S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head
Abstract
1. Introduction
2. Results
2.1. Decreased Serum S100A9 Levels in ONFH upon Hyperbaric Oxygen Therapy
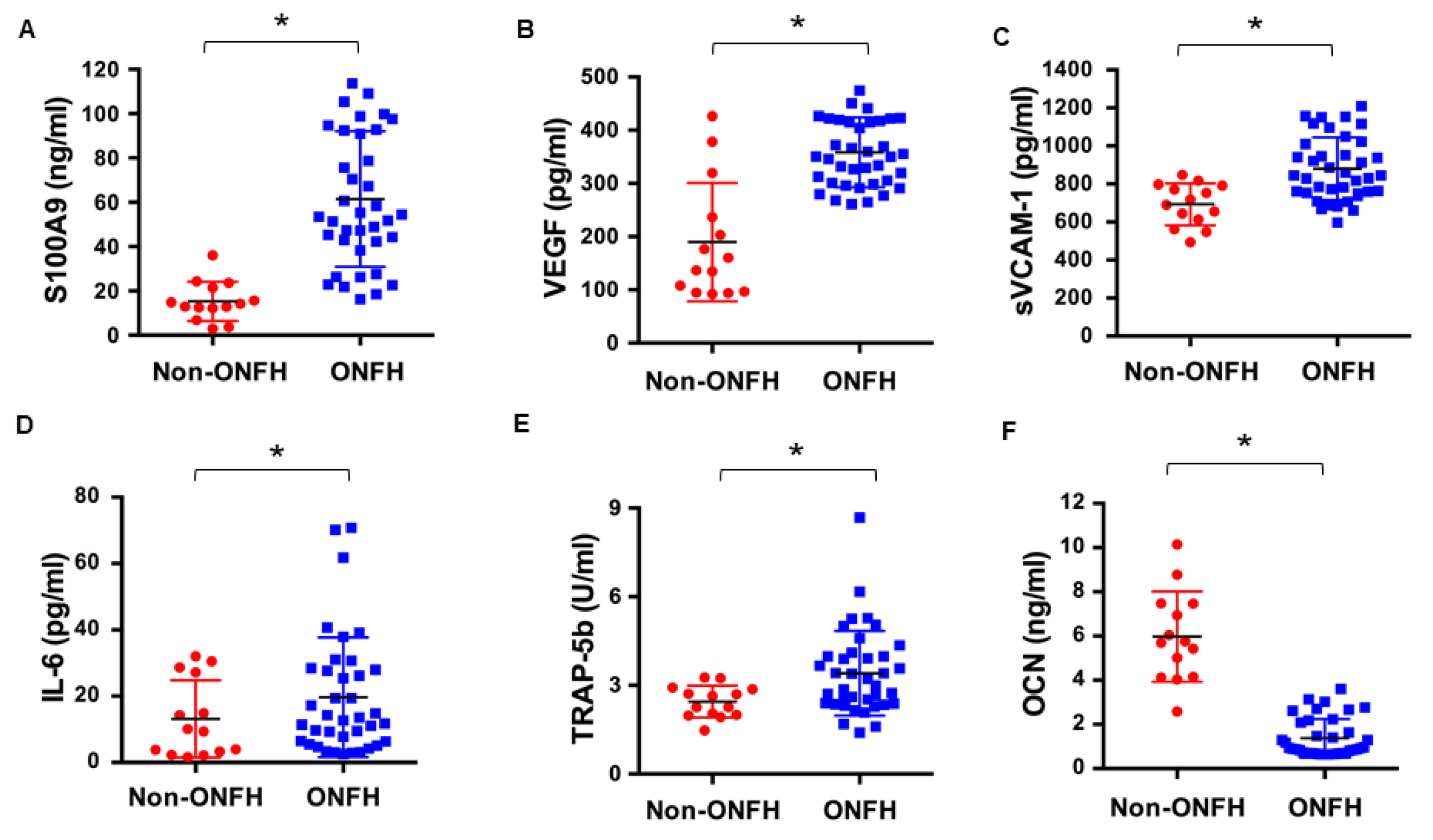
2.2. Increased Serum S100A9 Level Was Associated with Inflammation, Vessel Injury and Bone Turnover
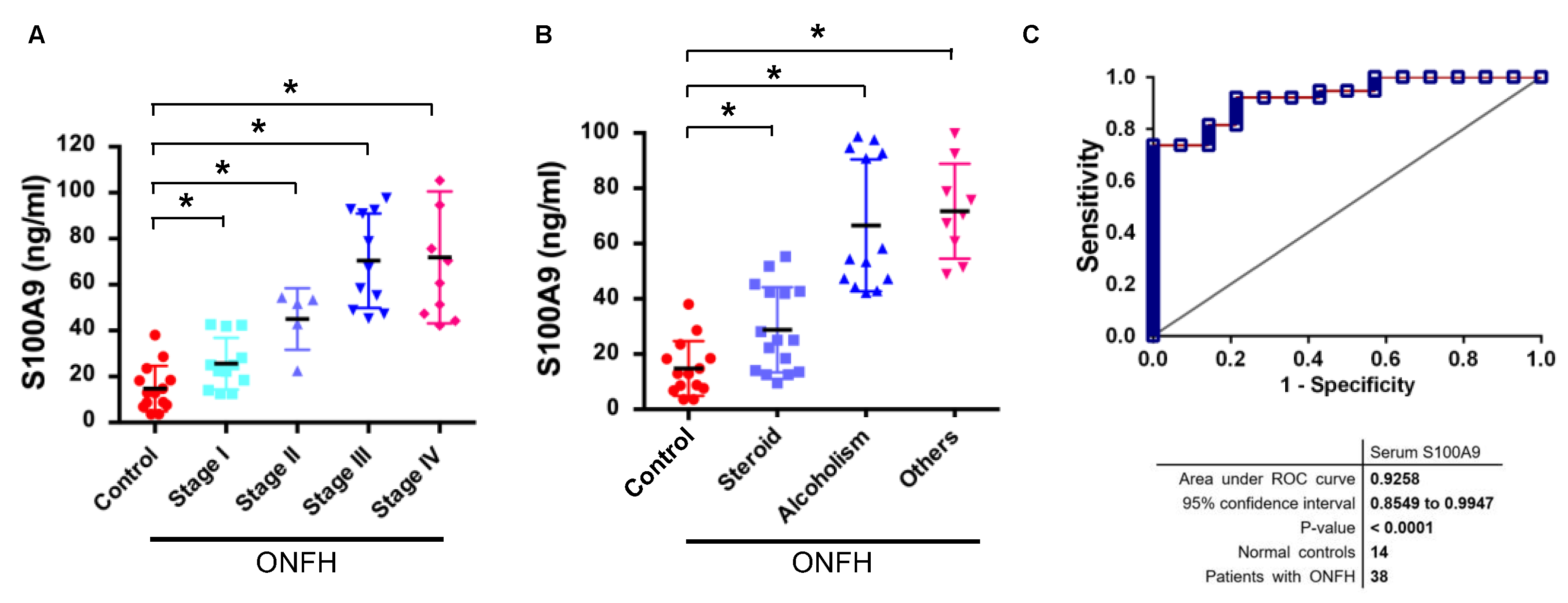
2.3. Increased S100A9 Levels Were Correlated with the Severity of ONFH
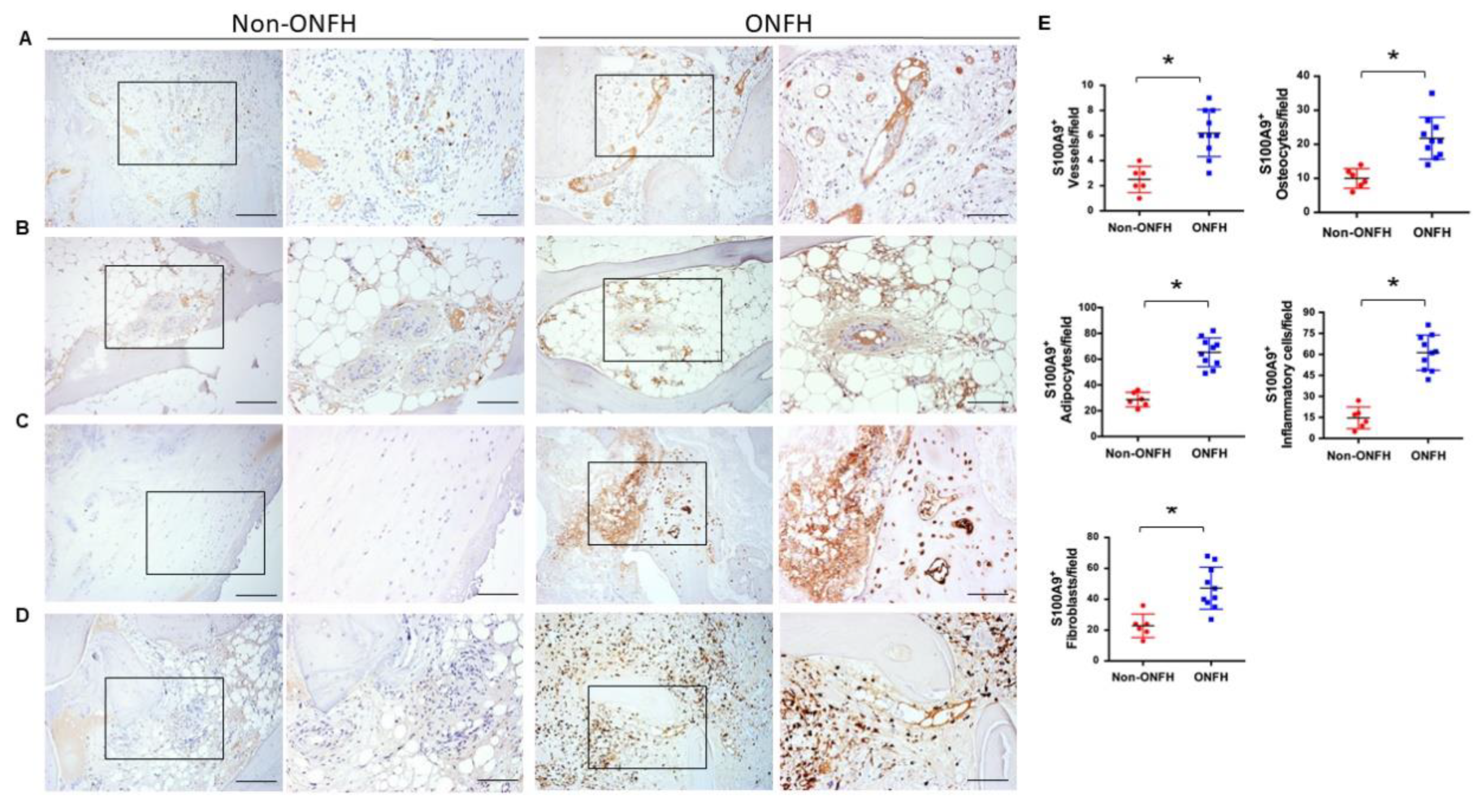
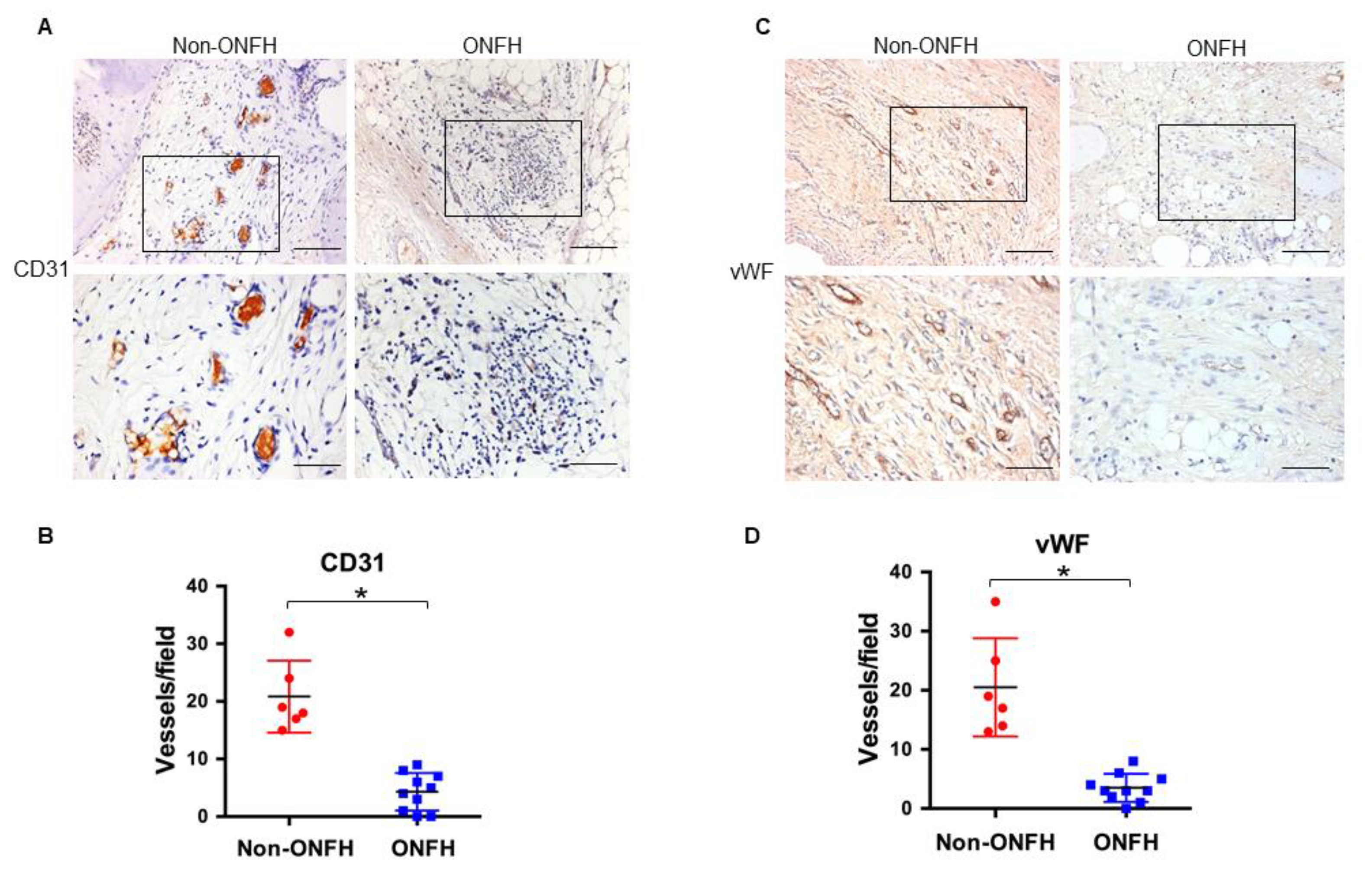
2.4. Strong S100A9 Immunostaining and Hypovasculature Histopathology in ONFH
2.5. S100A9 Inhibits Angiogenesis of Vessel Endothelial Cells and Aortic Rings
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Two-Dimensional Gel Electrophoresis and Tandem Mass Spectrometry
4.3. Tandem Mass Spectrometry
4.4. ELISA Quantification of Serum Proteins
4.5. Immunohistochemistry
4.6. Analysis of Angiogenesis of Human Vessel Endothelial Cells
4.7. Analysis of Vessel Formation of Rat Aortic Rings
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamb, J.N.; Holton, C.; O’Connor, P.; Giannoudis, P.V. Avascular necrosis of the hip. BMJ 2019, 365, I12178. [Google Scholar] [CrossRef] [PubMed]
- Hampton, S.N.; Nakonezny, P.A.; Richard, H.M.; Wells, J.E. Pain catastrophizing, anxiety, and depression in hip pathology. Bone Jt. J. 2019, 101, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Cherian, J.J.; Sierra, R.J.; Jones, L.C.; Lieberman, J.R. Nontraumatic osteonecrosis of the femoral head: Where do we stand today? A ten-year update. J. Bone Jt. Surg. Am. 2015, 97, 1604–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Hua, B.X.; Jaing, C.; Yuan, H.F.; Zhu, L.; Fan, W.S.; Ji, Z.F.; Wang, Z.; Yan, Z.Q. Serum biomarkers related to glucocorticoid-induced osteonecrosis of the femoral head: A prospective nested case-control study. J. Orthop. