The Role of MMP8 in Cancer: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. The Potential Use of MMP8 for Evaluating Cancer Prognosis
2.1.1. Analysis of Tumoral MMP8 on Protein and mRNA Level
2.1.1.1. No Clear Evidence for the Use of MMP8 Protein as a Prognostic Factor in Breast Cancer
2.1.1.2. The Prognostic Value of Tumoral MMP8 Protein Levels in Skin Cancer Depends on the Subtype
2.1.1.3. Tumoral MMP8 Protein Level is an Applicable Biomarker Only in Tongue SCC among All Head and Neck SCCs
2.1.1.4. Tumoral MMP8 Protein Level Associated with Malignancy in Ovarian and Liver Cancer and Variates in Colorectal and Gastric Cancer
2.1.1.5. MMP8 mRNA Expression is Rarely Detected in Patient Samples
2.1.2. MMP8 Levels in the Serum or Plasma
2.1.2.1. MMP8 Levels Increase with Malignancy in HNSCC Patients
2.1.2.2. High Serum MMP8 Level in Digestive System Cancers Predict Worse Prognosis
2.1.2.3. The Prognostic Value of Circulating MMP8 Levels in Other Cancers Requires More Studies
2.1.3. Genetics of MMP8 in Cancer
2.1.3.1. The SNP rs1122539 Protects from Breast and Bladder Cancer but Increases the Risk of Melanoma and Ovarian Cancer
2.1.3.2. Fluctuating Findings on the Effect of SNP rs1940475
2.1.3.3. Other MMP8 SNPs Also Decrease Cancer Risks
2.1.3.4. Studies on Somatic and Epigenetic Changes Strengthen the View of Active MMP8 as a Tumor-Suppressive Factor
2.2. In Vitro Experimental Evidence and In Vivo Mouse Studies Elucidate the Molecular Mechanisms of MMP8 in Cancers
2.2.1. Studies in Skin Cancer Paved the Way for the Idea of Tumor-Suppressive MMP8
2.2.2. MMP8 Has Fluctuating Expression Profiles in Breast Cancer Cells In Vitro, but Rather Consistent Tumor-Protective Effects In Vivo
2.2.3. Tumor-Protective Molecular Mechanisms of MMP8
2.2.4. Tumor-Promoting Molecular Mechanisms of MMP8
2.2.5. MMP8 Expression Is Not Detected at All in Some Cancer Cell Lines
2.3. The Potential of MMP8 in Cancer Treatment
2.3.1. Using MMP8 as a Pharmaceutical Adjuvant Shows Promising Results
2.3.2. Cancer Treatments Affect MMP8 Levels in Tumors
2.3.3. High MMP8 Levels Can Guide Treatment Choices and Indicate Harmful Post-Operative Reactions in Some Patients
2.3.4. MMP8 as an Anti-Target for MMP Inhibitors
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DFS | Disease-free survival |
| ECM | Extracellular matrix |
| EMT | Epithelial-mesenchymal transition |
| HNSCC | Head and neck squamous cell carcinoma |
| MMP | Matrix metalloproteinase |
| MMP8 | Matrix metalloproteinase 8 |
| MMPI | Matrix metalloproteinase inhibitor |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| OTSCC | Oral tongue squamous cell carcinoma |
| PMN | Polymorphonuclear neutrophils |
| RFS | Recurrence- or relapse-free survival |
| SCC | Squamous cell carcinoma |
| SNP | Single nucleotide polymorphism |
| TGF-β1 | Transforming growth factor β1 |
| TIMP-1 | Metalloproteinase inhibitor 1 |
| VEGF-C | Vascular endothelial growth factor C |
References
- Ahmad, A.S.; Ormiston-Smith, N.; Sasieni, P.D. Trends in the lifetime risk of developing cancer in Great Britain: Comparison of risk for those born from 1930 to 1960. Br. J. Cancer 2015, 112, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Wild, C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer 2019, 19, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Chie, W.; Chang, Y.; Chen, H. A novel method for evaluation of improved survival trend for common cancer: Early detection or improvement of medical care. J. Eval. Clin. Pract. 2007, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Benitez-Majano, S.; Muller, P.; Coleman, M.P.; Allemani, C.; Butler, J.; Peake, M.; Guren, M.G.; Glimelius, B.; Bergström, S.; et al. Is England closing the international gap in cancer survival? Br. J. Cancer 2015, 113, 848. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.A.; Weinstein, J.N. Biomarkers in Cancer Staging, Prognosis and Treatment Selection. Nat. Rev. Cancer 2005, 5, 845. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Nakagawa, S.; Sun, X.; Hoshida, Y. Cancer biomarker discovery and validation. Transl. Cancer Res. 2015, 4, 256–269. [Google Scholar]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.H.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Caner Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, Y.; Lee, E.; Park, J.; Seo, H.; Kim, H. Suppression of neuroinflammation by matrix metalloproteinase-8 inhibitor in aged normal and LRRK2 G2019S Parkinson’s disease model mice challenged with lipopolysaccharide. Biochem. Biophys. Res. Commun. 2017, 493, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.H.; Starr, A.E.; Kappelhoff, R.; Yan, R.; Roberts, C.R.; Overall, C.M. Matrix metalloproteinase 8 deficiency in mice exacerbates inflammatory arthritis through delayed neutrophil apoptosis and reduced caspase 11 expression. Arthritis Rheum. 2010, 62, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Quintero, J.; Owen, C. Matrix metalloproteinases in cystic fibrosis: Pathophysiologic and therapeutic perspectives. Met. Med. 2016, 3, 49–62. [Google Scholar]
- Van Lint, P.; Libert, C. Matrix metalloproteinase-8: Cleavage can be decisive. Cytokine Growth Factor Rev. 2006, 17, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ala-aho, R.; Kähäri, V. Collagenases in cancer. Biochimie 2005, 87, 273–286. [Google Scholar] [CrossRef]
- Kim, S.; Roh, J.; Park, C. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef]
- Ikeda, K.; Monden, T.; Kanoh, T.; Tsujie, M.; Izawa, H.; Haba, A.; Ohnishi, T.; Sekimoto, M.; Tomita, N.; Shiozaki, H.; et al. Extraction and Analysis of Diagnostically Useful Proteins from Formalin-fixed, Paraffin-embedded Tissue Sections. J. Histochem. Cytochem. 1998, 46, 397–403. [Google Scholar] [CrossRef]
- Kampf, C.; Olsson, I.; Ryberg, U.; Sjöstedt, E.; Pontén, F. Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J. Vis. Exp. JoVE 2012, 31, e3620. [Google Scholar] [CrossRef]
- Bernard, P.S.; Wittwer, C.T. Real-Time PCR Technology for Cancer Diagnostics. Clin. Chem. 2002, 48, 1178. [Google Scholar]
