Recent Advances in ROS-Responsive Cell Sheet Techniques for Tissue Engineering
Abstract
1. Introduction
2. Cell Sheet Harvesting Methods
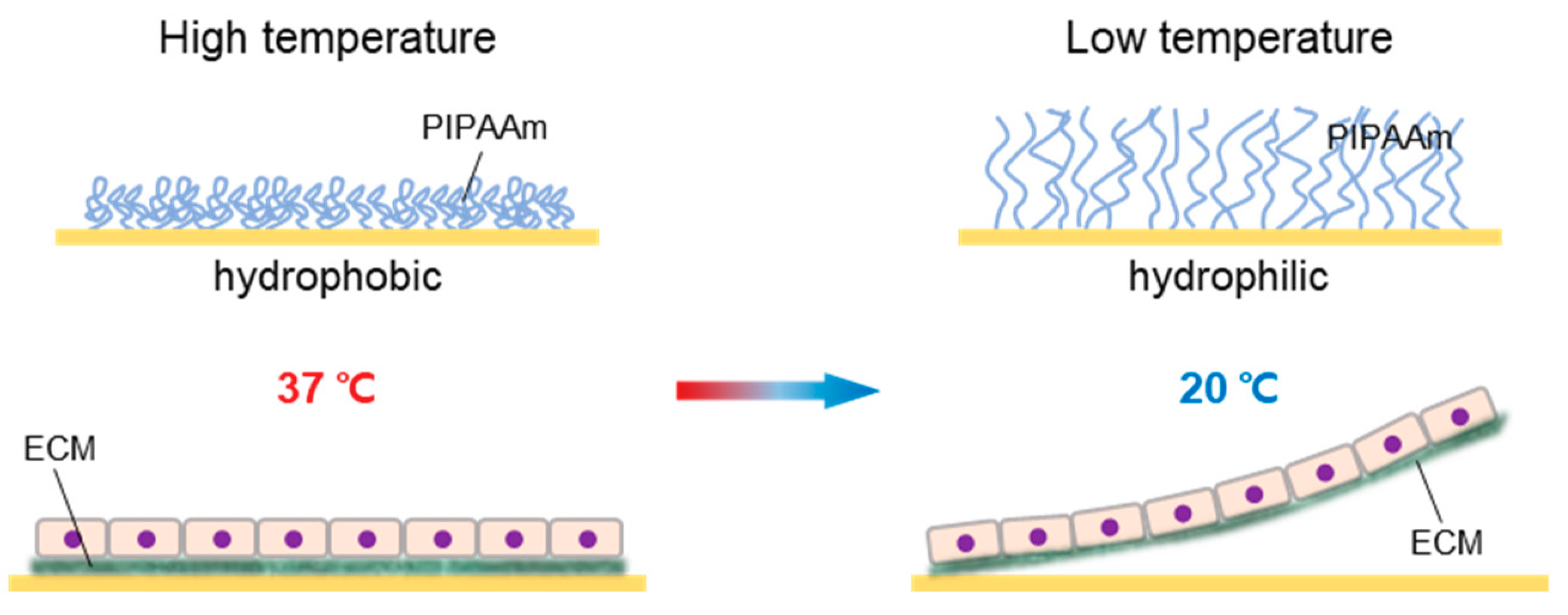
2.1. Thermo-Responsive Systems
2.2. Electro-Responsive Systems
2.3. pH-Responsive Systems
2.4. Magnetic Systems
3. ROS-Responsive Methods
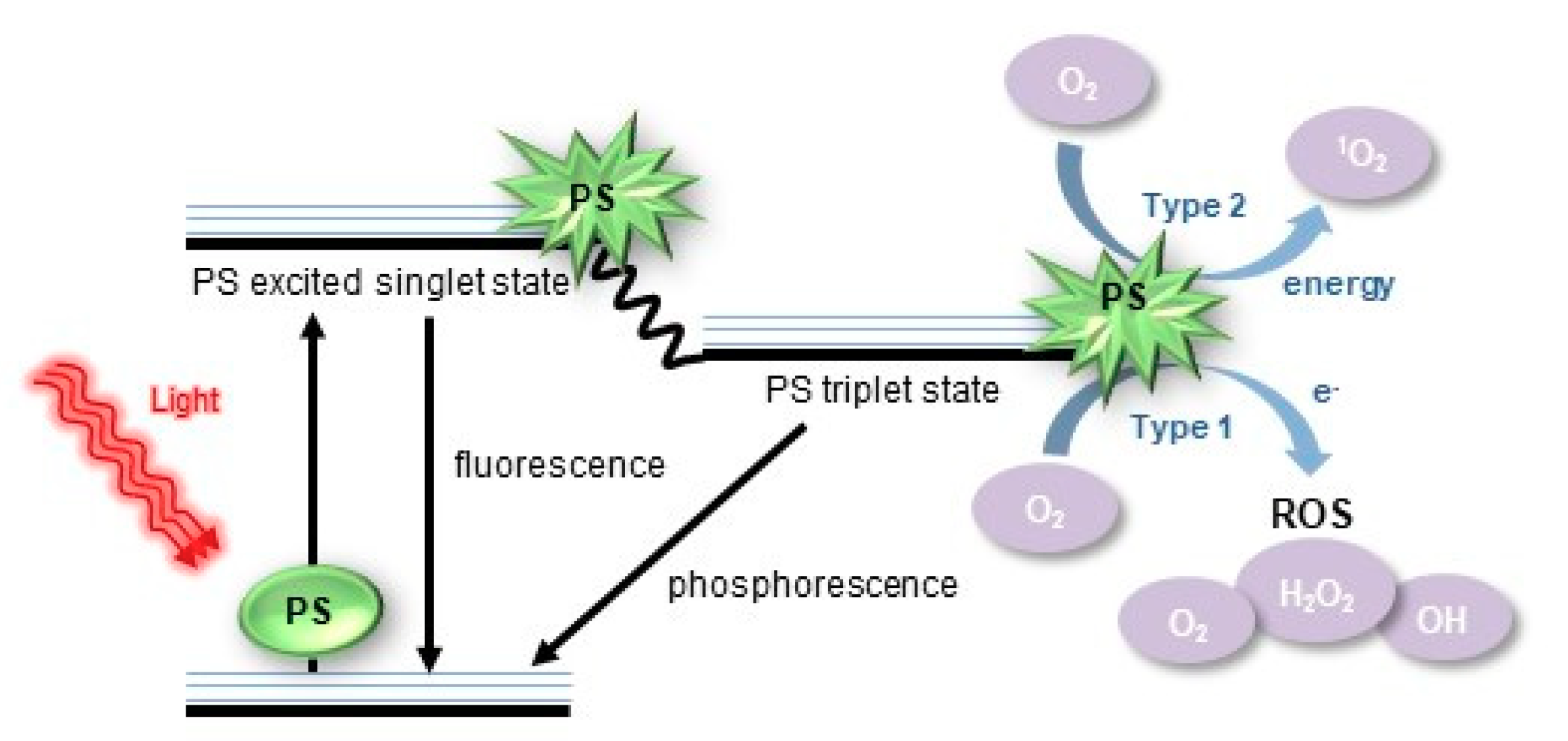
3.1. Definition of ROS
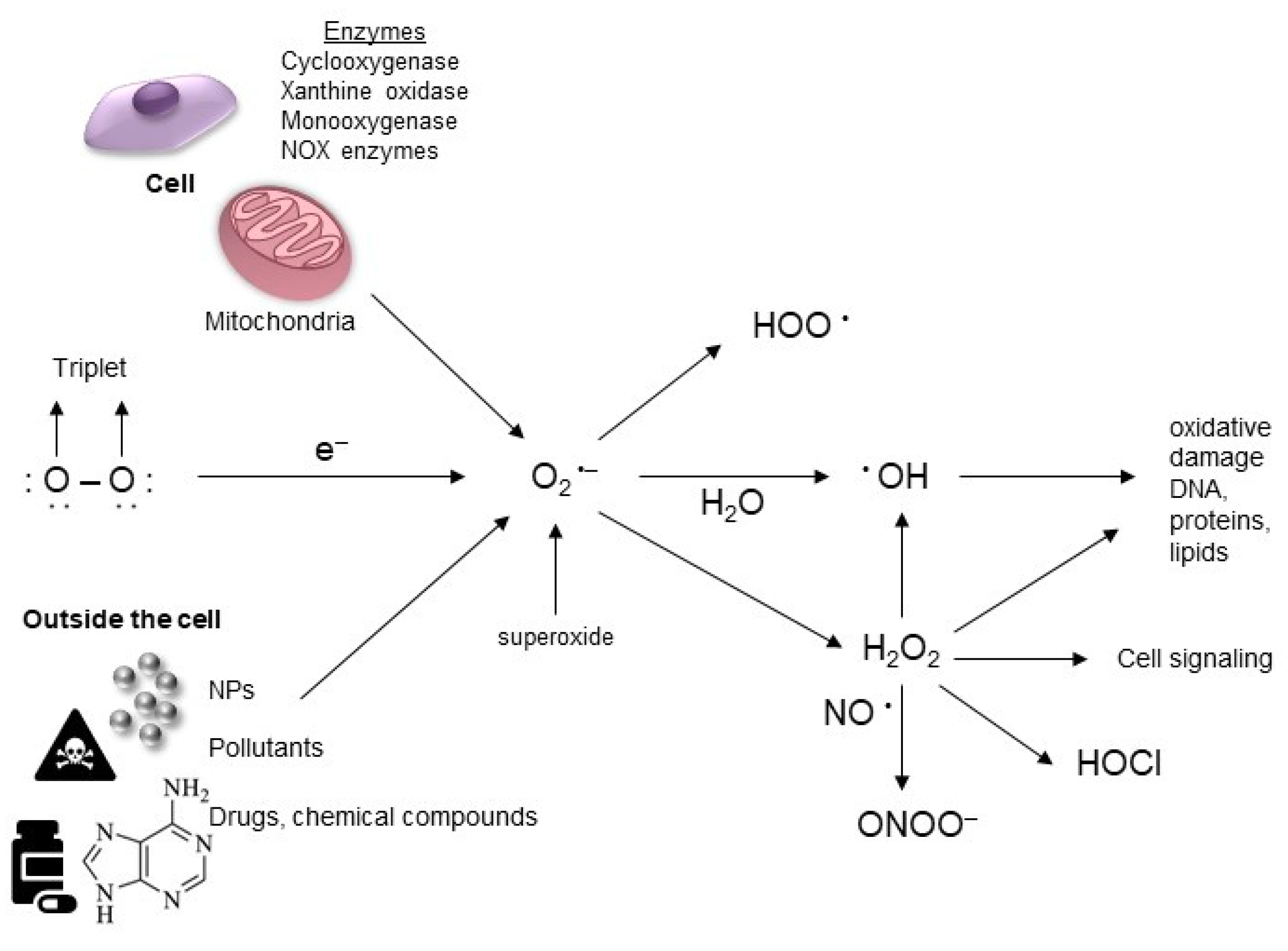
3.2. Source of ROS Generation
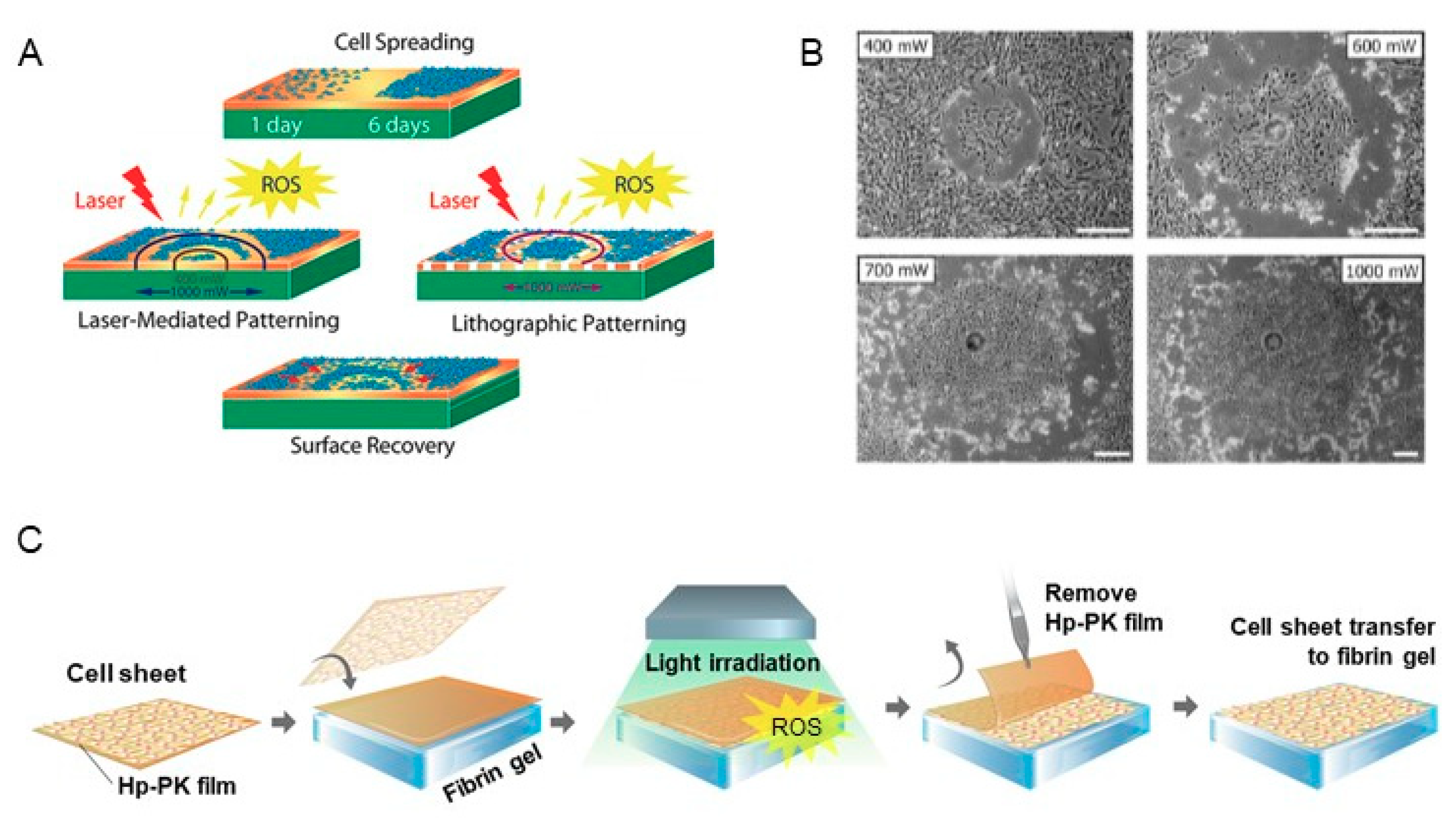
3.3. Cell Harvesting Methods by Extracellular ROS
3.4. Current Limitation for Clarifying the Mechanism by ROS
4. Final Remarks and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Sasagawa, T.; Sekine, H.; Sakaguchi, K.; Kikuchi, T.; Sekine, W.; Sekiya, S.; Yamato, M.; Umezu, M.; et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 2012, 7, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Shinoka, T.; Breuer, C. Tissue-engineered blood vessels in pediatric cardiac surgery. J. Biol. Med. 2008, 81, 161–166. [Google Scholar]
- Iwasa, J.; Engebretsen, L.; Shima, Y.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Chan, C.K.; Patil, N.; Goodman, S.B. Cell therapy for bone regeneration—Bench to bedside. J. Biomed. Mater. Res. B. Appl. Biomater. 2009, 89, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Isoi, Y.; Kikuchi, A.; Okano, T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperature-responsive culture dishes. Eur. Cell. Mater. 2005, 10, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of differentiated Madin-Darby canine kidney (MDCK) cell sheets: The noninvasive harvest from temperature-responsive culture dishes and transfer to other surfaces. J. Biomed. Mater. Res. 2001, 54, 37–46. [Google Scholar] [CrossRef]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Okano, T. Hepatocyte Transplantation: Cell Sheet Technology for Liver Cell Transplantation. Curr. Transplant. Rep. 2017, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qi, Y.; Niu, L.; Di, T.; Zhong, J.; Fang, T.; Yan, W. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015, 3, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Utoh, R.; Nagase, K.; Okano, T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 2014, 190, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, Y.; Yamato, M.; Okano, T. Cell attachment-detachment control on temperature-responsive thin surfaces for novel tissue engineering. Ann. Biomed. Eng. 2010, 38, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Egami, M.; Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Latest status of the clinical and industrial applications of cell sheet engineering and regenerative medicine. Arch. Pharm. Res. 2014, 37, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Nishida, K.; Yamato, M.; Sumide, T.; Utsumi, M.; Nozaki, T.; Kikuchi, A.; Okano, T.; Tano, Y. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature -responsive culture dishes. Biomaterials 2006, 27, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Honda, S.; Nakamura, Y.; López-Redondo, F.; Kohsaka, S.; Yamato, M.; Kikuchi, A.; Okano, T. Intact microglia are cultured and non-invasively harvested without pathological activation using a novel cultured cell recovery method. Biomaterials 2001, 22, 1213–1223. [Google Scholar] [CrossRef]
- Yamato, M.; Utsumi, M.; Kushida, A.; Konno, C.; Kikuchi, A.; Okano, T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma polymerized N-isopropylacrylamide: Synthesis and characterization of a smart thermally responsive coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Canavan, H.E.; Graham, D.J.; Cheng, X.; Ratner, B.D.; Castner, D.G. Comparison of native extracellular matrix with adsorbed protein films using secondary ion mass spectrometry. Langmuir 2007, 23, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tunc, M.; Humayun, M.; Cheng, X.; Ratner, B.D. A reversible thermosensitive adhesive for retinal implants: In vivo experience with plasma-deposited poly(N-isopropyl acrylamide). Retina 2008, 28, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Rayatpisheh, S.; Li, P.; Chan-Park, M.B. Argon-plasma-induced ultrathin thermal grafting of thermoresponsive pNIPAm coating for contractile patterned human SMC sheet engineering. Macromol. Biosci. 2012, 12, 937–945. [Google Scholar] [CrossRef] [PubMed]
