Genome-Wide Identification and Characterization of ABC Transporters in Nine Rosaceae Species Identifying MdABCG28 as a Possible Cytokinin Transporter linked to Dwarfing
Abstract
1. Introduction
2. Results
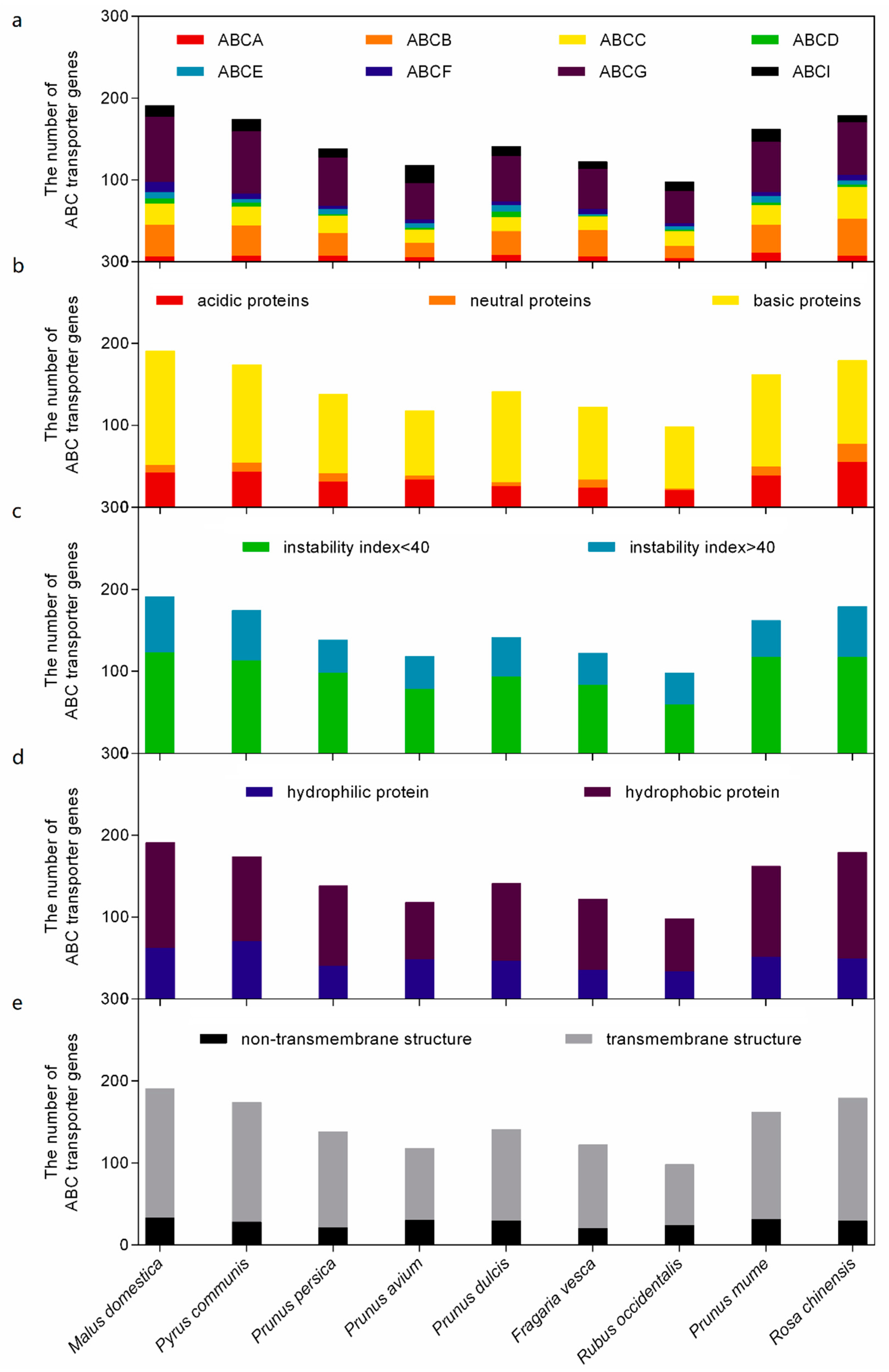
2.1. The ABC Transporter Superfamilies in Rosaceae
2.2. Chromosomal Localization, Motif Array, and Gene Structure of ABC Transporter Families
2.3. Tandem Duplication and Segmental Duplication of the ABC Transporter Families
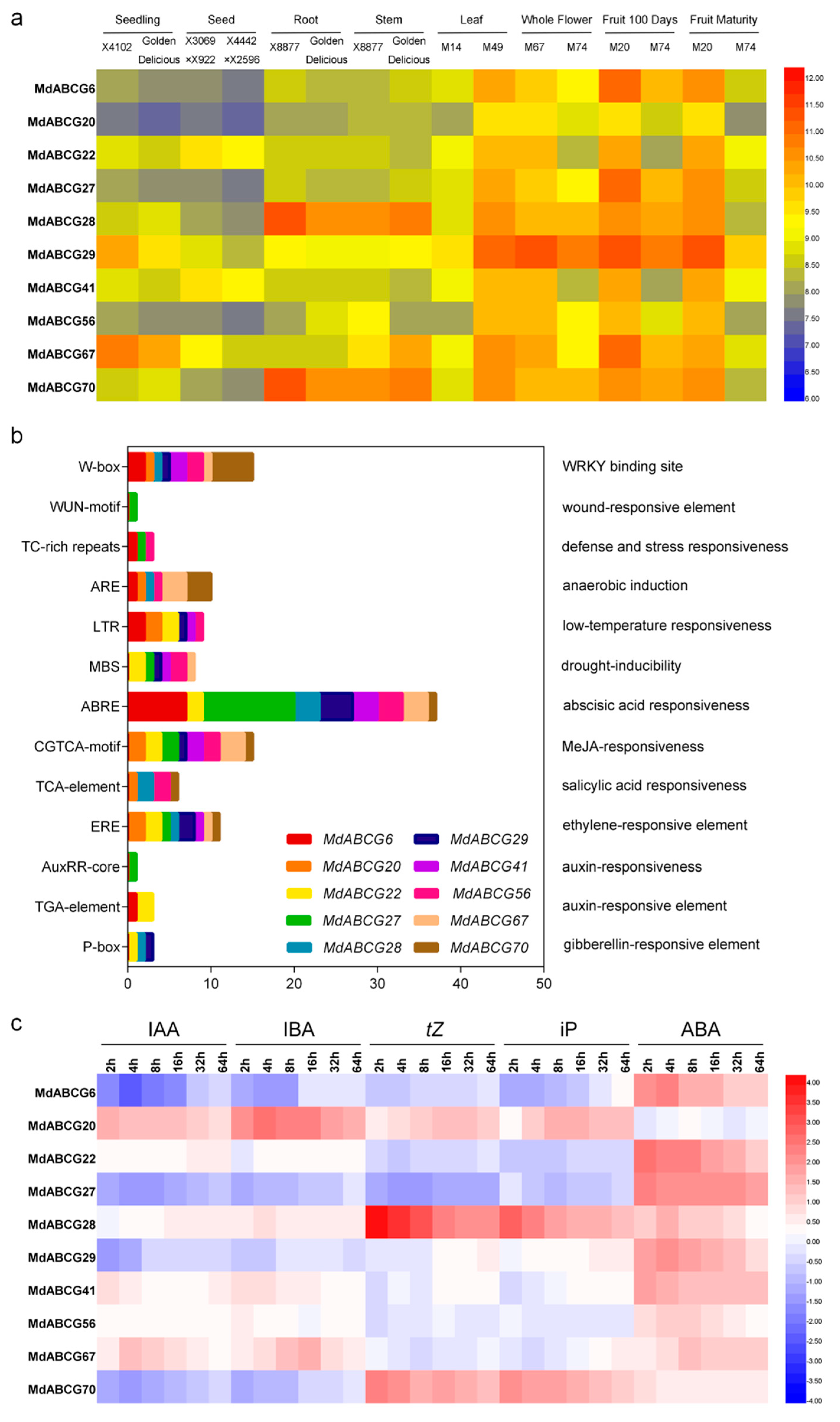
2.4. Identification and Characterization of Potential Phytohormone Transporters in MdABCG Subfamily Genes
2.5. Natural Variation in the Promoter Region of MdABCG28 Reduces the Gene Expression Level and Links to the Dwarfing Phenotype in Apple Rootstocks
3. Discussion
4. Materials and Methods
4.1. Database Searches and Identification of ABC Transporter Superfamilies in Rosaceae
4.2. Phylogenetic Analysis and Chromosome Localization
4.3. Motifs Analysis and Gene Structures
4.4. Tandem Duplication and Segmental Duplication Analysis
4.5. Putative Promoter Cis-Element Analysis
4.6. Transient Expression Assays of Promoter Activity
4.7. Growth Conditions and Treatments of Apple Callus
4.8. Generation of Transgenic Plants Using the atabcg14 Mutant
4.9. RNA Isolation and RT-PCR
4.10. Cytokinins Extraction and Quantification
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Verrier, P.J.; Bird, D.; Buria, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, U.; Lee, Y.; Martinoia, E.; et al. Plant ABC proteins—A unified nomenclature and updated inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F.; Linton, K.J. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 2004, 11, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, R.; Davies, T.G.E.; Coleman, J.O.D.; Rea, P.A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, K.; Dawson, R.J.P.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007, 17, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Kang, J.; Ko, D.; Lee, Y.; Martinoia, E. The role of ABCG-type ABC transporters in phytohormone transport. Biochem. Soc. Trans. 2015, 43, 924–930. [Google Scholar] [CrossRef]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J. 2011, 67, 885–894. [Google Scholar] [CrossRef]
