Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination
Abstract
1. Introduction
2. Results
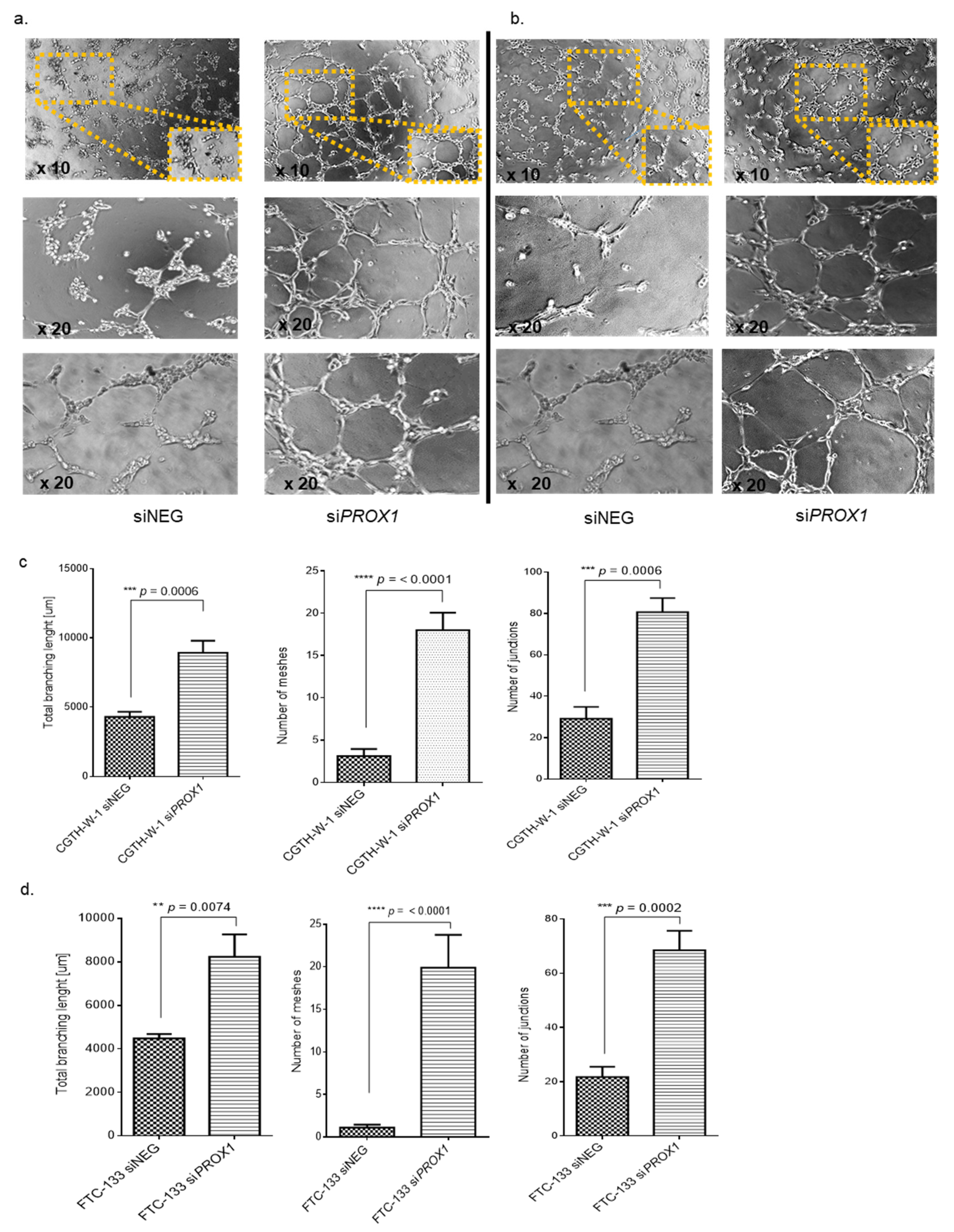
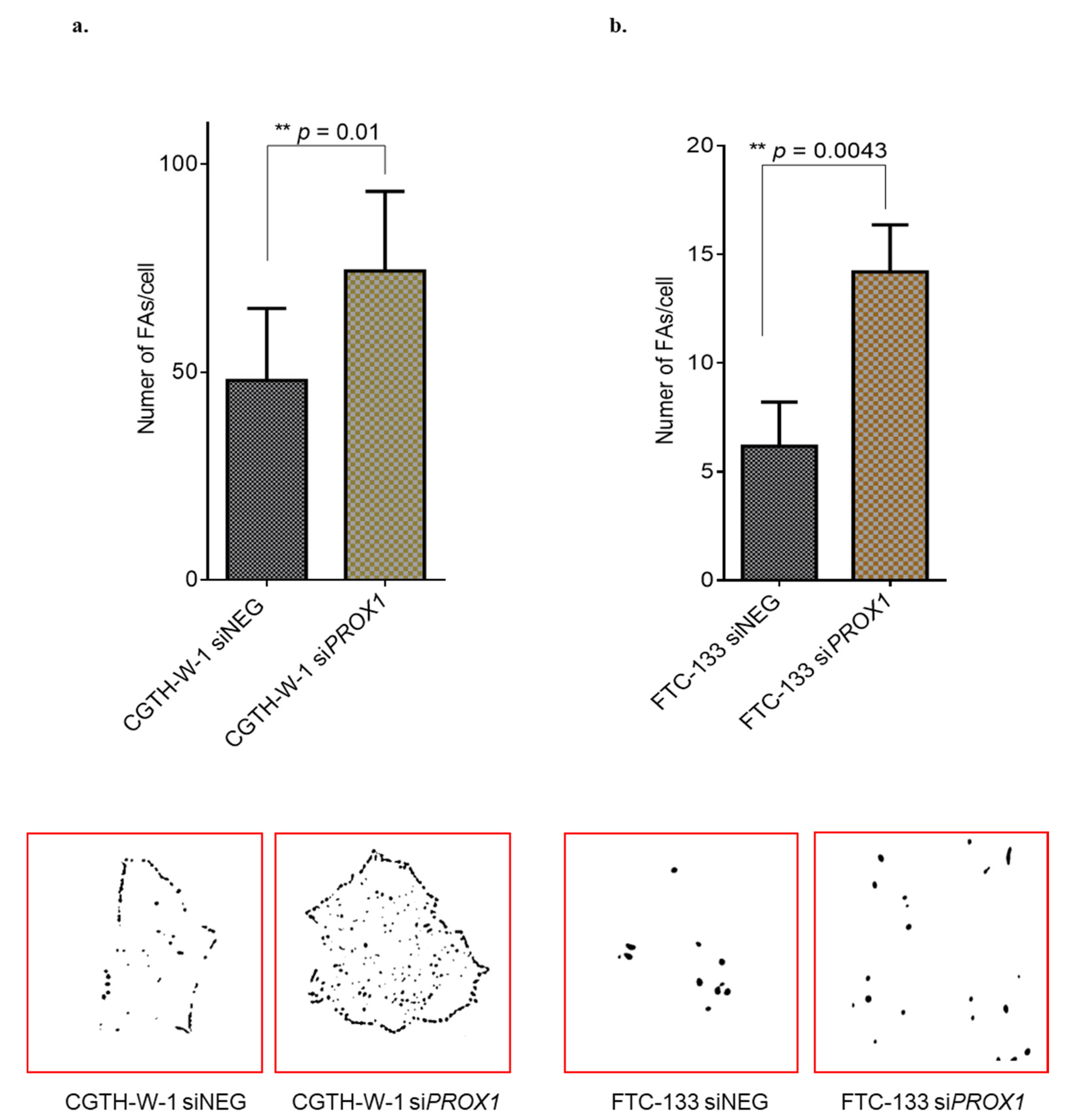
2.1. PROX1 Silencing in CGTH-W-1 Cells Has a Significant Impact on the In Vitro Angiogenesis
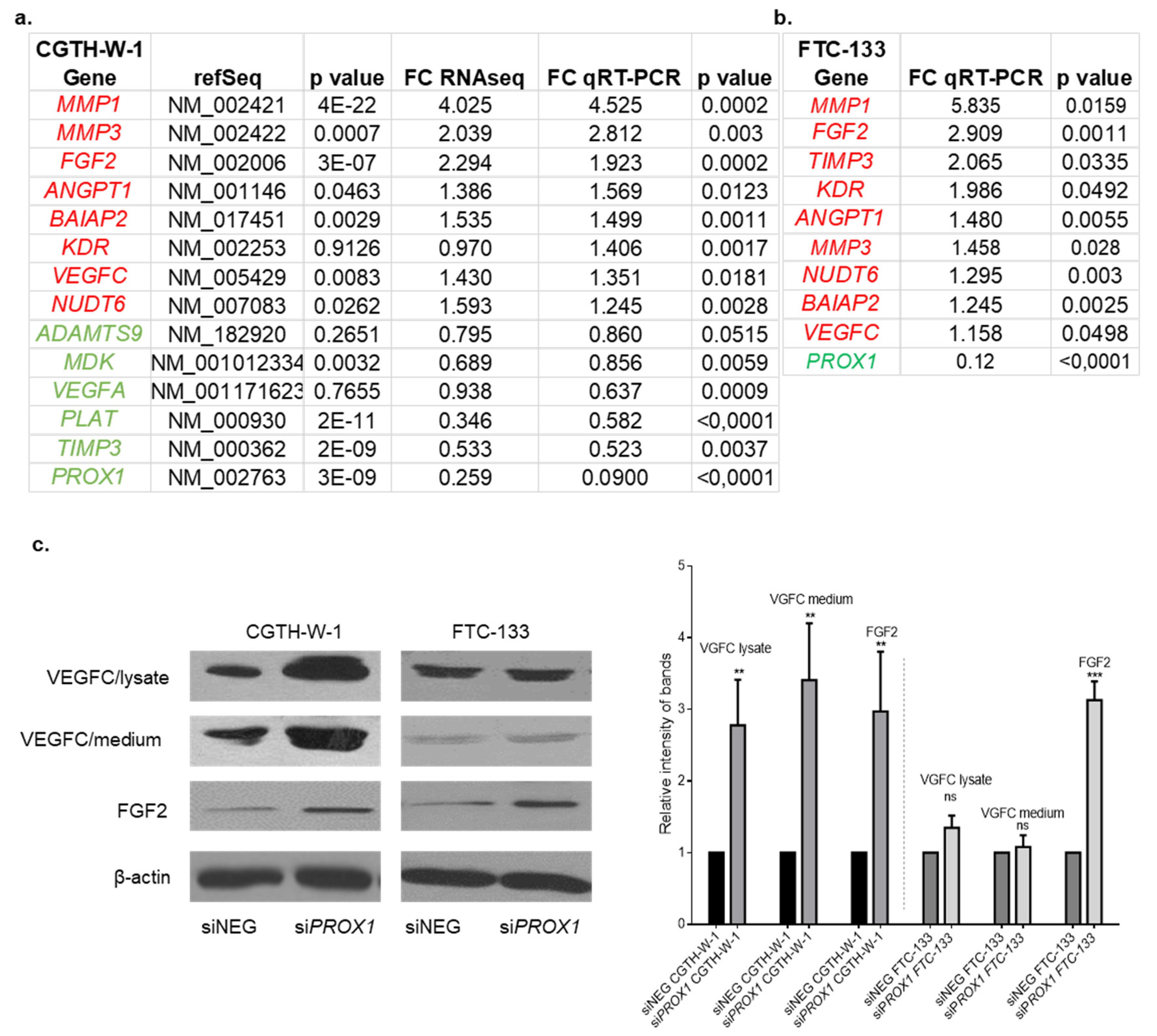