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Jones, L.C.; Chen, C.H.; Cheng, E.Y.; Cui, Q.; Drescher, W.; Fukushima, W.; Gangji, V.; Goodman, S.B.; Ha, Y.C.; et al. Etiologic classification criteria of ARCO on femoral head osteonecrosis part 2: Alcohol-associated osteonecrosis. J. Arthroplast. 2019, 49, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Fixation using alternative implants for the treatment of hip fractures (FAITH) investigators. Fracture fixation in the operative management of hip fracture (FAITH): An international, multicenter, randomized controlled trial. Lancet 2017, 389, 1519–1527. [Google Scholar] [CrossRef]
- Wyles, C.C.; Paradise, C.R.; Houdek, M.T.; Slager, S.L.; Terzic, A.; Behfar, A.; van Wijnen, A.J.; Sierra, R.J. CORR® ORS Richard A. Brand Award: Disruption in peroxisome proliferator-activated receptor-γ (PPARγ) increases osteonecrosis risk through genetic variance and pharmacologic modulation. Clin. Orthop. Relat. Res. 2019, 477, 1800–1812. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, S.; Zhao, D.; Wang, B. Evaluating the blood supply of the femoral head during different stages of necrosis using digital subtraction angiography. Orthopedics 2019, 42, e210–e215. [Google Scholar] [CrossRef]
- Pinheiro, M.; Dobson, C.A.; Perry, D.; Fagan, M.J. New insights into the biomechanics of Legg-Calve-Perthes’ disease: The role of epiphyseal skeletal immaturity in vascularity obstruction. Bone Jt. Res. 2018, 7, 148–156. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Li, X.; Li, J.; Yang, S.; Xing, X.; Fan, G.; Yokota, H.; Zhang, P. elF2α signaling regulates ischemic osteonecrosis through endoplasmic reticulum stress. Sci. Rep. 2017, 7, 5062. [Google Scholar] [CrossRef]
- Zha, X.; Sun, B.; Zhang, R.; Li, C.; Yan, Z.; Chen, J. Regulatory effect of microRNA-34a on osteogenesis and angiogenesis in glucocorticoid-induced osteonecrosis of the femoral head. J. Orthop. Res. 2018, 36, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liao, W.; Liu, X.Z.; Hu, J.; Zou, W.Z.; Ning, Y.; Yang, Y.; Li, Z.H. Down-regulation of exosomal microRNA-224-3p derived from bone marrow-derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. FASEB J. 2019, 33, 8055–8068. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Yang, J.; Yokota, H.; Zhang, P. Knee loading protects against osteonecrosis of the femoral head by enhancing remodeling and bone healing. Bone 2015, 81, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Fan, J.; Nan, K.; Li, J.; Shi, Z.; Dang, X.; Wang, K. Excessive activation of TLR4/NF-κB interactively suppresses the canonical Wnt/β-catenin pathway and induces SANFH in SD rats. Sci. Rep. 2017, 7, 11928. [Google Scholar] [CrossRef]
- Shichita, T.; Ito, M.; Morita, R.; Komai, K.; Noguchi, Y.; Ooboshi, H.; Koshida, R.; Takahashi, S.; Kodama, T.; Yoshimura, A. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 2017, 23, 723–732. [Google Scholar] [CrossRef]
- Ramadori, G.; Ljubicic, S.; Ricci, S.; Ramirez, B.; Rodriguez, A.; Becerri, S.; Valenti, V.; Moncada, R.; Silva, C.; Salavdor, J.; et al. S100A9 extends lifespan in insulin deficiency. Nat. Commun. 2019, 10, 3545. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Yang, X.; Zhang, C.; Jiao, Y.; Li, P.; Liu, Y.; Li, Z.; Qiao, B.; Bond Lau, W.; et al. S100a8/a9 signaling causes mitochondrial dysfunction and cardiomyocyte death in responses to ischemic/reperfusion injury. Circulation 2019, 140, 751–764. [Google Scholar] [CrossRef]
- Wang, L.; Luo, H.; Chen, X.; Jiang, Y.; Huang, Q. Functional characterization of S100A8 and S100A9 in altering monolayer permeability of human umbilical endothelial cells. PLoS ONE 2014, 9, e90472. [Google Scholar] [CrossRef]
- Fang, W.Y.; Chen, Y.W.; Hsiao, J.R.; Liu, C.S.; Kuo, Y.Z.; Wang, Y.C.; Chang, K.C.; Tsai, S.T.; Chang, M.Z.; Lin, S.H.; et al. Elevated S100A9 expression in tumor stroma functions as an early recurrence marker for early-stage oral cancer patients through increasing tumor cell invasion, angiogenesis, macrophage recruitiment and interleukin-6 production. Oncotarget 2015, 6, 28401–28424. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Farber, C.R.; Annex, B.H. Antiangiogenic VEGF165b regulates macrophages polarization via S100A8/S100A9 in peripheral artery disease. Circulation 2019, 139, 226–242. [Google Scholar] [CrossRef]
