MiRNAs as Players in Rhabdomyosarcoma Development
Abstract
1. Overview RMS (Rhabdomyosarcoma)
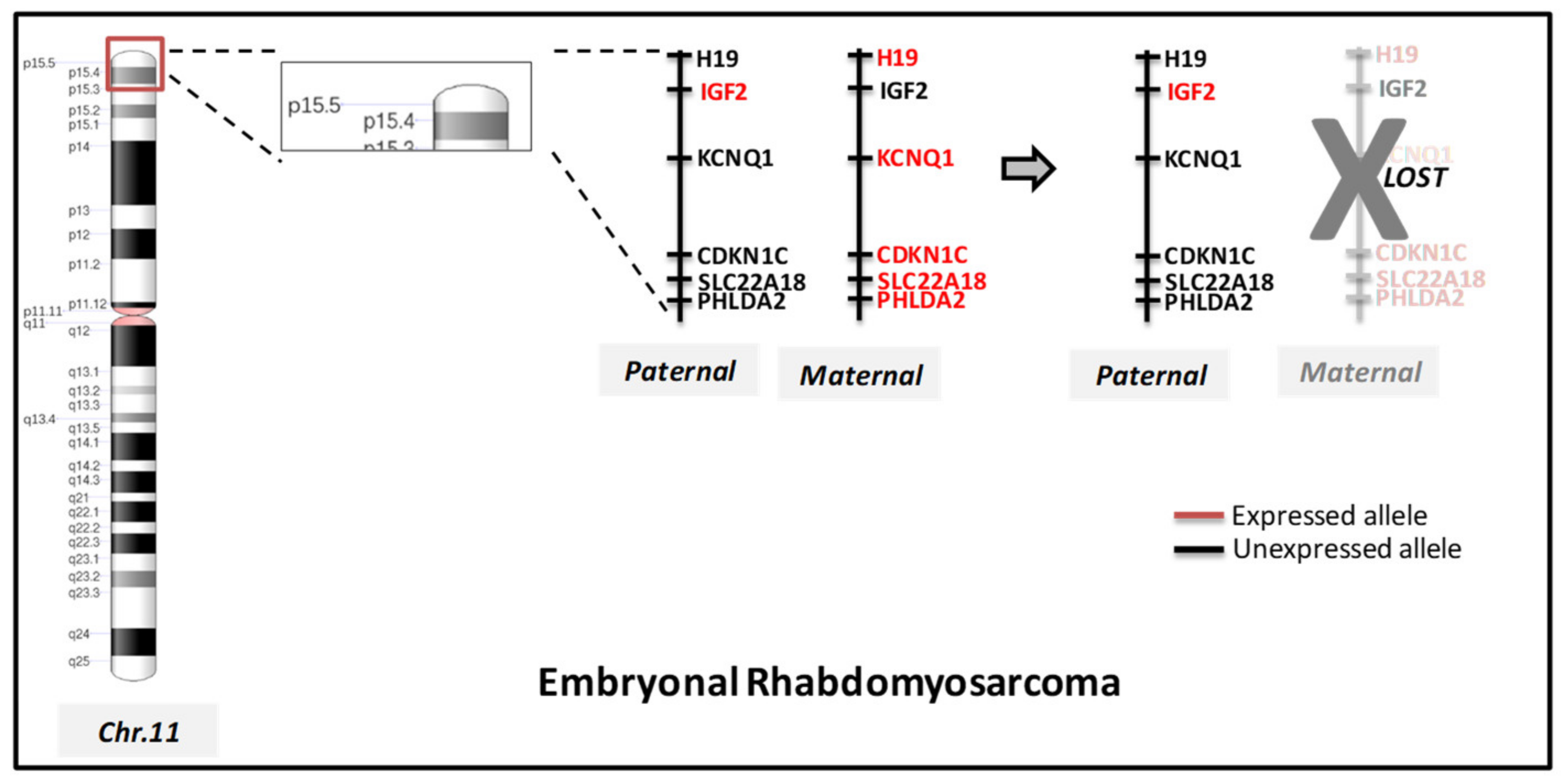
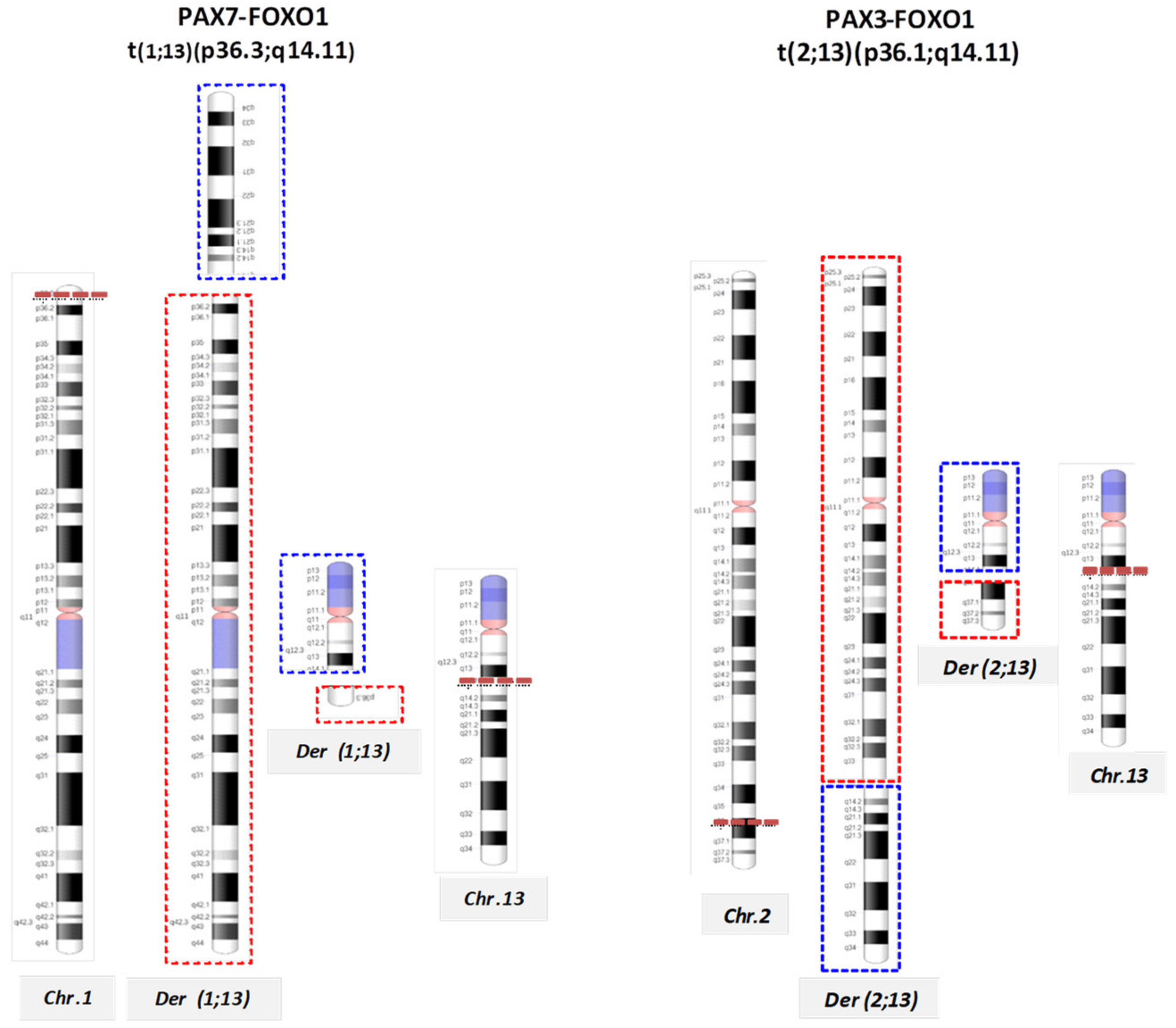
Molecular Cytogenetic Characterization of RMS
2. miRNAs (MicroRNAs) Involvement in Myogenesis
2.1. Myo-miRNAs
2.2. Non-myomiRNAs
3. miRNAs as Biomarker for RMS Development
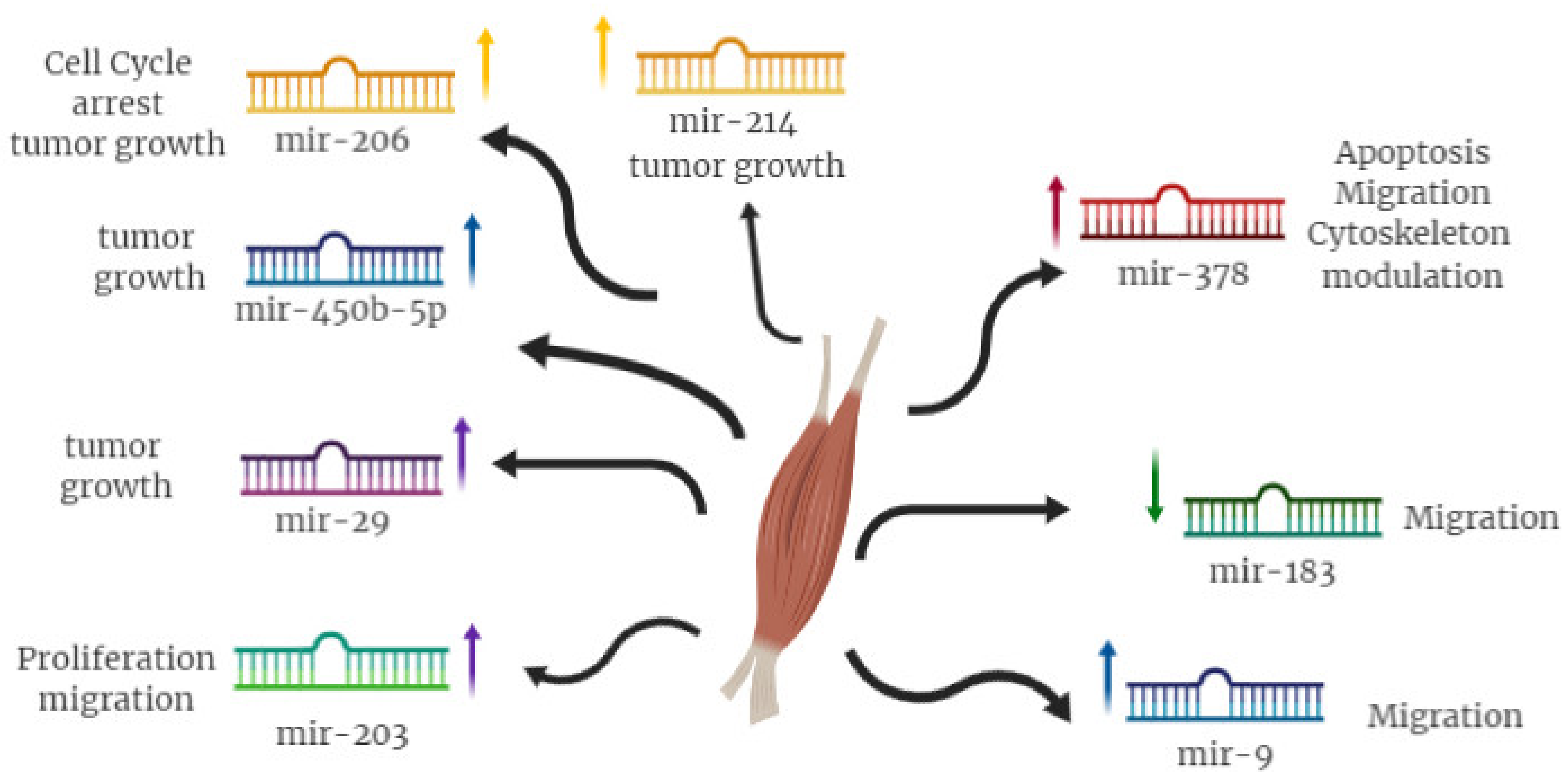
4. miRNA as Functional Players in RMS
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hawkins, D.S.; Spunt, S.L.; Skapek, S.X. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr. Blood Cancer 2013, 60, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, W.; Shen, J.K.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. Rhabdomyosarcoma: Advances in Molecular and Cellular Biology. Sarcoma 2015, 2015, 232010. [Google Scholar] [CrossRef] [PubMed]
- Megiorni, F.; Cialfi, S.; McDowell, H.P.; Felsani, A.; Camero, S.; Guffanti, A.; Pizer, B.; Clerico, A.; De Grazia, A.; Pizzuti, A.; et al. Deep Sequencing the microRNA profile in rhabdomyosarcoma reveals down-regulation of miR-378 family members. BMC Cancer 2014, 14, 880. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Leithner, A.; Posch, F.; Szkandera, J.; Liegl-Atzwanger, B.; Pichler, M. MicroRNAs in Different Histologies of Soft Tissue Sarcoma: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1960. [Google Scholar] [CrossRef]
- Gasparini, P.; Casanova, M.; Villa, R.; Collini, P.; Alaggio, R.; Zin, A.; Bonvini, P.; Antonescu, C.R.; Boldrini, R.; Caserini, R.; et al. Anaplastic lymphoma kinase aberrations correlate with metastatic features in pediatric rhabdomyosarcoma. Oncotarget 2016, 7, 58903–58914. [Google Scholar] [CrossRef][Green Version]
- Ferrari, A.; Miceli, R.; Meazza, C.; Zaffignani, E.; Gronchi, A.; Piva, L.; Collini, P.; Podda, M.G.; Massimino, M.; Luksch, R.; et al. Soft tissue sarcomas of childhood and adolescence: The prognostic role of tumor size in relation to patient body size. J. Clin. Oncol. 2009, 27, 371–376. [Google Scholar] [CrossRef]
- Ferrari, A.; Bleyer, A.; Patel, S.; Chiaravalli, S.; Gasparini, P.; Casanova, M. The challenge of the management of adolescents and young adults with soft tissue sarcomas. Pediatr. Blood Cancer 2018, 65, e27013. [Google Scholar] [CrossRef]