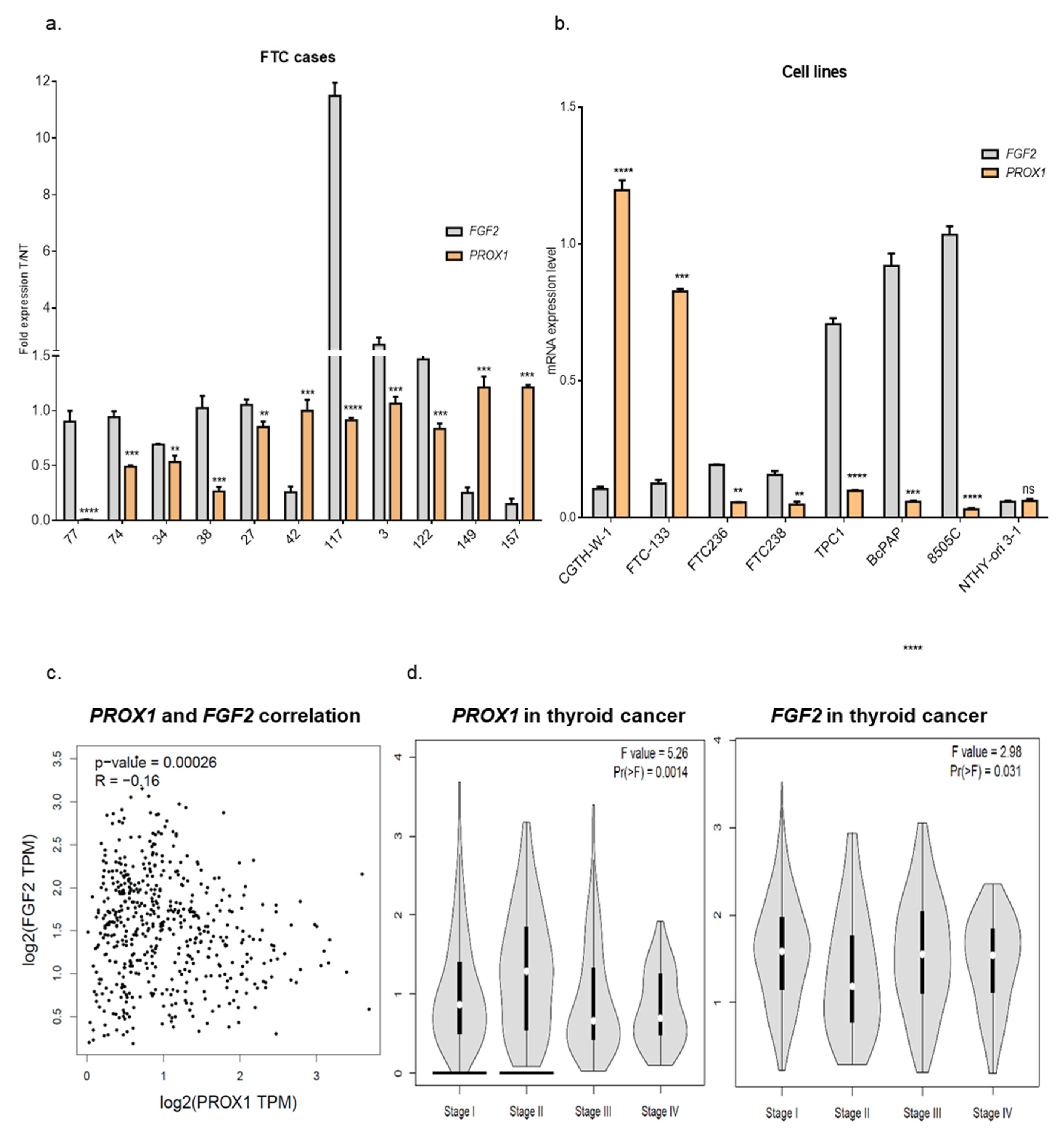
2.2. Genes Involved in Angiogenesis (FGF2, VEGFC, PLAT, BAIAP2) are Variably Expressed in Follicular Thyroid Cancer Tissues. Higher Expression of PROX1 Corresponds to a Lower Stage of Thyroid Cancer and is Negatively Correlated to FGF2 Expression.