- Langley, S.R.; Willeit, K.; Didangelos, A.; Matic, L.P.; Skroblin, P.; Barallobre-Barreiro, J.; Lengquist, M.; Rungger, G.; Kapustin, A.; Kedenko, L.; et al. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J. Clin. Investig. 2017, 127, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, C.; Gao, H.; Bilodeau, M.L.; Zhang, Z.; Croce, K.; Liu, S.; Morooka, T.; Sakuma, M.; Nakajima, K.; et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Investig. 2014, 124, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Pepper, R.J.; Wang, H.H.; Rajakaruna, G.K.; Papakrivopoulou, E.; Vogl, T.; Pusey, C.D.; Cook, H.T.; Salama, A.D. S100A8/A9 (calprotectin) is critical for development of glomerulonephritis and promotes inflammatory leukocyte-renal cell interactions. Am. J. Pathol. 2015, 185, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Spiekemann, C.; Roth, J. S100 proteins in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 528–541. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Vogl, T.; Roth, J.P.; Slöetjes, A.W.; van den Berg, W.B.; van der Kraan, P.M.; van Lent, P.L. Induction of canonical Wnt signaling by the alarmins S100A8/A9 in murine knee joints: Implications for osteoarthritis. Arthritis Rheumatol. 2016, 68, 152–163. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; Geven, E.J.; van den Bosch, M.H.; Eriksson, H.; Leanderson, T.; Vogl, T.; Roth, J.; van de Loo, F.A.; Koenders, M.I.; van der Kraan, P.M. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann. Rheum. Dis. 2015, 74, 2254–2258. [Google Scholar] [CrossRef]
- Bosco, G.; Vezzani, G.; Mrakic Sposta, S.; Rizzato, A.; Enten, G.; Abou-Samra, A.; Malacrida, S.; Quartesan, S.; Vezzoli, A.; Camporesi, E. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J. Enzym. Inhib. Med. Chem. 2018, 33, 1501–1505. [Google Scholar] [CrossRef]