- Ji, H.; Peng, Y.H.; Meckes, N.; Allen, S.; Stewart, C.N.; Traw, M.B. ATP-dependent binding cassette transporter G family member 16 increases plant tolerance to abscisic acid and assists in basal resistance against Pseudomonas syringae DC3000. Plant Physiol. 2014, 166, 879–888. [Google Scholar] [CrossRef]
- Zhang, K.W.; Novak, O.R.; Wei, Z.Y.; Gou, M.Y.; Zhang, X.B.; Yu, Y.; Yang, H.J.; Cai, Y.H.; Strnad, M.; Liu, C.J. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 2014, 5, 12. [Google Scholar] [CrossRef]
- Ko, D.; Kang, J.; Kiba, T.; Park, J.; Kojima, M.; Do, J.; Kim, K.Y.; Kwon, M.; Endler, A.; Song, W.Y.; et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA 2014, 111, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, K.; Strader, L.C.; Bailly, A.; Yang, H.B.; Blakeslee, J.; Langowski, L.; Nejedla, E.; Fujita, H.; Itoh, H.; Syono, K.; et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl. Acad. Sci. USA 2010, 107, 10749–10753. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Kilickaya, O. Whole-genome survey of the putative ATP-binding cassette transporter family genes in Vitis vinifera. PLoS ONE 2013, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Li, Y.; Zhao, L.H.; Hou, Z.M.; Yan, M.K.; Hu, B.Y.; Liu, Y.H.; Azam, S.M.; Zhang, Z.Y.; Rahman, Z.U.; et al. Genome-wide identification and expression profiling of ATP-binding cassette (ABC) transporter gene family in pineapple (Ananas comosus (L.) Merr.) reveal the role of AcABCG38 in pollen development. Front. Plant Sci. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Ofori, P.A.; Mizuno, A.; Suzuki, M.; Martinoia, E.; Reuscher, S.; Aoki, K.; Shibata, D.; Otagaki, S.; Matsumoto, S.; Shiratake, K. Genome-wide analysis of ATP binding cassette (ABC) transporters in tomato. PLoS ONE 2018, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Duan, W.K.; Lyu, S.W.; Li, Y.; Hou, X.L. Genome-wide identification, evolution, and expression analysis of the ATP-binding cassette transporter gene family in Brassica rapa. Front. Plant Sci. 2017, 8, 22. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhao, K.X.; Yang, Z.M. Identification of genomic ATP binding cassette (ABC) transporter genes and Cd-responsive ABCs in Brassica napus. Gene 2018, 664, 139–151. [Google Scholar] [CrossRef]
- George, A.M.; Jones, P.M. Perspectives on the structure-function of ABC transporters: The Switch and Constant Contact Models. Prog. Biophys. Mol. Biol. 2012, 109, 95–107. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus x domestica Borkh.). Nature Genet. 2010, 42, 833. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.W.; Shi, Z.B.; Zhang, S.; Ming, R.; Zhu, S.L.; Khan, M.A.; Tao, S.T.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant. 2016, 9, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, X.Z.; Wu, T.; Xu, X.F.; Han, Z.H.; Wang, Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Takei, K.; Kojima, M.; Sakakibara, H. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev. Cell 2013, 27, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Xu, Y.; Lu, Y.; Yu, H.X.; Gu, M.H.; Liu, Q.Q. The WRKY transcription factor OsWRKY78 regulates stem elongation and seed development in rice. Planta 2011, 234, 541–554. [Google Scholar] [CrossRef]
- Yu, F.F.; Huaxia, Y.F.; Lu, W.J.; Wu, C.G.; Cao, X.C.; Guo, X.Q. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Zhai, L.M.; Gan, Z.Y.; Zhang, G.F.; Yang, S.H.; Wang, Y.; Wu, T.; Zhang, X.Z.; Xu, X.F.; et al. Natural variation in cytokinin maintenance improves salt tolerance in apple rootstocks. Plant Cell Environ. 2019, 42, 424–436. [Google Scholar] [CrossRef]
- Zhang, M.L.; Lv, Y.D.; Wang, Y.; Rose, J.K.C.; Shen, F.; Han, Z.Y.; Zhang, X.Z.; Xu, X.F.; Wu, T.; Han, Z.H. TATA box insertion provides a selection mechanism underpinning adaptations to Fe deficiency. Plant Physiol. 2017, 173, 715–727. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099. [Google Scholar] [CrossRef]