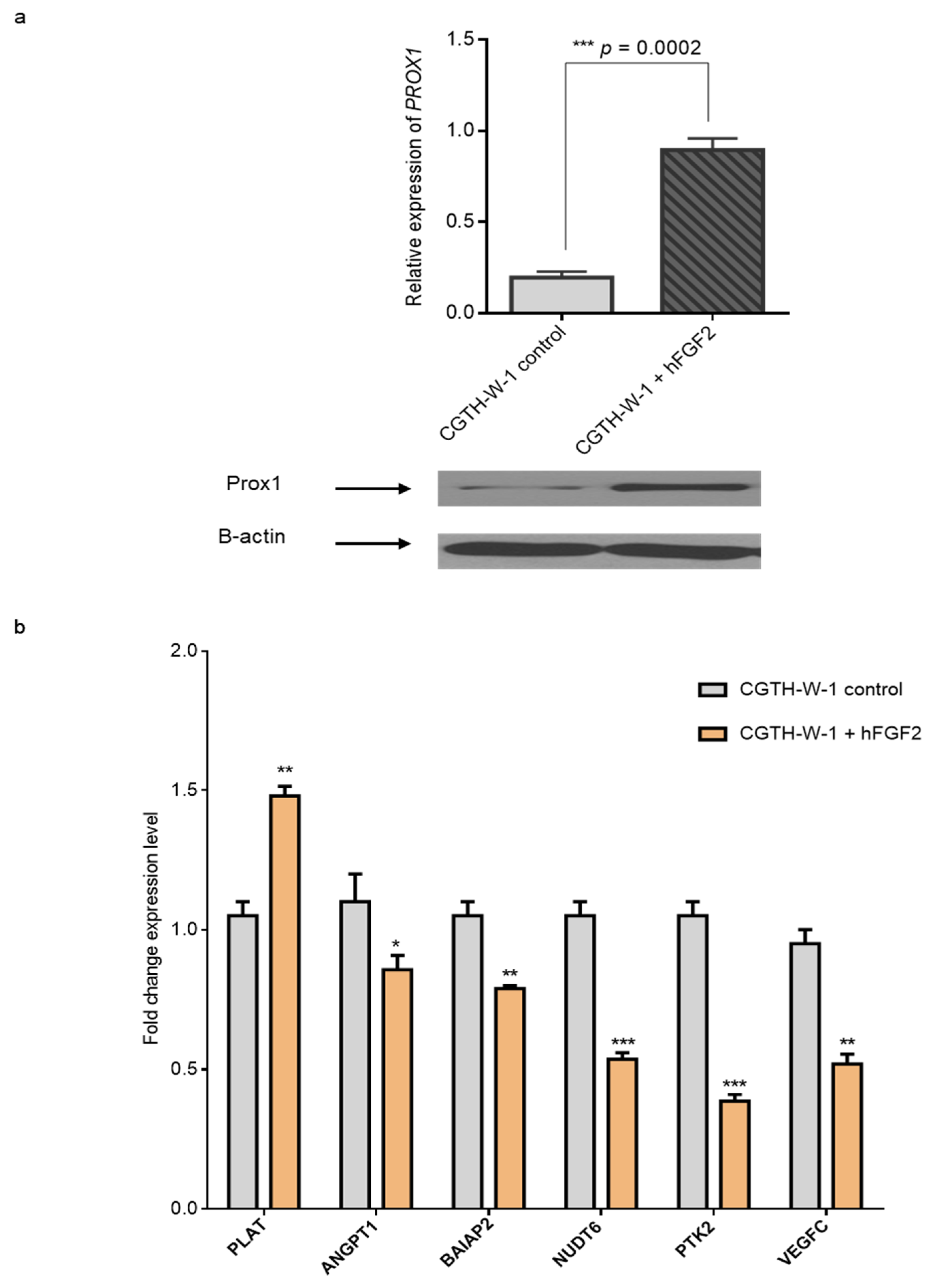
2.3. Treatment of CGTH-W-1 Cells with Pro-Angiogenic FGF2 Upregulates PROX1 Expression
3. Materials and Methods
3.1. RNA Extraction and cDNA Synthesis
3.2. Real-Time Quantitative PCR
3.3. Western Blotting
3.4. Transient Transfection of Small Interfering RNA
3.5. Tube Formation Assay
3.6. Angiogenesis Analysis
3.7. Culture with FGF2
3.8. Protocols of Focal Adhesion Measurement
3.9. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Enewold, L.; Zhu, K.; Ron, E.; Marrogi, A.J.; Stojadinovic, A.; Peoples, G.E.; Devesa, S.S. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol. Biomark. Prev.: A Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2009, 18, 784–791. [Google Scholar] [CrossRef]
- Gimm, O.; Dralle, H. Differentiated Thyroid Carcinoma. 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6979/ (accessed on 6 October 2019).