- Koren, L.; Ginsen, E.; Melamed, Y.; Norman, D.; Levin, D.; Peled, E. Hyperbaric oxygen for stage I and II femoral head osteonecrosis. Orthopedics 2015, 38, e200–e205. [Google Scholar] [CrossRef]
- Mont, M.A.; Marulanda, G.A.; Jones, L.C.; Saleh, K.J.; Gordon, N.; Hungerford, D.S.; Steinberg, M.E. Systematic analysis of classification systems for osteonecrosis of the femoral head. J. Bone Jt. Surg. Am. 2006, 88, 16–26. [Google Scholar]
- Guerado, E.; Caso, E. The physiopathology of avascular necrosis of the femoral head: An update. Injury 2016, 47, S16–S26. [Google Scholar] [CrossRef]
- Ko, J.Y.; Wang, F.S.; Wang, C.J.; Wong, T.; Chou, W.Y.; Tseng, S.L. Increased Dickkopf-1 expression accelerates bone cell apoptosis in femoral head osteonecrosis. Bone 2010, 46, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Sakai, T.; Ando, W.; Takao, M.; Nishi, T.; Nakamura, N.; Hamasaki, T.; Yoshikawa, H.; Sugano, N. Synovial joint fluid cytokine level in hip disease. Rheumatology 2014, 53, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhong, L.; Wang, Q.; Li, Z.; Shang, J.; Yang, Q.; Du, Z.; Wang, J.; Song, Y.; Zhang, G. The expression of chondrogenesis-related and arthritis-related genes in human ONFH cartilage with different Ficat stages. Peer J. 2019, 7, e6306. [Google Scholar] [CrossRef] [PubMed]
- Karasuyama, K.; Yamamoto, T.; Motomura, G.; Sonoda, K.; Kubo, Y.; Iwamoto, Y. The role of sclerotic changes in the starting mechanisms of collapse: A histomorphometric and FEM study on the femoral head of osteonecrosis. Bone 2015, 81, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kao, G.S.; Tu, Y.K.; Sung, P.H.; Wang, F.S.; Lu, Y.D.; Wu, C.T.; Lin, R.L.C.; Yi, H.K.; Lee, M.S. MicroRNA-mediated interacting circuits predict hypoxia and inhibited osteogenesis of stem cells, and dysregulated angiogenesis are involved in osteonecrosis of the femoral head. Int. Orthop. 