, B.P. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc. Res. Tech. 2002, 59, 264–272. [Google Scholar] [CrossRef]
- Mhatre, M.; Floyd, R.A.; Hensley, K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: Common links and potential therapeutic targets. J. Alzheimers Dis. 2004, 6, 147–157. [Google Scholar] [CrossRef]
- Santin, K.; da Rocha, R.F.; Cechetti, F.; Quincozes-Santos, A.; de Souza, D.F.; Nardin, P.; Rodrigues, L.; Leite, M.C.; Moreira, J.C.; Salbego, C.G.; et al. Moderate exercise training and chronic caloric restriction modulate redox status in rat hippocampus. Brain Res. 2011, 1421, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.H.; Emerson, S.S.; Jo, D.G.; Mattson, M.P.; de Cabo, R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc. Natl. Acad. Sci. USA 2006, 103, 19908–19912. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Forster, M.J.; Lal, H.; Sohal, R.S. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996, 333, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Matas, S.; Paul, R.K.; Molina-Martinez, P.; Palacios, H.; Gutierrez, V.M.; Corpas, R.; Pallas, M.; Cristofol, R.; de Cabo, R.; Sanfeliu, C. In vitro caloric restriction induces protective genes and functional rejuvenation in senescent SAMP8 astrocytes. Aging Cell 2015, 14, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014, 66, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Rasouri, S.; Lagouge, M.; Auwerx, J. [SIRT1/PGC-1: A neuroprotective axis?]. Med. Sci. (Paris) 2007, 23, 840–844. [Google Scholar] [CrossRef]
- Lafontaine-Lacasse, M.; Richard, D.; Picard, F. Effects of age and gender on Sirt 1 mRNA expressions in the hypothalamus of the mouse. Neurosci. Lett. 2010, 480, 1–3. [Google Scholar] [CrossRef]
- Cunha-Santos, J.; Duarte-Neves, J.; Carmona, V.; Guarente, L.; Pereira de Almeida, L.; Cavadas, C. Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway. Nat. Commun. 2016, 7, 11445. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Kahn, M.; Samiei, A.; Gao, J.; Ota, K.T.; Rei, D.; Tsai, L.H. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J. Neurosci. 2013, 33, 8951–8960. [Google Scholar] [CrossRef] [PubMed]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J.; et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.E.; Supinski, A.M.; Bonkowski, M.S.; Donmez, G.; Guarente, L.P. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009, 23, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.R.; Cabral-Costa, J.V.; Mazucanti, C.H.; Scavone, C.; Kawamoto, E.M. The Role of Steroid Hormones in the Modulation of Neuroinflammation by Dietary Interventions. Front. Endocrinol. (Lausanne) 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | GO Term | Expression Level Old vs. Young | Age/Brain Region | Species | Reference |
|---|---|---|---|---|---|
| Axl | GO:0002376, GO:0045087, GO:0006954 | Up | 24 months/whole brain | Mus musculus | [74] |
| Camp | GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ccl3 | GO:0006954, GO:0050729, GO:0006955 | Up | 24 months/whole brain | Mus musculus | [74] |
| Cd274 | GO:0006955 | Up | 24 months/whole brain | Mus musculus | [74] |
| Cd36 | GO:0006955 | Up | 24 months/whole brain | Mus musculus | [74] |
| Cd74 | GO:0002376, GO:0006955, GO:0019886, GO:0042613 | Up | 24 months/whole brain | Mus musculus | [74] |
| Chst1 | GO:0006954 | Up | 24 months/whole brain | Mus musculus | [74] |
| Clec7a | GO:0045087, GO:0006954 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ctse | GO:0019886 | Up | 24 months/whole brain | Mus musculus | [74] |
| Cxcl13 | GO:0006954, GO:0006955 | Up | 24 months/whole brain | Mus musculus | [74] |
| Cybb | GO:0045087, GO:0006954 | Up | 24 months/whole brain | Mus musculus | [74] |