- Jensen, E. Technical Review: In Situ Hybridization. Anat. Rec. 2014, 297, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Wallard, M.J.; Pennington, C.J.; Veerakumarasivam, A.; Burtt, G.; Mills, I.G.; Warren, A.; Leung, H.Y.; Murphy, G.; Edwards, D.R.; Neal, D.E.; et al. Comprehensive profiling and localisation of the matrix metalloproteinases in urothelial carcinoma. Br. J. Cancer 2006, 94, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Korpi, J.T.; Hagström, J.; Lehtonen, N.; Parkkinen, J.; Sorsa, T.; Salo, T.; Laitinen, M. Expression of matrix metalloproteinases-2, -8, -13, -26, and tissue inhibitors of metalloproteinase-1 in human osteosarcoma. Surg. Oncol. 2011, 20, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.P.; Berend, K.R.; Qi, W.; Harrelson, J.M. Collagenase specificity in chondrosarcoma metastasis. Braz. J. Med. Biol. Res. 1999, 32, 885–889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benson, C.S.; Babu, S.D.; Radhakrishna, S.; Selvamurugan, N.; Sankar, B.R. Expression of matrix metalloproteinases in human breast cancer tissues. Dis. Markers 2013, 34, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, A.; Fueyo, A.; Folgueras, A.R.; Garabaya, C.; Pennington, C.J.; Pilgrim, S.; Edwards, D.R.; Holliday, D.L.; Jones, J.L.; Span, P.N.; et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008, 68, 2755–2763. [Google Scholar] [CrossRef]
- McGowan, P.M.; Duffy, M.J. Matrix metalloproteinase expression and outcome in patients with breast cancer: Analysis of a published database. Ann. Oncol. 2008, 19, 1566–1572. [Google Scholar] [CrossRef]
- Decock, J.; Hendrickx, W.; Drijkoningen, M.; Wildiers, H.; Neven, P.; Smeets, A.; Paridaens, R. Matrix metalloproteinase expression patterns in luminal A type breast carcinomas. Dis. Markers 2007, 23, 189–196. [Google Scholar] [CrossRef]
- Duffy, M.J.; Blaser, J.; Duggan, C.; McDermott, E.; O’Higgins, N.; Fennelly, J.J.; Tschesche, H. Assay of matrix metalloproteases types 8 and 9 by ELISA in human breast cancer. Br. J. Cancer 1995, 71, 1025–1028. [Google Scholar] [CrossRef]
- Koskensalo, S.; Hagström, J.; Linder, N.; Lundin, M.; Sorsa, T.; Louhimo, J.; Haglund, C. Lack of MMP-9 expression is a marker for poor prognosis in Dukes’ B colorectal cancer. BMC Clin. Pathol. 2012, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, J.P.; Vornanen, J.; Tervahartiala, T.; Sorsa, T.; Bloigu, R.; Salo, T.; Tuomisto, A.; Mäkinen, M.J. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. Int. J. Cancer 2012, 131, E463–E474. [Google Scholar] [CrossRef] [PubMed]
- Verspaget, H.W.; Kubben, F.J.G.M.; Tschesche, H.; Verheijen, J.H.; Hanemaaijer, R.; Lamers, C.B.H.W. Matrix metalloproteinases increase with colorectal cancer progression. Fibrinolysis Proteolysis 1999, 13, 38. [Google Scholar]
- Takeha, S.; Fujiyama, Y.; Bamba, T.; Sorsa, T.; Nagura, H.; Ohtani, H. Stromal expression of MMP-9 and urokinase receptor is inversely associated with liver metastasis and with infiltrating growth in human colorectal cancer: A novel approach from immune/inflammatory aspect. Jpn. J. Cancer Res. 1997, 88, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, A.; Hagström, J.; Mustonen, H.; Kokkola, A.; Tervahartiala, T.; Sorsa, T.; Böckelman, C.; Haglund, C. Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol. 2018, 40. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, J.; Jin, L.; Jiang, Y. Polymorphisms in matrix metalloproteinases 2, 3, and 8 increase recurrence and mortality risk by regulating enzyme activity in gastric adenocarcinoma. Oncotarget 2017, 8, 105971–105983. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, S.; Sampieri, C.L.; Ochoa-Lara, M.; León-Córdoba, K.; Remes-Troche, J.M. Expression of the matrix metalloproteases 2, 14, 24, and 25 and tissue inhibitor 3 as potential molecular markers in advanced human gastric cancer. Dis. Markers 2014, 2014, 285906. [Google Scholar] [CrossRef]
- Kubben, F.J.; Sier, C.F.; van Duijn, W.; Griffioen, G.; Hanemaaijer, R.; van de Velde, C.J.; van Krieken, J.H.; Lamers, C.B.; Verspaget, H.W. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br. J. Cancer 2006, 94, 1035–1040. [Google Scholar] [CrossRef]
- Åström, P.; Juurikka, K.; Hadler-Olsen, E.S.; Svineng, G.; Cervigne, N.K.; Coletta, R.D.; Risteli, J.; Kauppila, J.H.; Skarp, S.; Kuttner, S.; et al. The interplay of matrix metalloproteinase-8, transforming growth factor-β and vascular endothelial growth factor-C cooperatively contributes to the aggressiveness of oral tongue squamous cell carcinoma. Br. J. Cancer 2017, 117, 1007–1016. [Google Scholar] [CrossRef]
- Omar, A.A.; Haglund, C.; Virolainen, S.; Häyry, V.; Atula, T.; Kontio, R.; Salo, T.; Sorsa, T.; Hagström, J. MMP-7, MMP-8, and MMP-9 in oral and cutaneous squamous cell carcinomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 459–467. [Google Scholar] [CrossRef]
- Lawal, A.; Adisa, A.; Kolude, B.; Adeyemi, B. Immunohistochemical expression of MMP-2 and MMP-8 in oral squamous cell carcinoma. J. Clin. Exp. Dent. 2015, 7, e203–e207. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, L.K.; Häyry, V.; Atula, T.; Haglund, C.; Keski-Säntti, H.; Leivo, I.; Mäkitie, A.; Passador-Santos, F.; Böckelman, C.; Salo, T.; et al. Prognostic significance of matrix metalloproteinase-2, -8, -9, and -13 in oral tongue cancer. J. Oral Pathol. Med. 2012, 41, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Korampalli, T.S.; Green, V.L.; Greenman, J.; Stafford, N.D. Protein profiling of angiogenesis-related growth factorsin laryngeal carcinoma: Pattern of protein expressionin relation to tumour progression. Int. J. Oncol. 2011, 39, 1033–1039. [Google Scholar] [PubMed]
- Korpi, J.T.; Kervinen, V.; Mäklin, H.; Väänänen, A.; Lahtinen, M.; Läärä, E.; Ristimäki, A.; Thomas, G.; Ylipalosaari, M.; Åström, P.; et al. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br. J. Cancer 2008, 98, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, Y.; Li, Y. Expression of matrix metalloproteinases in supraglottic carcinoma and its clinical implication for estimating lymph node metastases. Laryngoscope 2004, 114, 2243–2248. [Google Scholar] [CrossRef]
- Kayano, K.; Shimada, T.; Shinomiya, T.; Nakai, S.; Hisa, Y.; Aoki, T.; Seiki, M.; Okada, Y. Activation of pro-MMP-2 mediated by MT1-MMP in human salivary gland carcinomas: Possible regulation of pro-MMP-2 activation by TIMP-2. J. Pathol. 2004, 202, 403–411. [Google Scholar] [CrossRef]
- Moilanen, M.; Pirilä, E.; Grénman, R.; Sorsa, T.; Salo, T. Expression and regulation of collagenase-2 (MMP-8) in head and neck squamous cell carcinomas. J. Pathol. 2002, 197, 72–81. [Google Scholar] [CrossRef]
- Qin, G.; Luo, M.; Chen, J.; Dang, Y.; Chen, G.; Li, L.; Zeng, J.; Lu, Y.; Yang, J. Reciprocal activation between MMP-8 and TGF-β stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 2016, 374, 85–95. [Google Scholar] [CrossRef]
- Shah, S.A.; Spinale, F.G.; Ikonomidis, J.S.; Stroud, R.E.; Chang, E.I.; Reed, C.E. Differential matrix metalloproteinase levels in adenocarcinoma and squamous cell carcinoma of the lung. J. Thorac. Cardiovasc. Surg. 2010, 139, 984–990. [Google Scholar] [CrossRef]