- Chagne, D.; Crowhurst, R.N.; Pindo, M.; Thrimawithana, A.; Deng, C.; Ireland, H.; Fiers, M.; Dzierzon, H.; Cestaro, A.; Fontana, P.; et al. The draft genome sequence of European pear (Pyrus communis L. ‘Bartlett’). PLoS ONE 2014, 9, 12. [Google Scholar] [CrossRef]
- Verde, I.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Paris, R.; Aramini, V.; Gazza, L.; Rossini, L.; et al. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, K.; Isuzugawa, K.; Ikenaga, M.; Saito, Y.; Yamamoto, T.; Hirakawa, H.; Isobe, S. The genome sequence of sweet cherry (Prunus avium) for use in genomics-assisted breeding. DNA Res. 2017, 24, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Cigliano, R.A.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Edger, P.P.; VanBuren, R.; Colle, M.; Poorten, T.J.; Wai, C.M.; Niederhuth, C.E.; Alger, E.I.; Ou, S.J.; Acharya, C.B.; Wang, J.; et al. Single-molecule sequencing and optical mapping yields an improved genome of woodland strawberry (Fragaria vesca) with chromosome-scale contiguity. GigaScience 2017, 7, 7. [Google Scholar] [CrossRef]
- VanBuren, R.; Bryant, D.; Bushakra, J.M.; Vining, K.J.; Edger, P.P.; Rowley, E.R.; Priest, H.D.; Michael, T.P.; Lyons, E.; Filichkin, S.A.; et al. The genome of black raspberry (Rubus occidentalis). Plant J. 2016, 87, 535–547. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Chen, W.B.; Sun, L.D.; Zhao, F.Y.; Huang, B.Q.; Yang, W.R.; Tao, Y.; Wang, J.; Yuan, Z.Q.; Fan, G.Y.; et al. The genome of Prunus mume. Nat. Commun. 2012, 3, 8. [Google Scholar] [CrossRef]
- Saint-Oyant, L.H.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Xia, R.; Chen, H.; He, Y.H. TBtools, a toolkit for biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Tang, H.B.; Wang, X.Y.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2013, 41, D1152–D1158. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Lee, J.; Shin, M.K.; Ryu, D.K.; Kim, S.; Ryu, W.S. Insertion and deletion mutagenesis by overlap extension PCR. In In Vitro Mutagenesis Protocols, 3rd ed.; Braman, J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2010; Volume 634, pp. 137–146. [Google Scholar]