- von Recum, H.A.; Okano, T.; Kim, S.W.; Bernstein, P.S. Maintenance of retinoid metabolism in human retinal pigment epithelium cell culture. Exp. Eye. Res. 1999, 69, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.T.; Carroll, W.M.; Selezneva, I.; Gorelov, A.; Rochev, Y. Cell growth and detachment from protein-coated PNIPAAm-based copolymers. J. Biomed. Mater. Res. A 2007, 81, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.E.; Carroll, W.M.; Nikoloskya, N.; Yang, R.; O'Connell, C.; Gorelov, A.V.; Dockery, P.; Liptrot, C.; Lyng, F.M.; Garcia, A.; et al. Straightforward, one-step fabrication of ultrathin thermoresponsive films from commercially available pNIPAm for cell culture and recovery. ACS Appl. Mater. Interfaces 2011, 3, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.G.; Zhang, G. Responsive systems for cell sheet detachment. Organogenesis 2013, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.G.; Cavicchia, J.P.; Zhang, G.; Zhang Newby, B.M. Rapid cell sheet detachment using spin-coated pNIPAAm films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012, 8, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Lee, Y.B.; Choi, Y.S.; Engler, A.J.; Park, H.; Shin, H. Transfer stamping of human mesenchymal stem cell patches using thermally expandable hydrogels with tunable cell-adhesive properties. Biomaterials 2015, 54, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Shin, Y.M.; Kim, E.M.; Lim, J.; Lee, J.Y.; Shin, H. Facile Cell Sheet Harvest and Translocation Mediated by a Thermally Expandable Hydrogel with Controlled Cell Adhesion. Adv. Healthc. Mater. 2016, 5, 2320–2324. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.S.; Hodneland, C.D.; Mrksich, M. Electroactive monolayer substrates that selectively release adherent cells. Chembiochem. 2001, 2, 590–593. [Google Scholar] [CrossRef]