| H2-aa | GO:0002376, GO:0006955, GO:0019886, GO:0042613 | Up | 24 months/whole brain | Mus musculus | [74] |
| H2-ab1 | GO:0002376, GO:0006955, GO:0019886, GO:0042613 | Up | 24 months/whole brain | Mus musculus | [74] |
| H2-eb1 | GO:0002376, GO:0006955, GO:0019886, GO:0042613 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ifit3 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ifitm2 | GO:0002376 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ifitm3 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Lcn2 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Lgals3 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ltf | GO:0002376 | Up | 24 months/whole brain | Mus musculus | [74] |
| Ly9 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| Oasl2 | GO:0002376, GO:0045087, GO:0006955 | Up | 24 months/whole brain | Mus musculus | [74] |
| Rsad2 | GO:0002376, GO:0045087 | Up | 24 months/whole brain | Mus musculus | [74] |
| S100a8 | GO:0002376, GO:0045087, GO:0006954, GO:0050729 | Up | 24 months/whole brain | Mus musculus | [74] |
| S100a9 | GO:0002376, GO:0045087, GO:0006954, GO:0050729 | Up | 24 months/whole brain | Mus musculus | [74] |
| Spp1 | GO:0006954 | Up | 24 months/whole brain | Mus musculus | [74] |
| Gene Name | GO Term | Expression Level Old vs. Young | Age/Brain Region | Species | Reference |
|---|---|---|---|---|---|
| Akap8 | GO:0002376, GO:0045087 | Up | 24 months/Striatum | Mus musculus | [82] |
| App | GO:0045087 | Up | 24 months/Striatum | Mus musculus | [82] |
| B2m | GO:0006955, GO:0002376, GO:0045087 | Up | 24 months/ Hippocampus, Striatum | Mus musculus | [82] |
| Bcl6 | GO:0002376, GO:0006954 | Up | 24 months/ Cortex, Striatum | Mus musculus | [82] |
| Bmp6 | GO:0006954 | Up | 24 months/ Visual cortex, Striatum | Mus musculus | [82,83] |
| Bst2 | GO:0045087, GO:0002376 | Up | 24 months/Motor cortex | Mus musculus | [83] |
| C3 | GO:0045087, GO:0002376, GO:0006954 | Up | 24 months/Motor cortex, Visual cortex | Mus musculus | [83] |
| C4b | GO:0045087, GO:0006954 | Up | 24 months/Motor cortex, Visual cortex, Striatum | Mus musculus | [82,83] |
| Csf1 | GO:0002376, GO:0045087, GO:0006954 | Up | 24 months/Striatum | Mus musculus | [82] |
| Ctss | GO:0019882, GO:0006955 | Up | 24 months/ Hippocampus, Striatum | Mus musculus | [82] |
| Cxcl10 | GO:0006955, GO:0006954 | Up | 24 months/ Hippocampus, Striatum | Mus musculus | [82] |
| Cxcl12 | GO:0006955 | Down | 24 months/Hippocampus | Mus musculus | [82] |
| Cxcl5 | GO:0006954 | Up | 24 months/Visual cortex | Mus musculus | [83] |
| Defb1 | GO:0045087 | Up | 24 months/Motor cortex, Visual cortex | Mus musculus | [83] |
| Enpp2 | GO:0006955 | Down | 24 months/Hippocampus | Mus musculus | [82] |
| Erap1 | GO:0002376 | Up | 24 months/Striatum | Mus musculus | [82] |
| H2-d1 | GO:0019882, GO:0006955, GO:0002376 | Up | 24 months/Cortex, Hippocampus, Striatum | Mus musculus | [82] |
| H2-k1 | GO:0019882, GO:0006955, GO:0002376 | Up | 24 months/ Hippocampus, Striatum | Mus musculus | [82] |
| Hfe | GO:0019882 | Up | 24 months/Striatum | Mus musculus | [82] |
| Icosl | GO:0002376 | Up | 24 months/Striatum | Mus musculus | [82] |
| Ifit1 | GO:0045087, GO:0002376 | Up | 24 months/Visual cortex, Striatum | Mus musculus | [82,83] |
| Ifit3 | GO:0002376, GO:0045087 | Up | 24 months/Cortex, Striatum | Mus musculus | [82] |
| Ifitm3 | GO:0002376, GO:0045087 | Up | 24 months/Cortex, Striatum | Mus musculus | [82] |
| Ly86 | GO:0002376, GO:0045087, GO:0006954 | Up | 24 months/Striatum | Mus musculus | [82] |
| Nlrp6 | GO:0045087, GO:0002376, GO:0006954 | Up | 24 months/Visual cortex | Mus musculus | [83] |
| Oasl2 | GO:0045087, GO:0002376, GO:0006955 | Up | 24 months/Motor cortex, Visual cortex, Hippocampus, Striatum | Mus musculus | [82,83] |