- Stadlmann, S.; Pollheimer, J.; Moser, P.L.; Raggi, A.; Amberger, A.; Margreiter, R.; Offner, F.A.; Mikuz, G.; Dirnhofer, S.; Moch, H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 2003, 39, 2499–2505. [Google Scholar] [CrossRef]
- Stenman, M.; Paju, A.; Hanemaaijer, R.; Tervahartiala, T.; Leminen, A.; Stenman, U.; Konttinen, Y.T.; Sorsa, T. Collagenases (MMP-1, -8 and -13) and trypsinogen-2 in fluid from benign and malignant ovarian cysts. Tumor Biol. 2003, 24, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ansari, D.; Pawłowski, K.; Zhou, Q.; Sasor, A.; Welinder, C.; Kristl, T.; Bauden, M.; Rezeli, M.; Jiang, Y. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma. Oncotarget 2018, 9, 9789–9807. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.E.; Humphreys, M.J.; Campbell, F.; Neoptolemos, J.P.; Boyd, M.T. Comprehensive Analysis of Matrix Metalloproteinase and Tissue Inhibitor Expression in Pancreatic Cancer: Increased Expression of Matrix Metalloproteinase-7 Predicts Poor Survival. Clin. Cancer Res. 2004, 10, 2832–2845. [Google Scholar] [CrossRef] [PubMed]
- Ciążyńska, M.; Bednarski, I.A.; Wódz, K.; Kolano, P.; Narbutt, J.; Sobjanek, M.; Woźniacka, A.; Lesiak, A. Proteins involved in cutaneous basal cell carcinoma development. Oncol. Lett. 2018, 16, 4064–4072. [Google Scholar] [CrossRef] [PubMed]
- Kuivanen, T.T.; Jeskanen, L.; Kyllönen, L.; Impola, U.; Saarialho-Kere, U.K. Transformation-specific matrix metalloproteinases, MMP-7 and MMP-13, are present in epithelial cells of keratoacanthomas. Mod. Pathol. 2006, 19, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Impola, U.; Jeskanen, L.; Ravanti, L.; Syrjänen, S.; Baldursson, B.; Kähäri, V.M.; Saarialho-Kere, U. Expression of matrix metalloproteinase (MMP)-7 and MMP-13 and loss of MMP-19 and p16 are associated with malignant progression in chronic wounds. Br. J. Dermatol. 2005, 152, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Giambernardi, T.A.; Sakaguchi, A.Y.; Gluhak, J.; Pavlin, D.; Troyer, D.A.; Das, G.; Rodeck, U.; Klebe, R.J. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol. 2001, 20, 577–587. [Google Scholar] [CrossRef]
- Varani, J.; Hattori, Y.; Chi, Y.; Schmidt, T.; Perone, P.; Zeigler, M.E.; Fader, D.J.; Johnson, T.M. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: Comparison with normal skin. Br. J. Cancer 2000, 82, 657–665. [Google Scholar] [CrossRef]
- Roebuck, M.M.; Helliwell, T.R.; Chaudhry, I.H.; Kalogrianitis, S.; Carter, S.; Kemp, G.J.; Ritchie, D.A.; Jane, M.J.; Frostick, S.P. Matrix metalloproteinase expression is related to angiogenesis and histologic grade in spindle cell soft tissue neoplasms of the extremities. Am. J. Clin. Pathol. 2005, 123, 405–414. [Google Scholar] [CrossRef]
- Kebebew, E.; Peng, M.; Reiff, E.; Duh, Q.; Clark, O.H.; McMillan, A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann. Surg. 2005, 242, 353–363. [Google Scholar] [CrossRef]
- Ueno, H.; Yamashita, K.; Azumano, I.; Inoue, M.; Okada, Y. Enhanced production and activation of matrix metalloproteinase-7 (matrilysin) in human endometrial carcinomas. Int. J. Cancer 1999, 84, 470–477. [Google Scholar] [CrossRef]
- Sarper, M.; Allen, M.D.; Gomm, J.; Haywood, L.; Decock, J.; Thirkettle, S.; Ustaoglu, A.; Sarker, S.J.; Marshall, J.; Edwards, D.R.; et al. Loss of MMP-8 in ductal carcinoma in situ (DCIS)-associated myoepithelial cells contributes to tumour promotion through altered adhesive and proteolytic function. Breast Cancer Res. 2017, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Duggan, C.; Maguire, T.; Mulcahy, K.; Elvin, P.; McDermott, E.; Fennelly, J.J.; O’Higgins, N. Urokinase plasminogen activator as a predictor of aggressive disease in breast cancer. Enzym. Protein 1996, 49, 85–93. [Google Scholar] [CrossRef]
- Kuivanen, T.; Jeskanen, L.; Kyllönen, L.; Isaka, K.; Saarialho-Kere, U. Matrix metalloproteinase-26 is present more frequently in squamous cell carcinomas of immunosuppressed compared with immunocompetent patients. J. Cutan. Pathol. 2009, 36, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Hardt, M.; Lam, D.K.; Dolan, J.C.; Schmidt, B.L. Surveying proteolytic processes in human cancer microenvironments by microdialysis and activity-based mass spectrometry. Proteom. Clin. Appl. 2011, 5, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, T.; Lont, T.; Hagström, J.; Tervahartiala, T.; Sorsa, T.; Haglund, C.; Munck-Wickland, E.; Ramqvist, T.; Dalianis, T.; Aaltonen, L.M. Systemic matrix metalloproteinase-8 response in chronic tonsillitis. Infect. Dis. 2017, 49, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Maisi, P.; Sorsa, T.; Sutinen, M.; Tervahartiala, T.; Pirilä, E.; Teronen, O.; Hietanen, J.; Tjäderhane, L.; Salo, T. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J. Pathol. 2001, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, T.; Jang, J.; Jang, Y.; Lee, K.; Lee, S. Analysis of matrix metalloproteinase mRNAs expressed in hepatocellular carcinoma cell lines. Mol. Cells 2001, 12, 32–40. [Google Scholar]
- Merkerova, M.; Klamova, H.; Brdicka, R.; Bruchova, H. Targeting of gene expression by siRNA in CML primary cells. Mol. Biol. Rep. 2007, 34, 27–33. [Google Scholar] [CrossRef]
- Bruchova, H.; Borovanova, T.; Klamova, H.; Brdicka, R. Gene expression profiling in chronic myeloid leukemia patients treated with hydroxyurea. Leuk. Lymphoma 2002, 43, 1289–1295. [Google Scholar] [CrossRef]
- Linkov, F.; Gu, Y.; Arslan, A.A.; Liu, M.; Shore, R.E.; Velikokhatnaya, L.; Koenig, K.L.; Toniolo, P.; Marrangoni, A.; Yurkovetsky, Z.; et al. Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. Eur. Cytokine Netw. 2009, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Zhang, W.; Liao, Y.; Chen, J.; Shi, Y.; Luo, S. Serum cytokine profile in patients with breast cancer. Cytokine 2017, 89, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Decock, J.; Hendrickx, W.; Vanleeuw, U.; van Belle, V.; van Huffel, S.; Christiaens, M.R.; Ye, S.; Paridaens, R. Plasma MMP1 and MMP8 expression in breast cancer: Protective role of MMP8 against lymph node metastasis. BMC Cancer 2008, 8, 77. [Google Scholar] [CrossRef]
- Böckelman, C.; Beilmann-Lehtonen, I.; Kaprio, T.; Koskensalo, S.; Tervahartiala, T.; Mustonen, H.; Stenman, U.H.; Sorsa, T.; Haglund, C. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer 2018, 18, 679. [Google Scholar] [CrossRef] [PubMed]