- Seto, Y.; Inaba, R.; Okuyama, T.; Sassa, F.; Suzuki, H.; Fukuda, J. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials 2010, 31, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Schmutz, P.; Petronis, S.; Textor, M.; Keller, B.; Vörös, J. Locally Addressable Electrochemical Patterning Technique (LAEPT) applied to poly(L-lysine)-graft-poly(ethylene glycol) adlayers on titanium and silicon oxide surfaces. Biotechnol. Bioeng. 2005, 91, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Akiyama, Y.; Schuler, M.; Tang, C.; Textor, M.; Yamato, M.; Okano, T.; Vörös, J. Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv. Mater. 2008, 20, 560–565. [Google Scholar] [CrossRef]
- Guillaume-Gentil, O.; Gabi, M.; Zenobi-Wong, M.; Vörös, J. Electrochemically switchable platform for the micro-patterning and release of heterotypic cell sheets. Biomed. Microdevices 2011, 13, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, T.K.; Yang, J.M.; Liu, L.F. Acidic pH induces topoisomerase II-mediated DNA damage. Proc. Natl. Acad. Sci. U S A. 2003, 100, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Semenov, O.V.; Zisch, A.H.; Zimmermann, R.; Vörös, J.; Ehrbar, M. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials 2011, 32, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hayashida, M.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004, 10, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ito, A.; Lee, J.K.; Yoshida, T.; Miwa, K.; Ishiguro, H.; Numaguchi, Y.; Murohara, T.; Kodama, I.; Honda, H. Construction of multi-layered cardiomyocyte sheets using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 2007, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II-editorial review. Curr. Opin. Nephrol. Hypertens. 2005, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.F.; Laude, K.; McNally, J.S.; Harrison, D.G. Redox mechanisms in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Augusto, O.; Miyamoto, S.; Pantopoulos, K.; Schipper, H. Principles of Free Radical Biomedicine; Nova Science Publishers: Maringa, Brazil, 2011. [Google Scholar]