2018, 42, 1605–1614. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomasźewski, K.A.; Niedzwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration, and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Yuan, H.F.; Christian, V.R.; Guo, C.A.; Chu, Y.W.; Liu, R.H.; Yan, Z.Q. Involvement of microRNA-210 demethylation in steroid-associated osteonecrosis of the femoral head. Sci. Rep. 2016, 6, 20046. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Cornelia, R.; Swisher, S.; Kim, H. Regulation of VEGF expression by HIF-1α in the femoral head cartilage following ischemia osteonecrosis. Sci. Rep. 2012, 2, 650. [Google Scholar] [CrossRef]
- Adapala, N.S.; Kim, H.K. Comprehensive genome-wide transcriptome analysis of immature articular cartilage following ischemic osteonecrosis of the femoral head in piglets. PLoS ONE 2016, 11, 2153174. [Google Scholar] [CrossRef]
- Cremers, N.A.J.; van der Bosch, M.H.J.; van Dalen, S.; Di Ceglie Ascone, G.; van de Loo, F.; Koenders, M.; van der Kraan, P.; Sloetjes, A.; Vogl, T.; Roth, J. S100A8/A9 increases the mobilization of proinflammatory Ly6Chigh monocytes to the synovium during experimental osteoarthritis. Arthritis Res. Ther. 2017, 19, 217. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai Dilisio, M.F.; Sekundiak, T.D.; Agrawal, D.K. Increased expression of damage-associated molecular patterns (DAMPs) in osteoarthritis of human knee joint compared to hip joint. Mol. Cell. Biochem. 2017, 436, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Slöetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Park, B.S.; Kim, S.Y.; Gu, A.; Kim, D.H.; Lee, J.S.; Kim, I.S. Cytokines secreted by S100A9 via TLR4 in monocytes delays neutrophils apoptosis by inhibition of caspase9/3 pathway. Cytokine 2016, 86, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Landers-Ramos, R.Q.; Sapp, R.M.; Jenkins, N.T.; Murphy, A.E.; Cancre, L.; Chin, E.R.; Spangenburg, E.E.; Hagberg, J.M. Chronic endurance exercise affects paracrine action of CD31+ and CD34+ cells on endothelial tube formation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H407–H420. [Google Scholar] [CrossRef] [PubMed]