- Sirniö, P.; Tuomisto, A.; Tervahartiala, T.; Sorsa, T.; Klintrup, K.; Karhu, T.; Herzig, K.H.; Mäkelä, J.; Karttunen, T.J.; Salo, T.; et al. High-serum MMP-8 levels are associated with decreased survival and systemic inflammation in colorectal cancer. Br. J. Cancer 2018, 119, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Wang, X.L.; Li, S.B.; Yang, G.L.; Jin, S.; Gao, Z.Y.; Liu, X.B. Combined detection of IL-6 and IL-8 is beneficial to the diagnosis of early stage esophageal squamous cell cancer: A preliminary study based on the screening of serum markers using protein chips. OncoTargets Ther. 2018, 11, 5777–5787. [Google Scholar] [CrossRef] [PubMed]
- Nurmenniemi, S.; Koivula, M.K.; Nyberg, P.; Tervahartiala, T.; Sorsa, T.; Mattila, P.S.; Salo, T.; Risteli, J. Type I and III collagen degradation products in serum predict patient survival in head and neck squamous cell carcinoma. Oral Oncol. 2012, 48, 136–140. [Google Scholar] [CrossRef]
- Pradhan-Palikhe, P.; Vesterinen, T.; Tarkkanen, J.; Leivo, I.; Sorsa, T.; Salo, T.; Mattila, P.S. Plasma level of tissue inhibitor of matrix metalloproteinase-1 but not that of matrix metalloproteinase-8 predicts survival in head and neck squamous cell cancer. Oral Oncol. 2010, 46, 514–518. [Google Scholar] [CrossRef]
- Kuropkat, C.; Duenne, A.A.; Herz, U.; Renz, H.; Werner, J.A. Significant correlation of matrix metalloproteinase and macrophage colony-stimulating factor serum concentrations in patients with head and neck cancer. Neoplasma 2004, 51, 375–378. [Google Scholar]
- Kuropkat, C.; Plehn, S.; Herz, U.; Dünne, A.A.; Renz, H.; Werner, J.A. Tumor marker potential of serum matrix metalloproteinases in patients with head and neck cancer. Anticancer Res. 2002, 22, 2221–2227. [Google Scholar]
- Kołomecki, K.; Stepień, H.; Bartos, M.; Narebski, J. Evaluation of MMP-1, MMP-8, MMO-8, MMP-9 serum levels in patients with adrenal tumors prior to and after surgery. Neoplasma 2001, 48, 116–121. [Google Scholar] [PubMed]
- Lempinen, M.; Lyytinen, I.; Nordin, A.; Tervahartiala, T.; Mäkisalo, H.; Sorsa, T.; Isoniemi, H. Prognostic value of serum MMP-8, -9 and TIMP-1 in patients with hepatocellular carcinoma. Ann. Med. 2013, 45, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, P.; Koskivuo, I.; Syrjänen, K.; Tervahartiala, T.; Sorsa, T.; Pyrhönen, S. Serum matrix metalloproteinase-8 is associated with ulceration and vascular invasion of malignant melanoma. Melanoma Res. 2008, 18, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Gallelli, L.; Rende, P.; Scarcello, E.; Buffone, G.; Caliò, F.G.; Gasbarro, V.; Amato, B.; de Franciscis, S. Carotid body paragangliomas and matrix metalloproteinases. Ann. Vasc. Surg. 2014, 28, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Kang, E.S.; Kim, J.W.; Lee, K.T.; Lee, K.H.; Park, Y.S.; Park, J.O.; Lee, J.; Heo, J.S.; Choi, S.H.; et al. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics 2012, 12, 3590–3597. [Google Scholar] [CrossRef]
- Komorowski, J.; Pasieka, Z.; Jankiewicz-Wika, J.; Stȩpień, H. Matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases and angiogenic cytokines in peripheral blood of patients with thyroid cancer. Thyroid 2002, 12, 655–662. [Google Scholar] [CrossRef]
- Svatek, R.S.; Shah, J.B.; Xing, J.; Chang, D.; Lin, J.; McConkey, D.J.; Wu, X.; Dinney, C.P. A multiplexed, particle-based flow cytometric assay identified plasma matrix metalloproteinase-7 to be associated with cancer-related death among patients with bladder cancer. Cancer 2010, 116, 4513–4519. [Google Scholar] [CrossRef]
- Marín-Aguilera, M.; Reig, Ò.; Lozano, J.J.; Jiménez, N.; García-Recio, S.; Erill, N.; Gaba, L.; Tagliapietra, A.; Ortega, V.; Carrera, G.; et al. Molecular profiling of peripheral blood is associated with circulating tumor cells content and poor survival in metastatic castration-resistant prostate cancer. Oncotarget 2015, 6, 10604–10616. [Google Scholar] [CrossRef]
- Reichenberger, F.; Eickelberg, O.; Wyser, C.; Perruchoud, A.P.; Roth, M.; Tamm, M. Distinct endobronchial expression of matrix-metalloproteinases (MMP) and their endogenous inhibitors in lung cancer. Swiss Med. Wkly. 2001, 131, 273–279. [Google Scholar]
- Tsai, T.H.; Wang, Y.M.; Chang, W.S.; Tsai, C.W.; Wu, H.C.; Hsu, H.M.; Wang, Y.C.; Li, H.T.; Gong, C.L.; Bau, D.T.; et al. Association of Matrix Metalloproteinase-8 Genotypes with the Risk of Bladder Cancer. Anticancer Res. 2018, 38, 5159–5164. [Google Scholar] [CrossRef]
- Srivastava, P.; Kapoor, R.; Mittal, R.D. Association of single nucleotide polymorphisms in promoter of matrix metalloproteinase-2, 8 genes with bladder cancer risk in Northern India. Urol. Oncol. Semin. Orig. Invest. 2013, 31, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.K.; Liu, J.; Shao, L.; Dinney, C.P.; Lin, J.; Wang, Y.; Gu, J.; Grossman, H.B.; Wu, X. Matrix metalloproteinase polymorphisms are associated with bladder cancer invasiveness. Clin. Cancer Res. 2007, 13, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.K.; Shao, L.; Dinney, C.P.; Schabath, M.B.; Wang, Y.; Liu, J.; Gu, J.; Grossman, H.B.; Wu, X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006, 66, 11644–11648. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.L.; Liu, L.C.; Shih, T.C.; Chuang, C.L.; Chen, G.L.; Wang, H.C.; Pan, S.Y.; Shen, T.C.; Tsai, C.W.; Chang, W.S.; et al. The Association of Matrix Metalloproteinase-8 Promoter Genotypes in Breast Cancer. Anticancer Res. 2018, 38, 2181–2185. [Google Scholar] [PubMed]
- Beeghly-Fadiel, A.; Zheng, W.; Lu, W.; Long, J.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Cai, Q.; Gao, Y.T.; et al. Replication study for reported SNP associations with breast cancer survival. J. Cancer Res. Clin. Oncol. 2012, 138, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Debniak, T.; Jakubowska, A.; Serrano-Fernández, P.; Kurzawski, G.; Cybulski, C.; Chauhan, S.R.; Laxton, R.C.; Maleszka, R.; Lubinski, J.; Ye, S. Association of MMP8 gene variation with an increased risk of malignant melanoma. Melanoma Res. 2011, 21, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Dunning, A.M.; Ponder, B.A.J.; Easton, D.F.; Pharoah, P.D. Common genetic variation in candidate genes and susceptibility to subtypes of breast cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Decock, J.; Long, J.R.; Laxton, R.C.; Shu, X.O.; Hodgkinson, C.; Hendrickx, W.; Pearce, E.G.; Gao, Y.T.; Pereira, A.C.; Paridaens, R.; et al. Association of matrix metalloproteinase-8 gene variation with breast cancer prognosis. Cancer Res. 2007, 67, 10214–10221. [Google Scholar] [CrossRef] [PubMed]