- Wu, H.; Yin, J.-J.; Wamer, W.G.; Zeng, M.; Lo, Y.M. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signaling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105, 165–177. [Google Scholar] [PubMed]
- Bonner, J.C. Lung fibrotic responses to particle exposure. Toxicol. Pathol. 2007, 35, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, M.; Chuang, Y.C.; Lo, L.W. Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules 2019, 24, 520. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist. 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, S.C.; Babu, P.S.S.; Madhuri, B.; Marydasan, B.; Paul, A.K.; Nair, A.S.; Rao, K.S.; Srinivasan, A.; Chandrashekar, T.K.; Rao, C.M.; et al. In vitro demonstration of apoptosis mediated photodynamic activity and NIR nucleus imaging through a novel porphyrin. ACS Chem. Biol. 2013, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.; Zeng, Q.; Zhang, Y.; Tu, L.; Liu, T.; Kong, X.; Wang, Y.; Cao, F.; Lambrechts, S.A.; et al. Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003, 161, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cha, M.J.; Song, B.W.; Kim, I.K.; Chang, W.; Lim, S.; Choi, E.J.; Ham, O.; Lee, S.Y.; Chung, N.; et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells 2010, 28, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, T.A.; Kohler, D.; Skirtach, A.G.; Möhwald, H. Laser-induced cell detachment, patterning, and regrowth on gold nanoparticle functionalized surfaces. ACS Nano 2012, 6, 9585–9595. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Lee, M.H.; Kwon, B.J.; Seon, G.M.; Kim, M.S.; Kim, D.; Nam, K.C.; Park, J.C. Exogenous ROS-induced cell sheet transfer based on hematoporphyrin-polyketone film via a one-step process. Biomaterials 2018, 161, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Hong, S.H.; Lee, M.H.; Kwon, B.J.; Seon, G.M.; Kim, M.S.; Kim, D.; Nam, K.C.; Park, J.C. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Lei, Q.; Wang, S.B.; Hu, J.J.; Qiu, W.X.; Zhu, J.Y.; Yin, W.N.; Luo, X.; Zhang, X.Z. Dual-stage-light-guided tumor inhibition by mitochondria-targeted photodynamic therapy. Adv. Funct. Mater. 2015, 25, 2961–2971. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.X.; Discher, D.E. Conformational changes and signaling in cell and matrix physics. Curr. Biol. 2009, 19, R781–R789. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.J.; Wu, M.; Holowka, D.; Baird, B. Nanobiotechnology and cell biology: Micro- and nanofabricated surfaces to investigate receptor-mediated signaling. Annu. Rev. Biophys. 2008, 37, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Reactive oxygen species and signal transduction. IUBMB Life 2001, 52, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Rovira, I.I.; Finkel, T. Oxidants painting the cysteine chapel: Redox regulation of PTPs. Dev. Cell 2002, 2, 251–252. [Google Scholar] [CrossRef]
- Brakebusch, C.; Bouvard, D.; Stanchi, F.; Sakai, T.; Fässler, R. Integrins in invasive growth. J. Clin. Invest. 2002, 109, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. The focal adhesion kinase--a regulator of cell migration and invasion. IUBMB Life 2002, 53, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Kim, B.J.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Seon, G.M.; Kim, D.; Nam, K.C.; Wang, K.K.; Kim, Y.R.; et al. Controlled delivery of extracellular ROS based on hematoporphyrin-incorporated polyurethane film for enhanced proliferation of endothelial cells. ACS Appl. Mater. Interfaces 2016, 8, 28448–28457. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Redox signaling across cell membranes. Antioxid. Redox. Signaling 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta. 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent advances in reactive oxygen species measurement in biological systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Kalyanaraman, B. Real-time monitoring of reactive oxygen and nitrogen species in a multiwell plate using the diagnostic marker products of specific probes. Methods Enzymol. 2013, 526, 145–157. [Google Scholar] [PubMed]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C. Current methods to study reactive oxygen species--pros and cons. Preface. Biochim. Biophys. Acta 2014, 1840, 707. [Google Scholar] [CrossRef] [PubMed]