- Bornfeldt, K.E. 2013 Russell Ross memorial lecture in vascular biology: Cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Atheroscler. Thromb. Vasc. Biol. 2014, 34, 705–714. [Google Scholar] [CrossRef][Green Version]
- Di Ceglie, I.; Blom, A.B.; Davar, R.; Logie, C.; Martens, J.H.A.; Habibi, E.; Bottcher, L.M.; Roth, J.; Vogl, T.; Goodyear, C.S.; et al. The alarmin S100A9 hampers osteoclast differentiation from human circulating precursor cells by reducing the expression of RANK. FASEB J. 2019. [Google Scholar] [CrossRef]
- Wang, F.S.; Wu, R.W.; Yeh, D.W.; Chen, M.W.; Ke, H.C.; Wu, S.L.; Ko, J.Y. Heat shock protein 60 protects skeletal tissue against glucocorticoid-induced bone mass loss by regulating osteoblast survival. Bone 2011, 49, 1080–1089. [Google Scholar] [CrossRef]
- Kuo, H.C.; Yang, K.D.; Chiu, C.C.; Chang, W.C.; Sheen, J.M.; Ou, C.Y.; Kuo, H.C.; Chen, R.F.; Hsu, T.Y.; Chang, J.C.; et al. Use of proteomic differential displays to assess functional discrepancies and facilitate adjustments of different human mesenchymal stem cell types. J. Proteom. Res. 2011, 10, 1305–1315. [Google Scholar] [CrossRef]
- Weng, W.T.; Wu, C.S.; Wang, F.S.; Wu, C.Y.; Ma, Y.L.; Chan, H.H.; Wu, D.C.; Wu, J.C.; Chu, T.H.; Haung, C.S.; et al. α-melanocyte-stimulating hormone attenuates neovascularization by inducing nitric oxide deficiency via MC-Rs/PKA/NF-kB signaling. Int. J. Mol. Sci. 2018, 19, 3823. [Google Scholar] [CrossRef]
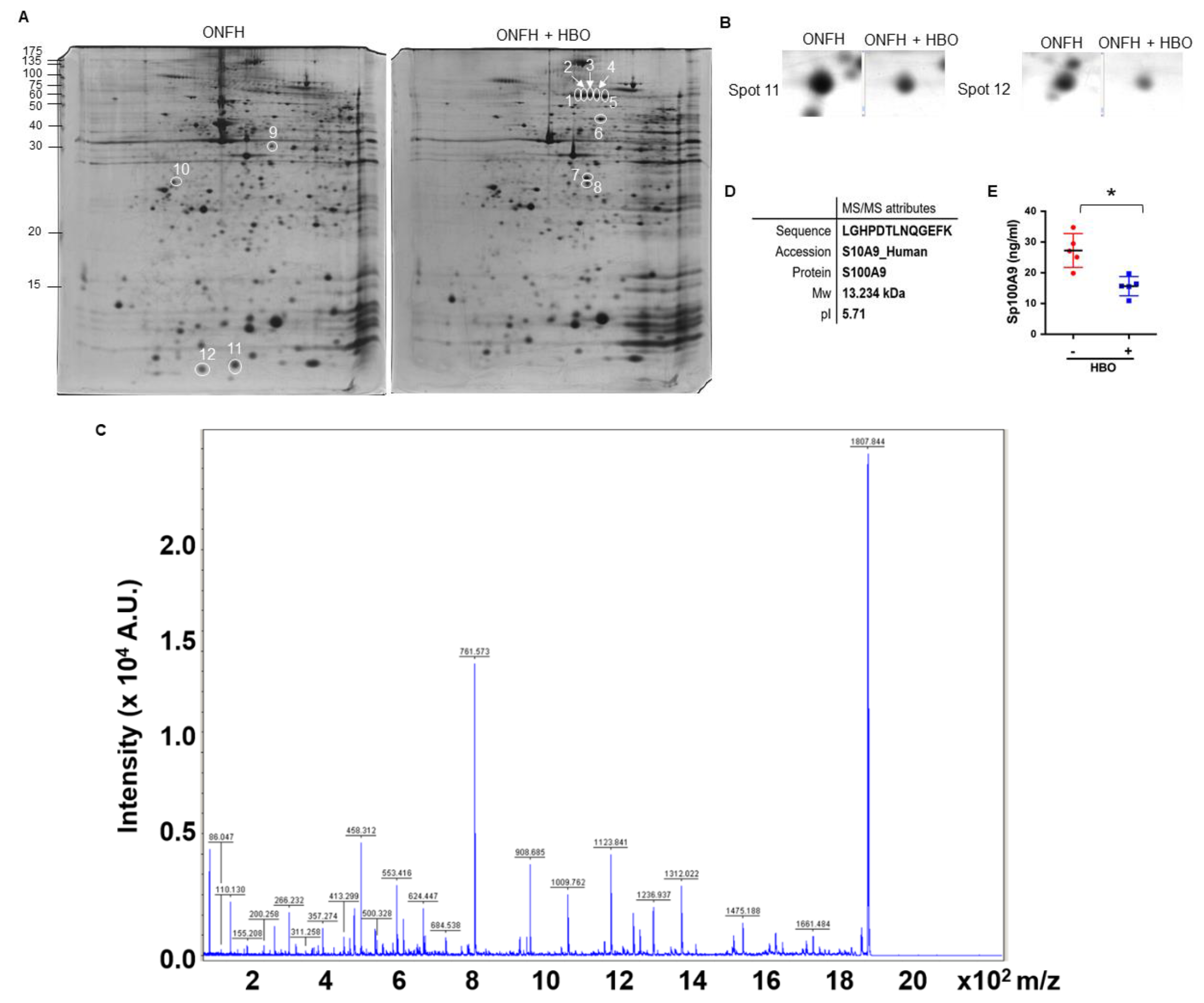

| Spot | Protein Name | Mw | pI | Accession |
|---|---|---|---|---|
| 1 | Filamin A (FLNA protein) | 88534 | 5.93 | gi|15779184 |
| 2 | Filamin A (FLNA protein) | 88534 | 5.93 | gi|15779184 |
| 3 | Filamin A (FLNA protein) | 88534 | 5.93 | gi|15779184 |
| 4 | Gelsolin isoform B | 80591 | 5.58 | gi|38044288 |