- Kubben, F.J.; Sier, C.F.; Meijer, M.J.; van Den Berg, M.; van der Reijden, J.J.; Griffioen, G.; van de Velde, C.J.; Lamers, C.B.; Verspaget, H.W. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br. J. Cancer 2006, 95, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.W.; Tsai, C.W.; Wu, C.N.; Shih, L.C.; Chen, Y.Y.; Liu, Y.F.; Hung, H.S.; Shen, M.Y.; Chang, W.S.; Bau, D.T. The Contribution of Matrix Metalloproteinase-8 Promoter Polymorphism to Oral Cancer Susceptibility. In Vivo 2017, 31, 585–590. [Google Scholar]
- Liu, H.; Huang, P.Y.; Tang, L.Q.; Chen, Q.Y.; Zhang, Y.; Zhang, L.; Guo, L.; Luo, D.H.; Mo, H.Y.; Xiang, Y.Q.; et al. Functional polymorphisms of matrix metalloproteinase-9 and survival in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Med. Oncol. 2013, 30, 685. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.S.; Chang, W.S.; Hsu, P.C.; Hung, Y.W.; Cheng, S.P.; Tsai, C.W.; Bau, D.T.; Gong, C.L. The Contribution of MMP-8 Promoter Genotypes to Childhood Leukemia. In Vivo 2017, 31, 1059–1064. [Google Scholar] [PubMed]
- Qiu, W.; Zhou, G.; Zhai, Y.; Zhang, X.; Xie, W.; Zhang, H.; Yang, H.; Zhi, L.; Yuan, X.; Zhang, X.; et al. No association of MMP-7, MMP-8, and MMP-21 polymorphisms with the risk of hepatocellular carcinoma in a Chinese population. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.C.; Hsia, T.C.; Chao, C.Y.; Chen, W.C.; Chen, C.Y.; Chen, W.C.; Lin, Y.T.; Hsiao, C.L.; Chang, W.S.; Tsai, C.W.; et al. The Contribution of MMP-8 Promoter Polymorphisms in Lung Cancer. Anticancer Res. 2017, 37, 3563–3567. [Google Scholar] [PubMed]
- González-Arriaga, P.; López-Cima, M.F.; Fernández-Somoano, A.; Pascual, T.; Marrón, M.G.; Puente, X.S.; Tardón, A. Polymorphism +17 C/G in Matrix Metalloprotease MMP8 decreases lung cancer risk. BMC Cancer 2008, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Y.; Lin, J.; Meyer, L.; Wu, X.; Lu, K.; Liang, D. Genetic variants in matrix metalloproteinase genes as disposition factors for ovarian cancer risk, survival, and clinical outcome. Mol. Carcinog. 2015, 54, 430–439. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, F.; Cuevas-Antonio, R.; Dominguez-Lopez, P.; Estrada-Moscoso, I.; Imani-Razavi, F.S.; Zeferino-Toquero, M.; Diaz-Cueto, L. Matrix metalloproteinase-8 promoter gene polymorphisms in Mexican women with ovarian cancer. Med. Oncol. 2014, 31, 132. [Google Scholar] [CrossRef]
- Qiu, Z.; Hu, J.; Xu, H.; Wang, W.; Nie, C.; Wang, X. Generation of antitumor peptides by connection of matrix metalloproteinase-9 peptide inhibitor to an endostatin fragment. Anti-Cancer Drugs 2013, 24, 677–689. [Google Scholar] [CrossRef]
- Murugan, A.K.; Dong, J.; Xie, J.; Xing, M. Uncommon gnaq, MMP8, AKT3, EGFR, and PIK3R1 mutations in thyroid cancers. Endocr. Pathol. 2011, 22, 97–102. [Google Scholar] [CrossRef]
- McLaughlin, J.; Rella, J.; Bakan, A.; Kong, L.; Zhu, L.; Frederick, D.; Yende, S.; Ferrell, R.; Bahar, I.; Shapiro, S.; et al. Impact of pro-domain stability of matrix metalloproteinase-8 on the outcome of sepsis. Critical Care 2011, 15, P278. [Google Scholar] [CrossRef][Green Version]
- Nan, H.; Niu, T.; Hunter, D.J.; Han, J. Missense polymorphisms in matrix metalloproteinase genes and skin cancer risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Palavalli, L.H.; Prickett, T.D.; Wunderlich, J.R.; Wei, X.; Burrell, A.S.; Porter-Gill, P.; Davis, S.; Wang, C.; Cronin, J.C.; Agrawal, N.S.; et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat. Genet. 2009, 41, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.V.; Baranovskaya, S.; Golubkov, V.S.; Wakeman, D.R.; Snyder, E.Y.; Williams, R.; Strongin, A.Y. Microarray-based transcriptional and epigenetic profiling of matrix metalloproteinases, collagens, and related genes in cancer. J. Biol. Chem. 2010, 285, 19647–19659. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, W. MicroRNA-539 suppresses osteosarcoma cell invasion and migration in vitro and targeting Matrix metallopeptidase-8. Int. J. Clin. Exp. Pathol. 2015, 8, 8075–8082. [Google Scholar] [PubMed]
- Giricz, O.; Lauer, J.L.; Fields, G.B. Comparison of metalloproteinase protein and activity profiling. Anal. Biochem. 2011, 409, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Nerlich, A.G.; Boukamp, P.; Lichtinghagen, R.; Tschesche, H.; Fritz, H.; Fink, E. Human keratinocyte cell lines differ in the expression of the collagenolytic matrix metalloproteinases-1,-8, and -13 and of TIMP-1. Biol. Chem. 2000, 381, 509–516. [Google Scholar] [PubMed]
- Balbín, M.; Fueyo, A.; Tester, A.M.; Pendás, A.M.; Pitiot, A.S.; Astudillo, A.; Overall, C.M.; Shapiro, S.D.; López-Otín, C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 2003, 35, 252–257. [Google Scholar] [CrossRef]
- Kotula, E.; Berthault, N.; Agrario, C.; Lienafa, M.C.; Simon, A.; Dingli, F.; Loew, D.; Sibut, V.; Saule, S.; Dutreix, M. DNA-PKcs plays role in cancer metastasis through regulation of secreted proteins involved in migration and invasion. Cell Cycle 2015, 14, 1961–1972. [Google Scholar] [CrossRef]
- Van Deventer, H.W.; Wu, Q.P.; Bergstralh, D.T.; Davis, B.K.; O’Connor, B.P.; Ting, J.P.; Serody, J.S. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. Am. J. Pathol. 2008, 173, 253–264. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.G.; Lichtinghagen, R.; Sommerhoff, C.P. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001, 21, 3821–3828. [Google Scholar]
- Bachmeier, B.E.; Albini, A.; Vené, R.; Benelli, R.; Noonan, D.; Weigert, C.; Weiler, C.; Lichtinghagen, R.; Jochum, M.; Nerlich, A.G. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp. Cell Res. 2005, 305, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, J.E.; Staren, E.D.; Appert, H.E. Matrix metalloproteinase expression in breast cancer. J. Surg. Res. 2003, 110, 383–392. [Google Scholar] [CrossRef]
- Thirkettle, S.; Decock, J.; Arnold, H.; Pennington, C.J.; Jaworski, D.M.; Edwards, D.R. Matrix metalloproteinase 8 (collagenase 2) induces the expression of interleukins 6 and 8 in breast cancer cells. J. Biol. Chem. 2013, 288, 16282–16294. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Goodison, S.; Nicholson, B.; Tarin, D.; Urquidi, V. Expression of matrix metalloproteinase 8 (MMP-8) and tyrosinase-related protein-1 (TYRP-1) correlates with the absence of metastasis in an isogenic human breast cancer model. Differentiation 2003, 71, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.M.; Anuszkiewicz, B.; Mentlein, R.; Yoneda, T.; Mehdorn, H.M.; Held-Feindt, J. Differential expression of matrix metalloproteinases in brain- and bone-seeking clones of metastatic MDA-MB-231 breast cancer cells. J. Neuro-Oncol. 2007, 81, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Koellensperger, E.; Bonnert, L.C.; Zoernig, I.; Marmé, F.; Sandmann, S.; Germann, G.; Gramley, F.; Leimer, U. The impact of human adipose tissue-derived stem cells on breast cancer cells: Implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017, 8, 121. [Google Scholar] [CrossRef]