| Psmb8 | GO:0002376, GO:0019882 | Up | 24 months/Visual cortex, Hippocampus, Striatum | Mus musculus | [82,83] |
| Psmb9 | GO:0002376, GO:0019882 | Up | 24 months/ Hippocampus, Striatum | Mus musculus | [82] |
| Rsad2 | GO:0045087, GO:0002376 | Up | 24 months/Visual cortex | Mus musculus | [83] |
| Serinc3 | GO:0002376, GO:0045087 | Up | 24 months/Striatum | Mus musculus | [82] |
| Serping1 | GO:0002376, GO:0045087 | Up | 24 months/Striatum | Mus musculus | [82] |
| Tspan2 | GO:0006954 | Up | 24 months/Striatum | Mus musculus | [82] |
| Tyrobp | GO:0045087 | Up | 24 months/Striatum | Mus musculus | [82] |
| Zc3hav1 | GO:0045087, GO:0002376 | Up | 24 months/Visual cortex, Striatum | Mus musculus | [82,83] |
| Gene Name | GO Term | Expression Level DR vs. AL | Age/Brain Region | Species | Reference |
|---|---|---|---|---|---|
| Prlr | GO:0034097 | Down | 15 months/Hippocampus | Mus musculus | [109] |
| Il2ra | GO:0034097 | Up | 15 months/Hippocampus | Mus musculus | [109] |
| Sigirr | GO:0034097 | Down | 15 months/Hippocampus | Mus musculus | [109] |
| Ptk2b | GO:0002376 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| Bcl6 | GO:0006954, GO:0050727, GO:0002376 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| Ccr1 | GO:0006954 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| Il1r1 | GO:0050727 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| Tnfrsf25 | GO:0006954 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| Gal | GO:0006954 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| H2-q10 | GO:0002376 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| S100a8 | GO:0006954, GO:0050727, GO:0002376 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| S100a9 | GO:0006954, GO:0050727, GO:0002376 | Up | 15 months/Hippocampus (CA1) | Mus musculus | [112] |
| C1qbp | GO:0006955 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Rt1-db1 | GO:0006955, GO:0019886, GO:0042613, GO:0002504 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Rt1-ba | GO:0006955, GO:0019886, GO:0042613, GO:0002504, GO:0019882 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Cxcl12 | GO:0006955 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Rt1-da | GO:0006955, GO:0042613, GO:0002504, GO:0019882 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Cd74 | GO:0006955, GO:0019886, GO:0042613, GO:0019882 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Rt1-bb | GO:0006955, GO:0019886, GO:0042613, GO:0002504, GO:0019882 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Fcer1g | GO:0019886 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Rab3b | GO:0019882 | Up | 28 months/Cerebral cortex | Rattus norvegicus | [111] |
| Tnfaip6 | GO:0006954 | Down | 19 months/Hypothalamus | Mus musculus | [110] |
| C1qg | GO:0002376 | Up | 19 months/Hypothalamus | Mus musculus | [110] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, E.; Jo, M.; Lee, S.; Lee, B.-R.; Kim, J.; Kim, H.-J. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. Int. J. Mol. Sci. 2019, 20, 464. https://doi.org/10.3390/ijms20030464
Bok E, Jo M, Lee S, Lee B-R, Kim J, Kim H-J. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. International Journal of Molecular Sciences. 2019; 20(3):464. https://doi.org/10.3390/ijms20030464
Chicago/Turabian StyleBok, Eugene, Myungjin Jo, Shinrye Lee, Bo-Ram Lee, Jaekwang Kim, and Hyung-Jun Kim. 2019. "Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link" International Journal of Molecular Sciences 20, no. 3: 464. https://doi.org/10.3390/ijms20030464
APA StyleBok, E., Jo, M., Lee, S., Lee, B.-R., Kim, J., & Kim, H.-J. (2019). Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. International Journal of Molecular Sciences, 20(3), 464. https://doi.org/10.3390/ijms20030464