- Sheen, J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001, 127, 1466–1475. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.Z.; Wang, Y.C.; Xu, H.F.; Jiang, S.H.; Fang, H.C.; Liu, J.X.; Zhang, Z.Y.; Chen, X.S. MdMYB4 enhances apple callus salt tolerance by increasing MdNHX1 expression levels. Plant Cell Tissue Organ Culture 2017, 131, 283–293. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Feng, Y.; Wei, J.; Zhang, G.F.; Sun, X.Y.; Wang, W.; Wu, C.Y.; Tang, M.; Gan, Z.Y.; Xu, X.Z.; Chen, S.M.; et al. Effects of cooling measures on ‘Nijisseiki’ pear (Pyrus pyrifolia) tree growth and fruit quality in the hot climate. Sci. Hortic. 2018, 238, 318–324. [Google Scholar] [CrossRef]
- Zizkova, E.; Dobrev, P.I.; Muhovski, Y.; Hosek, P.; Hoyerova, K.; Haisel, D.; Prochazkova, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biol. 2015, 15, 20. [Google Scholar] [CrossRef] [PubMed]


| Motif | Logo | Sequence | E-Value | Sites | Width |
|---|---|---|---|---|---|
| 1 | 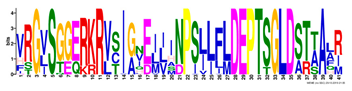 | VRGVSGGERKRVSIGNEJIINPSJLFLDEPTSGLDSTTALR | 4.9 × 10−1822 | 60 | 41 |
| 2 | 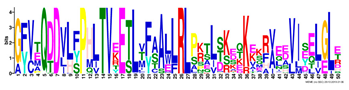 | GFVTQDDVLFPHLTVEETLVFAALLRLPKTLSKEQKEKRVEEVISELGLE | 1.1 × 10−1875 | 60 | 50 |
| 3 | 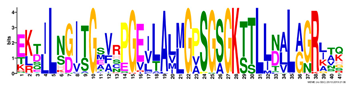 | EKTJLNGITGSVRPGEILALLGPSGSGKTTLLBALAGRLTQ | 1.2 × 10−1524 | 60 | 41 |
| 4 | 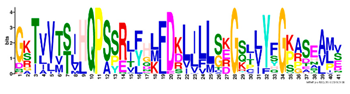 | GKTVVTSIHQPSSRLFHLFDKLJLLSKGSLLYFGKASEAMV | 1.5 × 10−1397 | 60 | 41 |
| 5 |  | WGFYPCFTALFTFPQERAMLTKERAAGMY | 8.3 × 10−1056 | 59 | 29 |
| 6 |  | VKKIPVFIIWIRYLSFVYYTYRLLLKVQY | 3.2 × 10−924 | 58 | 29 |
| 7 |  | WWZQFSILFQRGJKERRHEYFNWLRITQV | 3.9 × 10−814 | 41 | 29 |
| 8 |  | QGLGLAIGATLMDLKRATTLASVTVLTFM | 4.9 × 10−674 | 46 | 29 |
| 9 |  | RLSAYFLARTVVDLPLDLILPVAFLVITYWMAGLRPSAETF | 2.3 × 10−1088 | 58 | 41 |
| 10 | 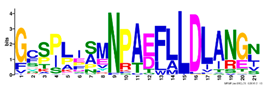 | GCSPLISMNPAEFLLDLANGN | 5.6 × 10−528 | 54 | 21 |
| 11 | 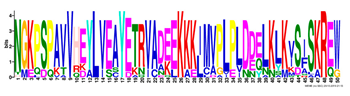 | NGKPSPAVVHEYLVEAYETRVADEEKKKJMVPLPLDDELKLKVSISKREW | 4.1 × 10−705 | 21 | 50 |
| 12 |  | KVSGSITYNGQTYSKFVKRRT | 2.1 × 10−485 | 59 | 21 |
| 13 | 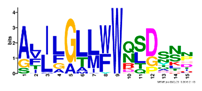 | ALJLGLLWWQSDSNN | 1.9 × 10−350 | 58 | 15 |