- Montel, V.; Kleeman, J.; Agarwal, D.; Spinella, D.; Kawai, K.; Tarin, D. Altered Metastatic Behavior of Human Breast Cancer Cells after Experimental Manipulation of Matrix Metalloproteinase 8 Gene Expression. Cancer Res. 2004, 64, 1687–1694. [Google Scholar] [CrossRef]
- Decock, J.; Hendrickx, W.; Thirkettle, S.; Gutiérrez-Fernández, A.; Robinson, S.D.; Edwards, D.R. Pleiotropic functions of the tumor- and metastasis-suppressing matrix metalloproteinase-8 in mammary cancer in MMTV-PyMT transgenic mice. Breast Cancer Res. 2015, 17, 38. [Google Scholar] [CrossRef]
- Soria-Valles, C.; Gutiérrez-Fernández, A.; Guiu, M.; Mari, B.; Fueyo, A.; Gomis, R.R.; López-Otín, C. The anti-metastatic activity of collagenase-2 in breast cancer cells is mediated by a signaling pathway involving decorin and miR-21. Oncogene 2014, 33, 3054–3063. [Google Scholar] [CrossRef]
- Merrell, M.A.; Ilvesaro, J.M.; Lehtonen, N.; Sorsa, T.; Gehrs, B.; Rosenthal, E.; Chen, D.; Shackley, B.; Harris, K.W.; Selander, K.S. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol. Cancer Res. 2006, 4, 437–447. [Google Scholar] [CrossRef]
- Binder, C.; Hagemann, T.; Husen, B.; Schulz, M.; Einspanier, A. Relaxin enhances in-vitro invasiveness of breast cancer cell lines by up-regulation of matrix metalloproteases. Mol. Hum. Reprod. 2002, 8, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Sabeh, F.; Ota, I.; Holmbeck, K.; Birkedal-Hansen, H.; Soloway, P.; Balbin, M.; Lopez-Otin, C.; Shapiro, S.; Inada, M.; Krane, S.; et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004, 167, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Ramanujum, R.; Lin, Y.; Liu, J.; He, S. Regulatory expression of MMP-8/MMP-9 and inhibition of proliferation, migration and invasion in human lung cancer A549 cells in the presence of HGF variants. Kaohsiung J. Med. Sci. 2013, 29, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Miki, Y.; Ishida, N.; Inoue, C.; Kobayashi, M.; Hata, S.; Yamada-Okabe, H.; Okada, Y.; Sasano, H. The Significance of MMP-1 in EGFR-TKI-Resistant Lung Adenocarcinoma: Potential for Therapeutic Targeting. Int. J. Mol. Sci. 2018, 19, 609. [Google Scholar] [CrossRef] [PubMed]
- Pellinen, T.; Rantala, J.K.; Arjonen, A.; Mpindi, J.; Kallioniemi, O.; Ivaska, J. A functional genetic screen reveals new regulators of β1-integrin activity. J. Cell Sci. 2012, 125, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Ylipalosaari, M.; Thomas, G.J.; Nystrom, M.; Salhimi, S.; Marshall, J.F.; Huotari, V.; Tervahartiala, T.; Sorsa, T.; Salo, T. αvβ6 integrin down-regulates the MMP-13 expression in oral squamous cell carcinoma cells. Exp. Cell Res. 2005, 309, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, S.; Turner, C.J.; Adams, R.H. Regulation of Angiogenesis by Eph-Ephrin Interactions. Trends Cardiovasc. Med. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Tanaka, M.; Sasaki, K.; Kamata, R.; Sakai, R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J. Cell Sci. 2007, 120, 2179–2189. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamata, R.; Yanagihara, K.; Sakai, R. Suppression of gastric cancer dissemination by ephrin-B1-derived peptide. Cancer Sci. 2010, 101, 87–93. [Google Scholar] [CrossRef]
- Pirilä, E.; Sharabi, A.; Salo, T.; Quaranta, V.; Tu, H.; Heljasvaara, R.; Koshikawa, N.; Sorsa, T.; Maisi, P. Matrix metalloproteinases process the laminin-5 γ 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 2003, 303, 1012–1017. [Google Scholar] [CrossRef]
- Van Valckenborgh, E.; Croucher, P.I.; de Raeve, H.; Carron, C.; de Leenheer, E.; Blacher, S.; Devy, L.; Noël, A.; De Bruyne, E.; Asosingh, K.; et al. Multifunctional role of matrix metalloproteinases in multiple myeloma: A study in the 5T2MM mouse model. Am. J. Pathol. 2004, 165, 869–878. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Hsiung, S.C.; Yeh, C.T.; Yen, C.F.; Chou, Y.H.; Lei, W.Y.; Pang, S.T.; Chuang, C.K.; Liao, S.K. Differential expression of CD44 and CD24 markers discriminates the epitheliod from the fibroblastoid subset in a sarcomatoid renal carcinoma cell line: Evidence suggesting the existence of cancer stem cells in both subsets as studied with sorted cells. Oncotarget 2017, 8, 15593–15609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, C.H.; Park, H.J.; Lee, J.R.; Kim, H.K.; Jeon, T.Y.; Jo, H.J.; Kim, D.H.; Kim, G.H.; Park, D.Y. Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. Br. J. Cancer 2014, 111, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Singh, R.; Singh, S.; Johnson-Holiday, C.M.; Grizzle, W.E.; Partridge, E.E.; Lillard, J.W. CCL25-CCR9 interaction modulates ovarian cancer cell migration, metalloproteinase expression, and invasion. World J. Surg. Oncol. 2010, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, U.P.; Stiles, J.K.; Grizzle, W.E.; Lillard, J.W., Jr. Expression and functional role of CCR9 in prostate cancer cell migration and invasion. Clin. Cancer Res. 2004, 10, 8743–8750. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Huang, W.; Huang, G.; Chen, T.; Lin, M. Up-regulation of matrix metalloproteinase-8 by betel quid extract and arecoline and its role in 2D motility. Oral Oncol. 2007, 43, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, M.; Sorsa, T.; Stenman, M.; Nyberg, P.; Lindy, O.; Vesterinen, J.; Paju, A.; Konttinen, Y.T.; Stenman, U.H.; Salo, T. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry 2003, 42, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.; Heikkilä, P.; Sorsa, T.; Luostarinen, J.; Heljasvaara, R.; Stenman, U.H.; Pihlajaniemi, T.; Salo, T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J. Biol. Chem. 2003, 278, 22404–22411. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, A.; Tsuji, M.; Nishitani, M.; Kanda, K.; Inoue, Y.; Kanayama, H.O.; Kagawa, S. Role of the matrix metalloproteinase and tissue inhibitors of metalloproteinase families in noninvasive and invasive tumors transplanted in mice with severe combined immunodeficiency. Urology 1998, 51, 849–853. [Google Scholar] [CrossRef]