- Barr, R.D.; Ferrari, A.; Ries, L.; Whelan, J.; Bleyer, W.A. Cancer in Adolescents and Young Adults: A Narrative Review of the Current Status and a View of the Future. JAMA Pediatr. 2016, 170, 495–501. [Google Scholar] [CrossRef]
- Yang, L.; Takimoto, T.; Fujimoto, J. Prognostic model for predicting overall survival in children and adolescents with rhabdomyosarcoma. BMC Cancer 2014, 14, 654. [Google Scholar] [CrossRef]
- Ferrari, A.; Gasparini, P.; Gill, J.; Gorlick, R. Challenges of Clinical Management of Adolescent and Young Adults with Bone and Soft Tissue Sarcoma. Cancer J. 2018, 24, 301–306. [Google Scholar] [CrossRef]
- Smith, A.C.; Squire, J.A.; Thorner, P.; Zielenska, M.; Shuman, C.; Grant, R.; Chitayat, D.; Nishikawa, J.L.; Weksberg, R. Association of alveolar rhabdomyosarcoma with the Beckwith-Wiedemann syndrome. Pediatr. Dev. Pathol. 2001, 4, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Grufferman, S.; Khoury, M.J.; Schwartz, A.G.; Kowalski, J.; Ruymann, F.B.; Maurer, H.M. Association of childhood rhabdomyosarcoma with neurofibromatosis type I and birth defects. Genet. Epidemiol. 1995, 12, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Trahair, T.; Andrews, L.; Cohn, R.J. Recognition of Li Fraumeni syndrome at diagnosis of a locally advanced extremity rhabdomyosarcoma. Pediatr. Blood Cancer 2007, 48, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Davicioni, E.; Anderson, M.J.; Finckenstein, F.G.; Lynch, J.C.; Qualman, S.J.; Shimada, H.; Schofield, D.E.; Buckley, J.D.; Meyer, W.H.; Sorensen, P.H.; et al. Molecular classification of rhabdomyosarcoma--genotypic and phenotypic determinants of diagnosis: A report from the Children’s Oncology Group. Am. J. Pathol. 2009, 174, 550–564. [Google Scholar] [CrossRef]
- Visser, M.; Sijmons, C.; Bras, J.; Arceci, R.J.; Godfried, M.; Valentijn, L.J.; A Voûte, P.; Baas, F. Allelotype of pediatric rhabdomyosarcoma. Oncogene 1997, 15, 1309–1314. [Google Scholar] [CrossRef][Green Version]
- Callaghan, R.C.; Caballero, J.; Donat, J.; Navarro, S.; Peris, T.; Gil-Benso, R.; San-Miguel, T.; Bataller-Calatayud, A.; Carcelen, A.P.; Cerdá-Nicolás, M.; et al. Chromosomal and genetic changes produced in tumoral progression of embryonal rhabdomyosarcoma. Histopathology 2013, 62, 816–819. [Google Scholar]