| 14 | 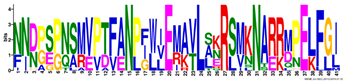 | NNBPSPNSMVPTFANPFWIEMAVLAKRSMKNARRMPELFGI | 3.9 × 10−318 | 15 | 41 |
| 15 | 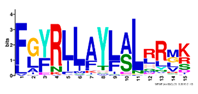 | FGYRLLAYLALRRMK | 5.3 × 10−276 | 54 | 15 |
| 16 | 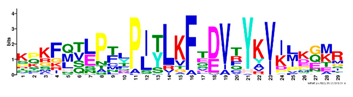 | KPKFQTLPTLPJTLKFTDVTYKVILKGMR | 1.8 × 10−520 | 48 | 29 |
| 17 |  | DEVPSGMALSRASSASLAFSFLFSGFTIP | 4.9 × 10−498 | 40 | 29 |
| 18 |  | SSRELFRASPSRESLLMKRNSFIYKFKSAQL | 1.0 × 10−390 | 26 | 31 |
| 19 |  | TGRTIVCTIHQPNIDIFEAFDELJLLKTGGRIIYSGPLGQHSSRVIEYFZ | 2.9 × 10−479 | 14 | 50 |
| 20 |  | GISGGQKKRLTTAEMJIGPTKALFMDEITNGLDSSTAFQIVNSLQQLVHI | 5.0 × 10−469 | 14 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Sun, Q.; Zhang, G.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; Wang, Y. Genome-Wide Identification and Characterization of ABC Transporters in Nine Rosaceae Species Identifying MdABCG28 as a Possible Cytokinin Transporter linked to Dwarfing. Int. J. Mol. Sci. 2019, 20, 5783. https://doi.org/10.3390/ijms20225783
Feng Y, Sun Q, Zhang G, Wu T, Zhang X, Xu X, Han Z, Wang Y. Genome-Wide Identification and Characterization of ABC Transporters in Nine Rosaceae Species Identifying MdABCG28 as a Possible Cytokinin Transporter linked to Dwarfing. International Journal of Molecular Sciences. 2019; 20(22):5783. https://doi.org/10.3390/ijms20225783
Chicago/Turabian StyleFeng, Yi, Qiran Sun, Guifen Zhang, Ting Wu, Xinzhong Zhang, Xuefeng Xu, Zhenhai Han, and Yi Wang. 2019. "Genome-Wide Identification and Characterization of ABC Transporters in Nine Rosaceae Species Identifying MdABCG28 as a Possible Cytokinin Transporter linked to Dwarfing" International Journal of Molecular Sciences 20, no. 22: 5783. https://doi.org/10.3390/ijms20225783
APA StyleFeng, Y., Sun, Q., Zhang, G., Wu, T., Zhang, X., Xu, X., Han, Z., & Wang, Y. (2019). Genome-Wide Identification and Characterization of ABC Transporters in Nine Rosaceae Species Identifying MdABCG28 as a Possible Cytokinin Transporter linked to Dwarfing. International Journal of Molecular Sciences, 20(22), 5783. https://doi.org/10.3390/ijms20225783