- Scotlandi, K.; Benini, S.; Manara, M.C.; Serra, M.; Nanni, P.; Lollini, P.L.; Nicoletti, G.; Landuzzi, L.; Chano, T.; Picci, P. Murine model for skeletal metastases of Ewing’s sarcoma. J. Orthop. Res. 2000, 18, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dutton, C.M.; Qi, W.N.; Block, J.A.; Brodt, P.; Durko, M.; Scully, S.P. Inhibition of MMP-1 expression by antisense RNA decreases invasiveness of human chondrosarcoma. J. Orthop. Res. 2003, 21, 1063–1070. [Google Scholar] [CrossRef]
- Bialek, J.; Kunanuvat, U.; Hombach-Klonisch, S.; Spens, A.; Stetefeld, J.; Sunley, K.; Lippert, D.; Wilkins, J.A.; Hoang-Vu, C.; Klonisch, T. Relaxin enhances the collagenolytic activity and in vitro invasiveness by upregulating matrix metalloproteinases in human thyroid carcinoma cells. Mol. Cancer Res. 2011, 9, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Tjomsland, V.; Pomianowska, E.; Aasrum, M.; Sandnes, D.; Verbeke, C.S.; Gladhaug, I.P. Profile of MMP and TIMP Expression in Human Pancreatic Stellate Cells: Regulation by IL-1α and TGFβ and Implications for Migration of Pancreatic Cancer Cells. Neoplasia 2016, 18, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, S.L.; Doucet, M.; Thorpe, M.; Weber, K.L. MMP-13 is over-expressed in renal cell carcinoma bone metastasis and is induced by TGF-β1. Clin. Exp. Metastasis 2008, 25, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Partridge, J.J.; Madsen, M.A.; Ardi, V.C.; Papagiannakopoulos, T.; Kupriyanova, T.A.; Quigley, J.P.; Deryugina, E.I. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J. Biol. Chem. 2007, 282, 35964–35977. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sauthoff, H.; Huang, Y.; Kutler, D.I.; Bajwa, S.; Rom, W.N.; Hay, J.G. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol. Ther. 2007, 15, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Mok, W.; Boucher, Y.; Jain, R.K. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007, 67, 10664–10668. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Goins, B.A.; Cameron, I.L.; Santoyo, C.; Bao, A.; Frohlich, V.C.; Fullerton, G.D. Ultrasound-guided intratumoral administration of collagenase-2 improved liposome drug accumulation in solid tumor xenografts. Cancer Chemother. Pharmacol. 2011, 67, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Wang, W.; Qiu, X.; Zhang, F.; TYustein, J.; Cameron, A.G.; Zhang, S.; Yu, D.; Zou, C.; Gao, X.; et al. Multiple target-specific molecular agents for detection and image analysis of breast cancer characteristics in mice. Curr. Mol. Med. 2013, 13, 446–458. [Google Scholar] [PubMed]
- Kim, M.H.; Gutierrez, A.M.; Goldfarb, R.H. Different mechanisms of soy isoflavones in cell cycle regulation and inhibition of invasion. Anticancer Res. 2002, 22, 3811–3817. [Google Scholar] [PubMed]
- Kim, M.H.; Albertsson, P.; Xue, Y.; Nannmark, U.; Kitson, R.P.; Goldfarb, R.H. Expression of neutrophil collagenase (MMP-8) in Jurkat T leukemia cells and its role in invasion. Anticancer Res. 2001, 21, 45–50. [Google Scholar] [PubMed]
- Kim, B.R.; Jeon, Y.K.; Nam, M.J. A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2011, 49, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Chai, H.; Cui, J.; Yao, J.; Ma, L.; Gao, W. Antitumor and anti-metastatic mechanisms of Rhizoma paridis saponins in Lewis mice. Environ. Toxicol. 2018, 33, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef]
- Musrati, A.A.; Tervahartiala, T.; Gürsoy, M.; Könönen, E.; Fteita, D.; Sorsa, T.; Uitto, V.J.; Gürsoy, U.K. Human neutrophil peptide-1 affects matrix metalloproteinase-2, -8 and -9 secretions of oral squamous cell carcinoma cell lines in vitro. Arch. Oral Biol. 2016, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Peters, C.; Ficnar, J.; Anlasik, S.; Bünemann, E.; Slotty, P.J.; Hänggi, D.; Steiger, H.J.; Sorg, R.V.; Stummer, W. Modulation of migratory activity and invasiveness of human glioma spheroids following 5-aminolevulinic acid-based photodynamic treatment. J. Neurosurg. 2011, 115, 281–288. [Google Scholar] [CrossRef] [PubMed]
- De Franciscis, S.; Grande, R.; Butrico, L.; Buffone, G.; Gallelli, L.; Scarcello, E.; Calio, F.G.; de Vito, D.; Compagna, R.; Amato, M.; et al. Resection of carotid body tumors reduces arterial blood pressure. An underestimated neuroendocrine syndrome. Int. J. Surg. 2014, 12, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.; Ko, J.; Rini, B.; Rayman, P.; Ireland, J.; Cohen, P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int. Immunopharmacol. 2011, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M.; Yamada, T.; Miki, T.; Sakai, T. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef]
- Reel, B.; Korkmaz, C.G.; Arun, M.Z.; Yildirim, G.; Ogut, D.; Kaymak, A.; Micili, S.C.; Ergur, B.U. The Regulation of Matrix Metalloproteinase Expression and the Role of Discoidin Domain Receptor 1/2 Signalling in Zoledronate-treated PC3 Cells. J. Cancer 2015, 6, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ohara, N.; Liu, J.; Amano, M.; Sitruk-Ware, R.; Yoshida, S.; Maruo, T. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol. Hum. Reprod. 2008, 14, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Tolvanen, K.; Virolainen, S.; Kuivanen, T.; Kyllönen, L.; Saarialho-Kere, U. Differential expression of stromal MMP-1, MMP-9 and TIMP-1 in basal cell carcinomas of immunosuppressed patients and controls. Virchows Arch. 2008, 452, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Vihinen, P.; Tervahartiala, T.; Sorsa, T.; Hansson, J.; Bastholt, L.; Aamdal, S.; Stierner, U.; Pyrhönen, S.; Syrjänen, K.; Lundin, J.; et al. Benefit of adjuvant interferon alfa-2b (IFN-α) therapy in melanoma patients with high serum MMP-8 levels. Cancer Immunol. Immunother. 2014, 64, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, M.; van Lint, P.; van Laere, I.; Wielockx, B.; Wilson, C.; López-Otin, C.; Shapiro, S.; Libert, C. Involvement of specific matrix metalloproteinases during tumor necrosis factor/IFNγ-based cancer therapy in mice. Mol. Cancer Ther. 2007, 6, 2563–2571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.; Wu, C.; Huang, S.; Wu, L.S. Polymorphisms of matrix metalloproteinases and their association with metastasis and the efficacy of androgen-deprivation therapy for prostate cancer in Taiwanese men. Urol. Sci. 2015, 26, 259–266. [Google Scholar] [CrossRef]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Völker, U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J. Proteom. 2015, 125, 98–103. [Google Scholar] [CrossRef]
- Pasternak, B.; Matthiessen, P.; Jansson, K.; Andersson, M.; Aspenberg, P. Elevated intraperitoneal matrix metalloproteinases-8 and -9 in patients who develop anastomotic leakage after rectal cancer surgery: A pilot study. Colorectal Dis. 2010, 12, e93–e98. [Google Scholar] [CrossRef]