- Hosoi, H.; Kakazu, N.; Konishi, E.; Tsuchihashi, Y.; Hada, S.; Amaya, E.; Nakabayahi, Y.; Misawa-Furihata, A.; Tabata-Maruyama, H.; Iehara, T.; et al. A novel PAX3 rearrangement in embryonal rhabdomyosarcoma. Cancer Genet. Cytogenet. 2009, 189, 98–104. [Google Scholar] [CrossRef]
- Sorensen, P.H.; Lynch, J.C.; Qualman, S.J.; Tirabosco, R.; Lim, J.F.; Maurer, H.M.; Bridge, J.A.; Crist, W.M.; Triche, T.J.; Barr, F.G. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the children’s oncology group. J. Clin. Oncol. 2002, 20, 2672–2679. [Google Scholar] [CrossRef]
- Missiaglia, E.; Williamson, D.; Chisholm, J.; Wirapati, P.; Pierron, G.; Petel, F.; Concordet, J.-P.; Thway, K.; Oberlin, O.; Pritchard-Jones, K.; et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J. Clin. Oncol. 2012, 30, 1670–1677. [Google Scholar] [CrossRef]
- Wachtel, M.; Dettling, M.; Koscielniak, E.; Stegmaier, S.; Treuner, J.; Simon-Klingenstein, K.; Bühlmann, P.; Niggli, F.K.; Schäfer, B.W. Gene expression signatures identify rhabdomyosarcoma subtypes and detect a novel t(2;2)(q35;p23) translocation fusing PAX3 to NCOA1. Cancer Res. 2004, 64, 5539–5545. [Google Scholar] [CrossRef]
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014, 4, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, D.; Jiang, J.; Hu, J.; Zhang, W.; Chen, Y.; Cui, X.; Qi, Y.; Zou, H.; Zhang, W.; et al. Analysis of molecular cytogenetic alteration in rhabdomyosarcoma by array comparative genomic hybridization. PLoS ONE 2014, 9, e94924. [Google Scholar] [CrossRef] [PubMed]
- Paulson, V.; Chandler, G.; Rakheja, D.; Galindo, R.L.; Wilson, K.; Amatruda, J.F.; Cameron, S. High-resolution array CGH identifies common mechanisms that drive embryonal rhabdomyosarcoma pathogenesis. Genes Chromosomes Cancer 2011, 50, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Selfe, J.; Hamdi, M.; Williamson, D.; Schaaf, G.; Fang, C.; Koster, J.; Summersgill, B.; Messahel, B.; Versteeg, R.; et al. Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: An approach to identify candidate genes involved in tumor development. Genes Chromosomes Cancer 2009, 48, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.G.; Duan, F.; Smith, L.M.; Gustafson, D.; Pitts, M.; Hammond, S.; Gastier-Foster, J.M. Genomic and clinical analyses of 2p24 and 12q13-q14 amplification in alveolar rhabdomyosarcoma: A report from the Children’s Oncology Group. Genes Chromosomes Cancer 2009, 48, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chatterjee, B.; Wang, Y.; Stevenson, H.S.; Edelman, D.C.; Meltzer, P.S.; Barr, F.G. Distinct methylation profiles characterize fusion-positive and fusion-negative rhabdomyosarcoma. Mod. Pathol. 2015, 28, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Missiaglia, E.; De Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; Thway, K.; Orbach, D.; Laé, M.; Fréneaux, P.; et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158. [Google Scholar] [CrossRef]
- Chen, X.; Stewart, E.; Shelat, A.A.; Qu, C.; Bahrami, A.; Hatley, M.; Wu, G.; Bradley, C.; Mc Evoy, J.; Pappo, A.; et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell 2013, 24, 710–724. [Google Scholar] [CrossRef]
- Nakano, K.; Takahashi, S. Translocation-Related Sarcomas. Int. J. Mol. Sci. 2018, 19, 3784. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. Chromosomal rearrangements and microRNAs: A new cancer link with clinical implications. J. Clin. Investig. 2007, 117, 2059–2066. [Google Scholar] [CrossRef]
- Dela, C.F.; Matushansky, I. MicroRNAs in chromosomal translocation-associated solid tumors: Learning from sarcomas. Discov. Med. 2011, 12, 307–317. [Google Scholar]
- Buckingham, M. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 440–448. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhou, L.; Chen, E.Z.; Sun, K.; Jiang, P.; Wang, L.; Su, X.; Sun, H.; Wang, H. A Novel YY1-miR-1 regulatory circuit in skeletal myogenesis revealed by genome-wide prediction of YY1-miRNA network. PLoS ONE 2012, 7, e27596. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206, The skeletal muscle-specific myomiR. Biochim. Biophys. Acta 2008, 1779, 682–691. [Google Scholar] [CrossRef]
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar] [CrossRef]