- Basnet, A.; Pandita, A.; Fullmer, J.; Sivapiragasam, A. Squamous cell carcinoma of the thyroid as a result of anaplastic transformation from BRAF-positive papillary thyroid cancer. Case Rep. Oncol. Med. 2017, 2017, 4276435. [Google Scholar] [CrossRef]
- Hall, F.T.; Freeman, J.L.; Asa, S.L.; Jackson, D.G.; Beasley, N.J. Intratumoral Lymphatics and Lymph Node Metastases in Papillary Thyroid Carcinoma. Arch. Otolaryngol. Head Neck Surg. 2017, 129, 716–719. [Google Scholar] [CrossRef][Green Version]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012, 31, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Zinovieva, R.D.; Duncan, M.K.; Johnson, T.R.; Torres, R.; Polymeropoulos, M.H.; Tomarev, S.I. Structure and chromosomal localization of the human homeobox gene Prox 1. Genomics 1996, 35, 517–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torii, M.A.; Matsuzaki, F.; Osumi, N.; Kaibuchi, K.; Nakamura, S.; Casarosa, S.; Guillemot, F.; Nakafuku, M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development 1999, 126, 443–456. [Google Scholar] [PubMed]
- Risebro, C.A.; Searles, R.G.; Melville, A.A.D.; Ehler, E.; Jina, N.; Shah, S.; Pallas, J.; Hubank, M.; Dillard, M.; Harvey, N.L.; et al. Prox1 maintains muscle structure and growth in the developing heart. Development 2009, 136, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Livesey, F.J.; Cepko, C.L.; Oliver, G. Prox1 function controls progenitor cell proliferation and horizontal, cell genesis in the mammalian retina. Nat. Genet. 2003, 34, 53–58. [Google Scholar] [CrossRef]
- Wigle, J.T.; Chowdhury, K.; Gruss, P.; Oliver, G. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 1999, 21, 318–322. [Google Scholar] [CrossRef]
- Sosa-Pineda, B.; Wigle, J.T.; Oliver, G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 2000, 25, 254–255. [Google Scholar] [CrossRef]
- Wang, J.F.; Kilic, G.; Aydin, M.; Burke, Z.; Oliver, G.; Sosa-Pineda, B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev. Biol. 2005, 286, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002, 225, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Li, Y.H.; Hatano, S.; Toshihito, O.; Yuge, M.; Ito, E.; Utsumi, M.; Saito, H.; Kinoshita, T. Mutations and aberrant DNA methylation of the PROX1 gene in hematologic malignancies. Genes Chromosomes Cancer 2003, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Versmold, B.; Felsberg, J.; Mikeska, T.; Ehrentraut, D.; Kohler, J.; Hampl, J.A.; Rohn, G.; Niederacher, D.; Betz, B.; Hellmich, M.; et al. Epigenetic silencing of the candidate tumor suppressor gene PROX1 in sporadic breast cancer. Int. J. Cancer 2007, 121, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, T.; Bao, P.; Fukuchi, M.; Altan, B.; Ozawa, D.; Rokudai, S.; Bai, T.; Kumakura, Y.; Honjo, H.; Hara, K.; et al. Nuclear PROX1 is Associated with Hypoxia-Inducible Factor 1 alpha Expression and Cancer Progression in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22, S1566–S1573. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Buchler, P.; Giese, N.; Giese, T.; Wilting, J.; Buchler, M.W.; Friess, H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int. J. Oncol. 2006, 28, 883–890. [Google Scholar] [CrossRef]
- Laerm, A.; Helmbold, P.; Goldberg, M.; Dammann, R.; Holzhausen, H.J.; Ballhausen, W.G. Prospero-related homeobox 1 (PROX1) is frequently inactivated by genomic deletions and epigenetic silencing in carcinomas of the bilary system. J. Hepatol. 2007, 46, 89–97. [Google Scholar] [CrossRef]
- Elsir, T.; Qu, M.; Berntsson, S.G.; Orrego, A.; Olofsson, T.; Lindstrom, M.S.; Nister, M.; von Deimling, A.; Hartmann, C.; Ribom, D.; et al. PROX1 is a predictor of survival for gliomas WHO grade II. Br. J. Cancer 2011, 104, 1747–1754. [Google Scholar] [CrossRef]
- Petrova, T.V.; Nykanen, A.; Norrmen, C.; Ivanov, K.I.; Andersson, L.C.; Haglund, C.; Puolakkainen, P.; Wempe, F.; von Melchner, H.; Gradwohl, G.; et al. Transcription factor PROM induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 2008, 13, 407–419. [Google Scholar] [CrossRef]