| 5 | Filamin A (FLNA protein) | 88534 | 5.93 | gi|15779184 |
| 6 | Gelsolin isoform B | 80591 | 5.58 | gi|38044288 |
| 7 | Protein disulfide isomerase-associated 3 isoform 1 | 54929 | 6.42 | gi|114656687 |
| 8 | Annexin III chain A | 36309 | 5.63 | gi|157830132 |
| 9 | Capping protein (actin filament) muscle Z-line | 29277 | 6.45 | gi|55665440 |
| 10 | Actin beta (ACTB protein) | 40194 | 5.55 | gi|15277503 |
| 11 | S100A9 | 13234 | 5.71 | S10A9_HUMAN |
| 12 | S100A9 | 13234 | 5.71 | S10A9_HUMAN |
| Healthy Controls | ONFH | |||||
|---|---|---|---|---|---|---|
| Total | Stage I | Stage II | Stage III | Stage IV | ||
| Patients | 14 | 38 | 12 | 5 | 11 | 10 |
| Age | 34.5 ± 5 | 50.4 ± 17.8 | 40.9 ± 10.9 | 58.2 ± 10.4 | 47.8 ± 12.2 | 57.8 ± 15 |
| (28–47) | (25–78) | (25–63) | (40–70) | (29–78) | (37–76) | |
| Gender | ||||||
| Males | 7 | 18 | 2 | 3 | 7 | 6 |
| Females | 7 | 20 | 10 | 2 | 4 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-W.; Lian, W.-S.; Kuo, C.-W.; Chen, Y.-S.; Ko, J.-Y.; Wang, F.-S. S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head. Int. J. Mol. Sci. 2019, 20, 5786. https://doi.org/10.3390/ijms20225786
Wu R-W, Lian W-S, Kuo C-W, Chen Y-S, Ko J-Y, Wang F-S. S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head. International Journal of Molecular Sciences. 2019; 20(22):5786. https://doi.org/10.3390/ijms20225786
Chicago/Turabian StyleWu, Re-Wen, Wei-Shiung Lian, Chung-Wen Kuo, Yu-Shan Chen, Jih-Yang Ko, and Feng-Sheng Wang. 2019. "S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head" International Journal of Molecular Sciences 20, no. 22: 5786. https://doi.org/10.3390/ijms20225786
APA StyleWu, R.-W., Lian, W.-S., Kuo, C.-W., Chen, Y.-S., Ko, J.-Y., & Wang, F.-S. (2019). S100 Calcium Binding Protein A9 Represses Angiogenic Activity and Aggravates Osteonecrosis of the Femoral Head. International Journal of Molecular Sciences, 20(22), 5786. https://doi.org/10.3390/ijms20225786