- Sweetman, D.; Goljanek, K.; Rathjen, T.; Oustanina, S.; Braun, T.; Dalmay, T.; Münsterberg, A. Specific requirements of MRFs for the expression of muscle specific microRNAs, miR-1, miR-206 and miR-133. Dev. Biol. 2008, 321, 491–499. [Google Scholar] [CrossRef]
- O’Rourke, J.R.; Georges, S.A.; Seay, H.R.; Tapscott, S.J.; McManus, M.T.; Goldhamer, D.J.; Swanson, M.S.; Harfe, B.D. Essential role for Dicer during skeletal muscle development. Dev. Biol. 2007, 311, 359–368. [Google Scholar] [CrossRef]
- Rao, P.K.; Missiaglia, E.; Shields, L.; Hyde, G.; Yuan, B.; Shepherd, C.J.; Shipley, J.; Lodish, H.F. Distinct roles for miR-1 and miR-133a in the proliferation and differentiation of rhabdomyosarcoma cells. FASEB J. 2010, 24, 3427–3437. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.-Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.I.; Georges, S.A.; Asawachaicharn, A.; Analau, E.; Tapscott, S.J. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J. Cell Biol. 2006, 175, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Crist, C.G.; Montarras, D.; Pallafacchina, G.; Rocancourt, D.; Cumano, A.; Conway, S.J.; Buckingham, M. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc. Natl. Acad. Sci. USA 2009, 106, 13383–13387. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.F.; Tellam, R.L. MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J. Biol. Chem. 2008, 283, 9836–9843. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cao, J.H.; Li, X.Y.; Zhao, S.H. Inhibition of miR-214 expression represses proliferation and differentiation of C2C12 myoblasts. Cell Biochem. Funct. 2011, 29, 378–383. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.-J.; Xiong, A.-W.; Zhang, Z.-D.; Yue, S.; Zhu, M.-S.; Cheng, S.Y. MicroRNA-214 promotes myogenic differentiation by facilitating exit from mitosis via down-regulation of proto-oncogene N-ras. J. Biol. Chem. 2010, 285, 26599–26607. [Google Scholar] [CrossRef]
- Naguibneva, I.; Ameyar-Zazoua, M.; Polesskaya, A.; Ait-Si-Ali, S.; Groisman, R.; Souidi, M.; Cuvellier, S.; Harel-Bellan, A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 2006, 8, 278–284. [Google Scholar] [CrossRef]