- Miettinen, M.; Wang, Z.F. Prox1 Transcription Factor as a Marker for Vascular Tumors-Evaluation of 314 Vascular Endothelial and 1086 Nonvascular Tumors. Am. J. Surg. Pathol. 2012, 36, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.L.; Myung, E.; Park, S.Y.; Kim, N.; Oak, C.Y.; Myung, D.S.; Cho, S.B.; Lee, W.S.; Kweon, S.S.; Kim, H.S.; et al. Impact of prospero homeobox-1 on tumor cell behavior and prognosis in colorectal cancer. Am. J. Cancer Res. 2015, 5, 3286–3300. [Google Scholar] [PubMed]
- Rudzinska, M.; Ledwon, J.K.; Gawel, D.; Sikorska, J.; Czarnocka, B. The role of prospero homeobox 1 (PROX1) expression in follicular thyroid carcinoma cells. Oncotarget 2017, 8, 114136–114155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavora, B.; Batista, S.; Reynolds, L.E.; Jadeja, S.; Robinson, S.; Kostourou, V.; Hart, I.; Fruttiger, M.; Parsons, M.; Hodivala-Dilke, K.M. Endothelial FAK is required for tumour angiogenesis. EMBO Mol. Med. 2010, 2, 516–528. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grzanka, M.; Stachurska, A.; Mikula, M.; Paczkowska, K.; Stępień, T.; Paziewska, A.; Ostrowski, J.; Czarnocka, B. Molecular Signature of Prospero Homeobox 1 (PROX1) in Follicular Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2212. [Google Scholar] [CrossRef]
- Chu, L.H.; Rivera, C.G.; Popel, A.S.; Bader, J.S. Constructing the angiome: a global angiogenesis protein interaction network. Physiol. Genomics 2012, 44, 915–924. [Google Scholar] [CrossRef]
- Horzum, U.; Ozdil, B.; Pesen-Okvur, D. Step-by-step quantitative analysis of focal adhesions. MethodsX 2014, 1, 56–59. [Google Scholar] [CrossRef]
- Petrova, T.V.; Makinen, T.; Makela, T.P.; Saarela, J.; Virtanen, I.; Ferrell, R.E.; Finegold, D.N.; Kerjaschki, D.; Yla-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef]
- Johnson, N.C.; Dillard, M.E.; Baluk, P.; McDonald, D.M.; Harvey, N.L.; Frase, S.L.; Oliver, G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008, 22, 3282–3291. [Google Scholar] [CrossRef]
- Audette, D.S.; Anand, D.; So, T.; Rubenstein, T.B.; Lachke, S.A.; Lovicu, F.J.; Duncan, M.K. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 2016, 143, 318–328. [Google Scholar] [CrossRef]
- Paul, L.; Walker, E.M.; Drosos, Y.; Cyphert, H.A.; Neale, G.; Stein, R.; South, J.; Grosveld, G.; Herrera, P.L.; Sosa-Pineda, B. Lack of Prox1 Downregulation Disrupts the Expansion and Maturation of Postnatal Murine β-Cells. Diabetes 2016, 65, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Toller, M.; Graziano, F.; Russo, F.; Pepe, M.; Biordi, L.; Marchioni, E.; Curcio, F.; Ulisse, S.; Ambesi-Impiombato, F. Expression of matrix metalloproteinases and their specific inhibitors in normal and different human thyroid tumor cell lines. Thyroid 2004, 14, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yagi, M.; Akiyama, N.; Hirosaki, T.; Higashi, S.; Lin, C.Y.; Dickson, R.B.; Kitamura, H.; Miyazaki, K. Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci. 2017, 97, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J. Clin. Investig. 1999, 103, 1237–1241. [Google Scholar] [CrossRef]
- Mignatti, P.; Rifkin, D.B. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzym. Protein 1996, 49, 117–137. [Google Scholar] [CrossRef]
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415. [Google Scholar] [CrossRef]
- Koo, B.H.; Coe, D.M.; Dixon, L.J.; Somerville, R.P.; Nelson, C.M.; Wang, L.W.; Young, M.E.; Lindner, D.J.; Apte, S.S. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am. J. Pathol. 2010, 176, 1494–1504. [Google Scholar] [CrossRef]
- Fagiani, E.; Lorentz, P.; Kopfstein, L.; Christofori, G. Angiopoietin-1 and -2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011, 71, 5717–5727. [Google Scholar] [CrossRef]
- Cao, Y.; Linden, P.; Farnebo, J.; Cao, R.; Eriksson, A.; Kumar, V.; Qi, J.-H.; Claesson-Welsh, L.; Alitalo, K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 14389–14394. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Baguma-Nibasheka, M.; MacFarlane, L.A.; Murphy, P.R. Regulation of Fibroblast Growth Factor-2 Expression and Cell Cycle Progression by an Endogenous Antisense RNA. Genes 2012, 3, 505–520. [Google Scholar] [CrossRef]