| Probe | Specificity | Advantages | Disadvantages |
|---|---|---|---|
| Fluorescent probes | O2•―, H2O2 | Cell permeable, intensity quantifiable, product stable | Products complex, low specificity, interfered by ONOO― |
| Chemiluminescent probes | O2•―, ·OH | Cell permeable | Low selectivity and sensitivity, intermediates not stable |
| Spectrophotometry methods | O2•―, H2O2 | Sensitive, fast, single product | Low specificity |
| Chromatography methods | ·OH | Fast, sensitive | Products complex |
| Electrochemical biosensors | O2•― | Sensitive, fast | Complex to prepare |
| Electron spin resonance | ROS, RNS | Specific, sensitive | Expensive |
| Fluorescent proteins | H2O2, redox status changes | Dynamic, real-time, cell friendly | Slow in reaction, restriction in receptor cells, non-sensitive |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, M.-A.; Lee, M.H.; Park, J.-C. Recent Advances in ROS-Responsive Cell Sheet Techniques for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5656. https://doi.org/10.3390/ijms20225656
Koo M-A, Lee MH, Park J-C. Recent Advances in ROS-Responsive Cell Sheet Techniques for Tissue Engineering. International Journal of Molecular Sciences. 2019; 20(22):5656. https://doi.org/10.3390/ijms20225656
Chicago/Turabian StyleKoo, Min-Ah, Mi Hee Lee, and Jong-Chul Park. 2019. "Recent Advances in ROS-Responsive Cell Sheet Techniques for Tissue Engineering" International Journal of Molecular Sciences 20, no. 22: 5656. https://doi.org/10.3390/ijms20225656
APA StyleKoo, M.-A., Lee, M. H., & Park, J.-C. (2019). Recent Advances in ROS-Responsive Cell Sheet Techniques for Tissue Engineering. International Journal of Molecular Sciences, 20(22), 5656. https://doi.org/10.3390/ijms20225656