- Crippa, S.; Cassano, M.; Messina, G.; Galli, D.; Galvez, B.G.; Curk, T.; Altomare, C.; Ronzoni, F.; Toelen, J.; Gijsbers, R.; et al. miR669a and miR669q prevent skeletal muscle differentiation in postnatal cardiac progenitors. J. Cell Biol. 2011, 193, 1197–1212. [Google Scholar] [CrossRef]
- Naguibneva, I.; Polesskaya, A.; meyar-Zazoua, M.; Souidi, M.; Groisman, R.; Cuvellier, S.; Ait-Si-Ali, S.; Pritchard, L.L.; Harel-Bellan, A. Micro-RNAs and muscle differentiation. J. Soc. Biol. 2007, 201, 367–376. [Google Scholar] [CrossRef]
- Bersani, F.; Lingua, M.F.; Morena, D.; Foglizzo, V.; Miretti, S.; Lanzetti, L.; Carrà, G.; Morotti, A.; Ala, U.; Provero, P.; et al. Deep Sequencing Reveals a Novel miR-22 Regulatory Network with Therapeutic Potential in Rhabdomyosarcoma. Cancer Res. 2016, 76, 6095–6106. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Vinklarek, J.; Bienertova-Vasku, J.; Slaby, O. MicroRNAs involved in skeletal muscle development and their roles in rhabdomyosarcoma pathogenesis. Pediatric Blood Cancer 2013, 60, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Taulli, R.; Bersani, F.; Foglizzo, V.; Linari, A.; Vigna, E.; Ladanyi, M.; Tuschl, T.; Ponzetto, C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J. Clin. Investig. 2009, 119, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Dong, X.D. (Eric); Chen, X.; Wang, L.; Lu, C.; Wang, J.; Qu, J.; Tu, L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J. Biol. Chem. 2009, 284, 29596–29604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sarver, A.L.; Alamgir, S.; Subramanian, S. Downregulation of microRNAs miR-1, -206 and -29 stabilizes PAX3 and CCND2 expression in rhabdomyosarcoma. Lab. Investig. 2012, 92, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Garzon, R.; Sun, H.; Ladner, K.J.; Singh, R.; Dahlman, J.; Cheng, A.; Hall, B.M.; Qualman, S.J.; Chandler, D.S.; et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 2008, 14, 369–381. [Google Scholar] [CrossRef]
- Sarver, A.L.; Li, L.; Subramanian, S. MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 2010, 70, 9570–9580. [Google Scholar] [CrossRef]
- Armeanu-Ebinger, S.; Herrmann, D.; Bonin, M.; Leuschner, I.; Warmann, S.W.; Fuchs, J.; Seitz, G. Differential expression of miRNAs in rhabdomyosarcoma and malignant rhabdoid tumor. Exp. Cell Res. 2012, 318, 2567–2577. [Google Scholar] [CrossRef]