- Cork, S.M.; Van Meir, E.G. Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J. Mol. Med. 2011, 89, 743–752. [Google Scholar] [CrossRef]
- Gorka, B.; Skubis-Zegadlo, J.; Mikula, M.; Bardadin, K.; Paliczka, E.; Czarnocka, B. NrCAM, a neuronal system cell-adhesion molecule, is induced in papillary thyroid carcinomas. Br. J. Cancer 2007, 97, 531–538. [Google Scholar] [CrossRef]
- Zhu, D.; Li, C.; Swanson, A.M.; Villalba, R.M.; Guo, J.; Zhang, Z.; Matheny, S.; Murakami, T.; Stephenson, J.R.; Daniel, S. BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J. Clin. Investig. 2015, 125, 1497–1508. [Google Scholar] [CrossRef]
- Kerzerho, J.; Adotevi, O.; Castelli, F.A.; Dosset, M.; Bernardeau, K.; Szely, N.; Lang, F.; Tartour, E.; Maillere, B. The angiogenic growth factor and biomarker midkine is a tumor-shared antigen. J. Immunol. 2010, 185, 418–423. [Google Scholar] [CrossRef]
- Rivera, C.G.; Bader, J.S.; Popel, A.S. Angiogenesis-associated crosstalk between collagens, CXC chemokines, and thrombospondin domain-containing proteins. Ann. Biomed. Eng. 2011, 39, 2213–2222. [Google Scholar] [CrossRef][Green Version]
- De Almeida, C.J.G. Caveolin-1 and caveolin-2 can be antagonistic partners in inflammation and beyond. Front. Immunol. 2017, 8, 1530. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Dehart, J.P.; Murphy, J.M.; Lim, S.-T.S. Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights. J. Histochem. Cytochem. 2015, 63, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ji, H.; Feng, N.; Zhang, Y.; Yang, X.; Andersson, P.; Sun, Y.; Tritsaris, K.; Hansen, A.J.; Dissing, S.; et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 15894–15899. [Google Scholar] [CrossRef] [PubMed]
- Korah, R.; Choi, L.; Barrios, J.; Wieder, R. Expression of FGF-2 alters focal adhesion dynamics in migration-restricted. Breast Cancer Res. Treat. 2004, 88, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Reiland, J.; Doty Kempf, M.R.; Denkins, Y.; Marchetti, D. FGF2 binding, signaling, and angiogenesis are modulated by heparanase in metastatic melanoma cells. Neoplasia 2006, 8, 596. [Google Scholar] [CrossRef][Green Version]
- Hussein, O.; Komarova, S.V. Breast cancer at bone metastatic sites: recent discoveries and treatment targets. J. Cell Commun. Signal. 2011, 5, 85–99. [Google Scholar] [CrossRef]
- Tivari, S.; Korah, R.; Lindy, M.; Wieder, R. An in vitro dormancy model of estrogen-sensitive breast cancer in the bone marrow: A tool for molecular mechanism studies and hypothesis generation. J. Vis. Exp. 2015, 100, e52672. [Google Scholar] [CrossRef]
- Gonzalez-Perez, R.; Lanier, V.; Newman, G. Leptin’s pro-angiogenic signature in breast cancer. Cancers 2013, 5, 1140–1162. [Google Scholar] [CrossRef]
- Skog, M.; Bono, P.; Lundin, M.; Lundin, J.; Louhimo, J.; Linder, N.; Petrova, T.V.; Andersson, L.C.; Joensuu, H.; Alitalo, K. Expression and prognostic value of transcription factor PROX1 in colorectal cancer. Br. J. Cancer 2011, 105, 1346. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudzińska, M.; Mikula, M.; Arczewska, K.D.; Gajda, E.; Sabalińska, S.; Stępień, T.; Ostrowski, J.; Czarnocka, B. Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination. Int. J. Mol. Sci. 2019, 20, 5619. https://doi.org/10.3390/ijms20225619
Rudzińska M, Mikula M, Arczewska KD, Gajda E, Sabalińska S, Stępień T, Ostrowski J, Czarnocka B. Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination. International Journal of Molecular Sciences. 2019; 20(22):5619. https://doi.org/10.3390/ijms20225619
Chicago/Turabian StyleRudzińska, Magdalena, Michał Mikula, Katarzyna D. Arczewska, Ewa Gajda, Stanisława Sabalińska, Tomasz Stępień, Jerzy Ostrowski, and Barbara Czarnocka. 2019. "Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination" International Journal of Molecular Sciences 20, no. 22: 5619. https://doi.org/10.3390/ijms20225619
APA StyleRudzińska, M., Mikula, M., Arczewska, K. D., Gajda, E., Sabalińska, S., Stępień, T., Ostrowski, J., & Czarnocka, B. (2019). Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination. International Journal of Molecular Sciences, 20(22), 5619. https://doi.org/10.3390/ijms20225619