- Gasparini, P.; Fortunato, O.; De Cecco, L.; Casanova, M.; Iannó, M.F.; Carenzo, A.; Centonze, G.; Milione, M.; Collini, P.; Boeri, M.; et al. Age-Related Alterations in Immune Contexture Are Associated with Aggressiveness in Rhabdomyosarcoma. Cancers 2019, 11, 1380. [Google Scholar] [CrossRef]
- Goljanek-Whysall, K.; Sweetman, D.; Munsterberg, A.E. microRNAs in skeletal muscle differentiation and disease. Clin. Sci. 2012, 123, 611–625. [Google Scholar] [CrossRef]
- Diao, Y.; Guo, X.; Jiang, L.; Wang, G.; Zhang, C.; Wan, J.; Jin, Y.; Wu, Z. miR-203, a tumor suppressor frequently down-regulated by promoter hypermethylation in rhabdomyosarcoma. J. Biol. Chem. 2014, 289, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.; Li, J.F.; Guo, L.L.; Xiao, H.T.; Dong, L.; Wang, F.; Huang, F.B.; Cao, D.; Qin, T.; Yin, X.H.; et al. TGF-beta1 suppression of microRNA-450b-5p expression: A novel mechanism for blocking myogenic differentiation of rhabdomyosarcoma. Oncogene 2014, 33, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Liu, J.; Hua, H.; Li, S.-E.; Zhao, J.; Yue, S.; Yu, T.-T.; Jin, Y.-C.; Cheng, S.Y. MiR-214 and N-ras regulatory loop suppresses rhabdomyosarcoma cell growth and xenograft tumorigenesis. Oncotarget 2014, 5, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Cytogenetic Alteration | Chromosomes Involved | Genes Involved |
|---|---|---|---|
| Embryonal | Gains | whole chromosomes: 2,7,8,12,13, 19, and 20 | GOK, PTCH, TP53 |
| Losses | whole chromosomes: 1,6,9,14,14 | ||
| Loss of heterozygosity LOH | 11p15.5, 11q, 16q | IGF2, H19, CDKN1C | |
| complex translocation | t(2;12;8) | PAX3 | |
| t(2;20)(q35;p12) | PAX3 | ||
| Alveolar | Rearrangements | t(2;13)(q35;q14); | PAX3-FOXO1 |
| t(1;13)(p36;q14), double minutes | PAX7-FOXO1 | ||
| t(2;2)(q35;p23) | PAX3-NCOA1 | ||
| t(2;8)(q35;q13) | PAX3-NCOA2 | ||
| t(X;2)(q13;q35) | PAX3-FOXO4 | ||
| t(8;13;9)(p11.2;q14;q32) | FGFR1-FOXO1 | ||
| Gains | 12q13.3–q14.1 and 8p11.2–q11.2 | CDK4, MYCN, GLI, MDM2, FGFR1, and FGFR4 |
| miRNA | Target Gene | Function |
|---|---|---|
| miR-1 | HDAC4 | Promote myoblasts differentiation |
| HAND2 | Inhibition of cardiomyocites proliferation | |
| PAX3 | Inhibition of RMS proliferation | |
| PAX7 | Promote muscle cells differentiation | |
| CCND2 | Inhibition of RMS growth | |
| cMET | Inhibition of RMS development | |
| miR-133 | SRF | Promote myoblasts differentiation |
| miR-206 | PAX7 | Promote muscle cells differentiation |
| CCND2 | Inhibition of RMS proliferation | |
| cMET | Inhibition of RMS development | |
| CX43 | Induction of myoblasts differentiation | |
| Polα1 | ||
| FSTL1 | ||
| UTRN |
| miRNA | Target Gene | Function |
|---|---|---|
| miR-27b | PAX3 | Inhibition of myoblast differentiation |
| miR-26a | EZH2 | Promoter of myogenesis |
| miR-214 | EZH2 | Induction of myoblast differentiation |
| miR-181 | HOX11 | Induction of myoblast differentiation |
| miR-669a | MyoD | Inhibition of skeletal muscle differentiation |
| miR-29 | YY1 | Induction of myoblast differentiation |
| PAX3 | Inhibition of RMS proliferation | |
| CCND2 | ||
| miR-183 | PTEN | Inhibition of RMS cell migration |
| EGR1 | ||
| miR-203 | P63 | Inhibition of RMS proliferation |
| miR-9 | E-cadherin | Inhibition of RMS migration |
| miR-450b | TGF-β1 | Inhibition RMS development |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparini, P.; Ferrari, A.; Casanova, M.; Limido, F.; Massimino, M.; Sozzi, G.; Fortunato, O. MiRNAs as Players in Rhabdomyosarcoma Development. Int. J. Mol. Sci. 2019, 20, 5818. https://doi.org/10.3390/ijms20225818
Gasparini P, Ferrari A, Casanova M, Limido F, Massimino M, Sozzi G, Fortunato O. MiRNAs as Players in Rhabdomyosarcoma Development. International Journal of Molecular Sciences. 2019; 20(22):5818. https://doi.org/10.3390/ijms20225818
Chicago/Turabian StyleGasparini, Patrizia, Andrea Ferrari, Michela Casanova, Francesca Limido, Maura Massimino, Gabriella Sozzi, and Orazio Fortunato. 2019. "MiRNAs as Players in Rhabdomyosarcoma Development" International Journal of Molecular Sciences 20, no. 22: 5818. https://doi.org/10.3390/ijms20225818
APA StyleGasparini, P., Ferrari, A., Casanova, M., Limido, F., Massimino, M., Sozzi, G., & Fortunato, O. (2019). MiRNAs as Players in Rhabdomyosarcoma Development. International Journal of Molecular Sciences, 20(22), 5818. https://doi.org/10.3390/ijms20225818