- Davies, B.; Brown, P.D.; East, N.; Crimmin, M.J.; Balkwill, F.R. A Synthetic Matrix Metalloproteinase Inhibitor Decreases Tumor Burden and Prolongs Survival of Mice Bearing Human Ovarian Carcinoma Xenografts. Cancer Res. 1993, 53, 2087. [Google Scholar]
- Levitt, N.C.; Eskens, F.A.; O’Byrne, K.J.; Propper, D.J.; Denis, L.J.; Owen, S.J.; Choi, L.; Foekens, J.A.; Wilner, S.; Wood, J.M.; et al. Phase I and pharmacological study of the oral matrix metalloproteinase inhibitor, MMI270 (CGS27023A), in patients with advanced solid cancer. Clin. Cancer Res. 2001, 7, 1912–1922. [Google Scholar]
- Dive, V.; Andarawewa, K.L.; Boulay, A.; Matziari, M.; Beau, F.; Guerin, E.; Rousseau, B.; Yiotakis, A.; Rio, M.C. Dosing and scheduling influence the antitumor efficacy of a phosphinic peptide inhibitor of matrix metalloproteinases. Int. J. Cancer 2005, 113, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Scoffafava, A.; Supuran, C. Carbonic Anhydrase and Matrix Metalloproteinase Inhibitors: Sulfonylated Amino Acid Hydroxamates with MMP Inhibitory Properties Act as Efficient Inhibitors of CA Isozymes I, II, and IV, and N-Hydroxysulfonamides Inhibit Both These Zinc Enzymes. J. Med. Chem. 2000, 43, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Teronen, O.; Heikkilä, P.; Konttinen, Y.T.; Laitinen, M.; Salo, T.; Hanemaaijer, R.; Teronen, A.; Maisi, P.; Sorsa, T. MMP Inhibition and Downregulation by Bisphosphonates. Ann. NY Acad. Sci. 1999, 878, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.P.; Villamil, C.I.; Barta, T.E.; Bedell, L.J.; Boehm, T.L.; DeCrescenzo, G.A.; Freskos, J.N.; Getman, D.P.; Hockerman, S.; Heintz, R.; et al. Synthesis and structure-activity relationships of β- and α-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J. Med. Chem. 2005, 48, 6713–6730. [Google Scholar] [CrossRef] [PubMed]
- Reich, R.; Katz, Y.; Hadar, R.; Breuer, E. Carbamoylphosphonate matrix metalloproteinase inhibitors 3: In vivo evaluation of cyclopentylcarbamoylphosphonic acid in experimental metastasis and angiogenesis. Clin. Cancer Res. 2005, 11, 3925–3929. [Google Scholar] [CrossRef] [PubMed]
- Breuer, E.; Salomon, C.J.; Katz, Y.; Chen, W.; Lu, S.; Röschenthaler, G.V.; Hadar, R.; Reich, R. Carbamoylphosphonates, a new class of in vivo active matrix metalloproteinase inhibitors. 1. Alkyl- and cycloalkylcarbamoylphosphonic acids. J. Med. Chem. 2004, 47, 2826–2832. [Google Scholar] [CrossRef]
- Rubino, M.T.; Agamennone, M.; Campestre, C.; Campiglia, P.; Cremasco, V.; Faccio, R.; Laghezza, A.; Loiodice, F.; Maggi, D.; Panza, E.; et al. Biphenyl Sulfonylamino Methyl Bisphosphonic Acids as Inhibitors of Matrix Metalloproteinases and Bone Resorption. ChemMedChem 2011, 6, 1258–1268. [Google Scholar] [CrossRef]
- Matziari, M.; Beau, F.; Cuniasse, P.; Dive, V.; Yiotakis, A. Evaluation of P1′-Diversified Phosphinic Peptides Leads to the Development of Highly Selective Inhibitors of MMP-11. J. Med. Chem. 2004, 47, 325–336. [Google Scholar] [CrossRef]
- Wang, J.; Radomski, M.W.; Medina, C.; Gilmer, J.F. MMP inhibition by barbiturate homodimers. Bioorganic Med. Chem. Lett. 2013, 23, 444–447. [Google Scholar] [CrossRef]
- Tauro, M.; Laghezza, A.; Loiodice, F.; Agamennone, M.; Campestre, C.; Tortorella, P. Arylamino methylene bisphosphonate derivatives as bone seeking matrix metalloproteinase inhibitors. Bioorganic Med. Chem. 2013, 21, 6456–6465. [Google Scholar] [CrossRef]
- Von Reedern, E.G.; Grams, F.; Brandstetter, H.; Moroder, L. Design and synthesis of malonic acid-based inhibitors of human neutrophil collagenase (MMP8). J. Med. Chem. 1998, 41, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Suojanen, J.; Salo, T.; Koivunen, E.; Sorsa, T.; Pirilä, E. A novel and selective membrane type-1 matrix metalloproteinase (MT1-MMp) inhibitor reduces cancer cell motility and tumor growth. Cancer Biol. Ther. 2009, 8, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Nuti, E.; Casalini, F.; Santamaria, S.; Gabelloni, P.; Bendinelli, S.; da Pozzo, E.; Costa, B.; Marinelli, L.; la Pietra, V.; Novellino, E.; et al. Synthesis and biological evaluation in U87MG glioma cells of (ethynylthiophene)sulfonamido-based hydroxamates as matrix metalloproteinase inhibitors. Eur. J. Med. Chem. 2011, 46, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Erić, S.; Ke, S.; Barata, T.; Solmajer, T.; Stanković, J.A.; Juranić, Z.; Savić, V.; Zloh, M. Target fishing and docking studies of the novel derivatives of aryl-aminopyridines with potential anticancer activity. Bioorganic Med. Chem. 2012, 20, 5220–5228. [Google Scholar] [CrossRef] [PubMed]
- Fainaru, O.; Pencovich, N.; Hantisteanu, S.; Yona, G.; Hallak, M. Immature myeloid cells derived from mouse placentas and malignant tumors demonstrate similar proangiogenic transcriptional signatures. Fertil. Steril. 2013, 99, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yan, M.; Pu, C.; Wang, J.; van den Steen, P.E.; Opdenakker, G.; Xu, H. Chemically synthesized matrix metalloproteinase and angiogenesis-inhibiting peptides as anticancer agents. Anti-Cancer Agents Med. Chem. 2014, 14, 483–494. [Google Scholar] [CrossRef]
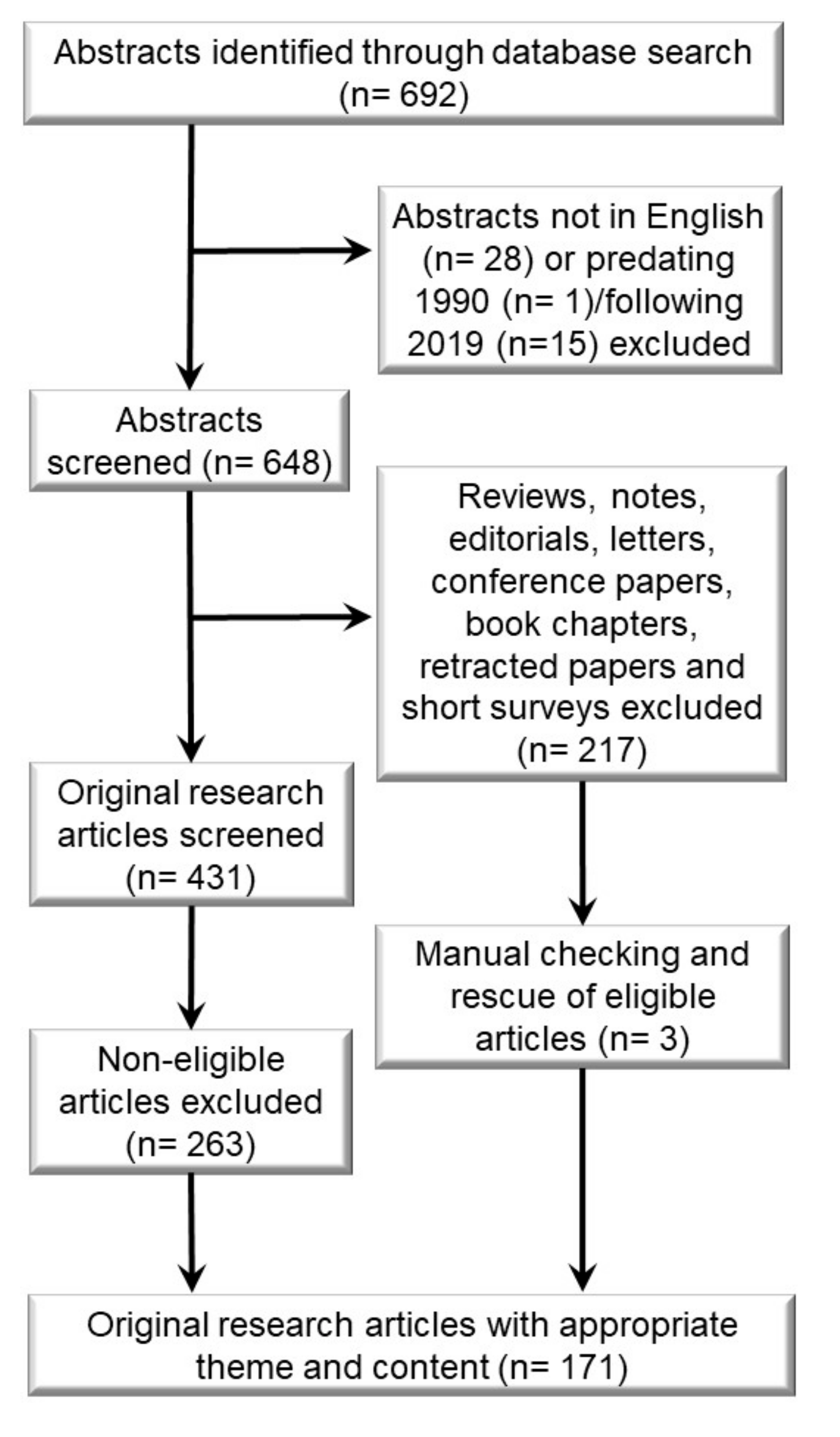
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
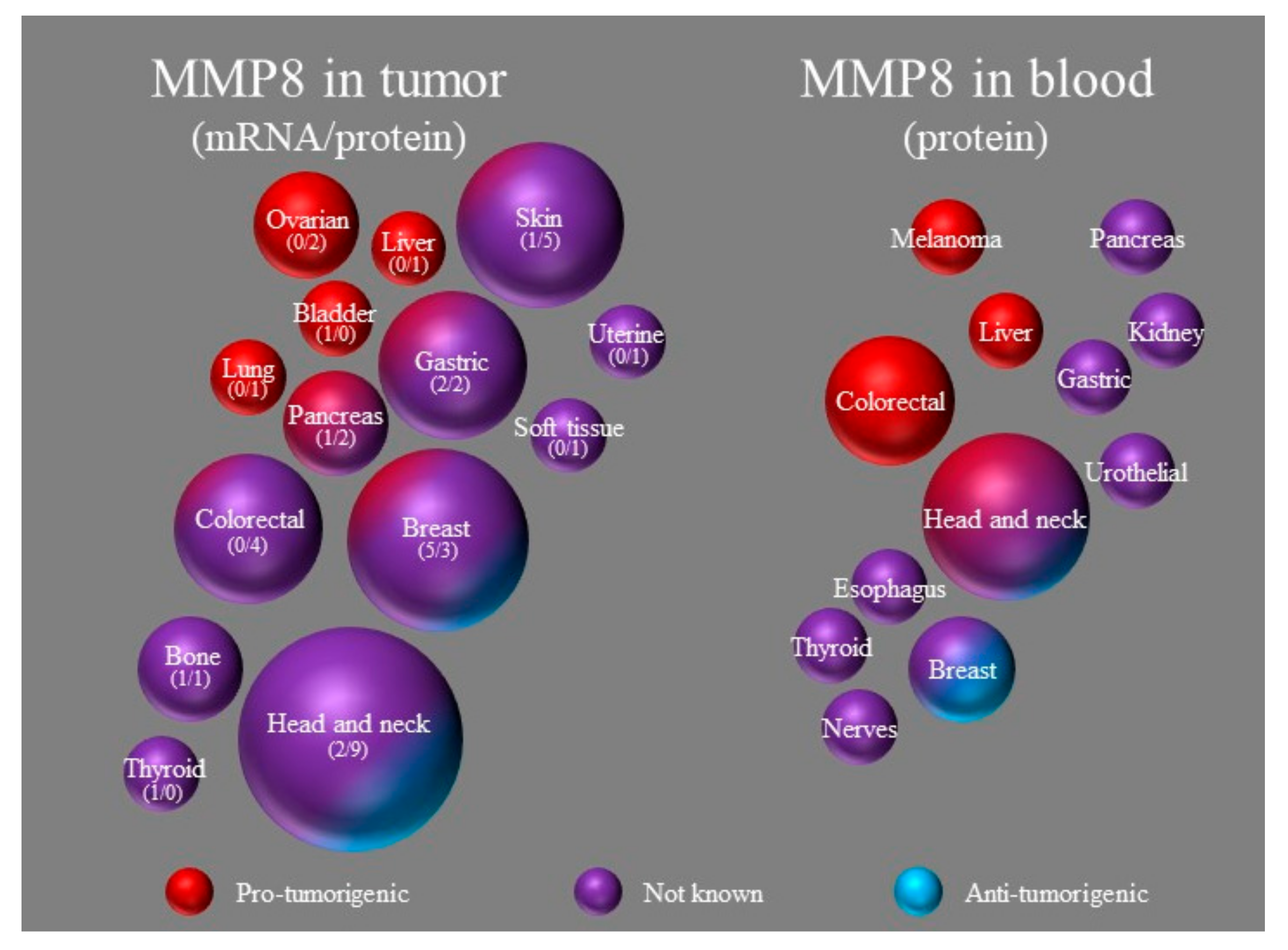
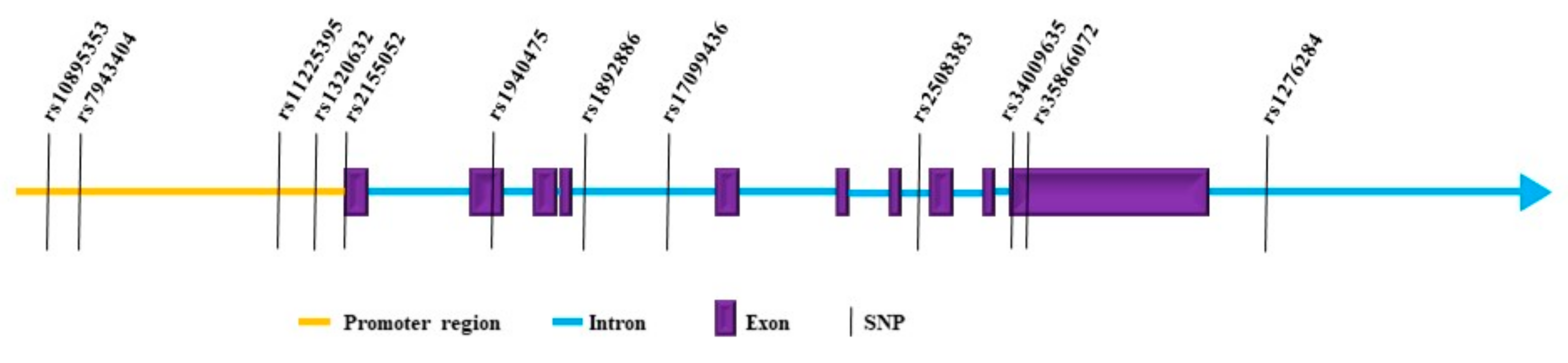
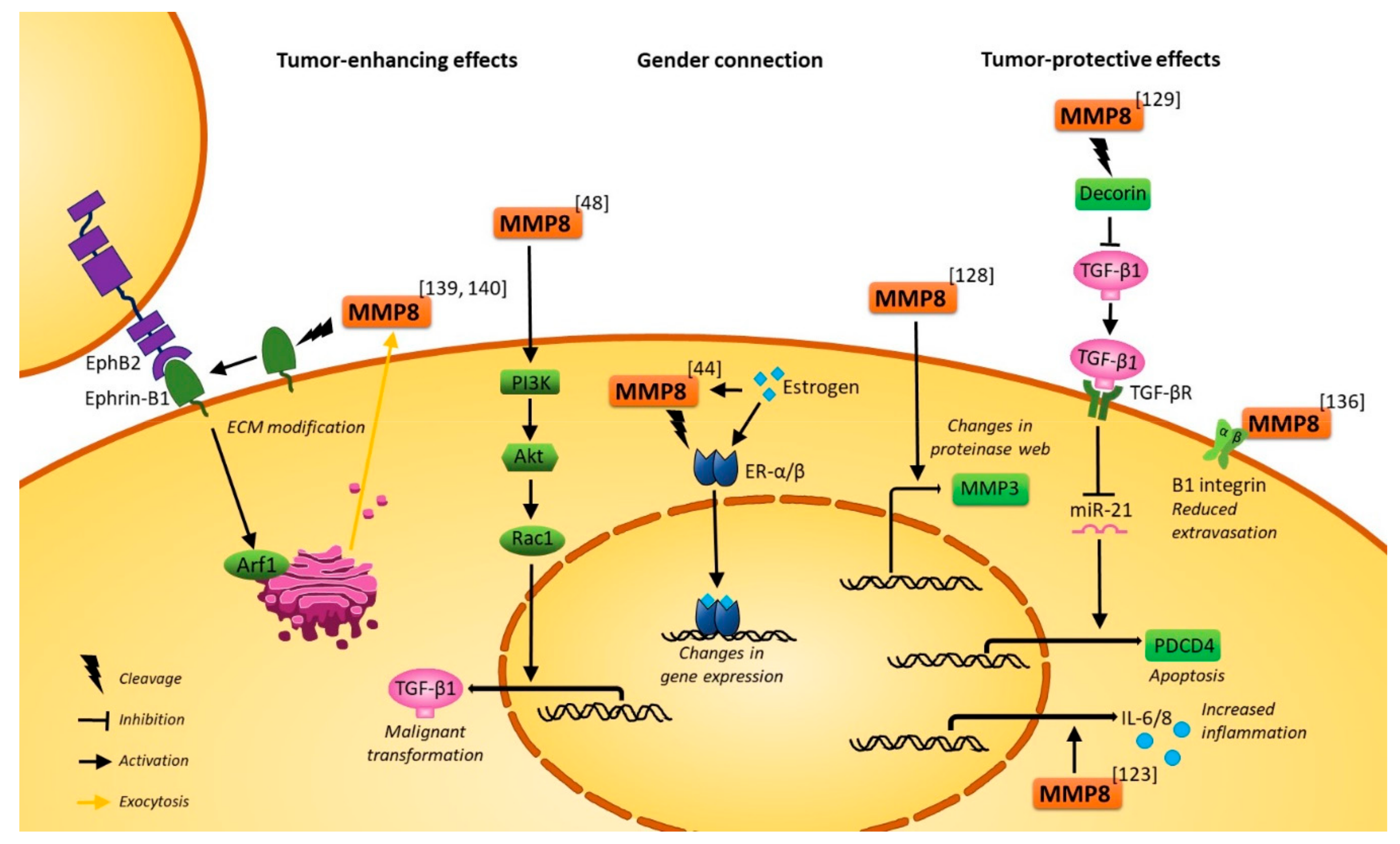
| Cancer | Focus | Method | Study Size (Patients + Healthy Controls) | Expression and Prognosis (p-Value) | Authors |
|---|---|---|---|---|---|
| Bladder | mRNA | RT-PCR | 113 + 20 | Positively correlates with tumor grade (p < 0.001). | Wallard et al. 2006 [22] |
| Bone | Protein | IHC | 25 + 0 | 5/10 resection samples and 11/22 biopsies but 0/3 metastases show MMP8 staining. | Korpi et al. 2011 [23] |
| mRNA | RT-PCR | 29 + 0 | 5/29 chondrosarcoma tumors show expression. | Scully et al. 1999 [24] | |
| Breast | mRNA | RT-PCR | 39 + 16 | Trend to positive correlation with tumor grade (ns). | Benson et al. 2013 [25] |
| Protein, mRNA | RT-PCR, WB, IHC | 20 + 5 | No difference in mRNA. Protein correlates with stage (p ≤ 0.05). | Köhrmann et al. 2009 [26] | |
| Protein, mRNA | IHC, RT-PCR | 280 IHC, 250 RT-PCR + 10 | mRNA expression negatively correlates with LN involvement (p = 0.006). Better survival of patients without adjuvant therapy (p = 0.009). | Gutierrez-Fernandez et al. 2008 [27] | |
| mRNA | Microarray | 295 + 0 | No correlation to clinicopathological features. | McGowan & Duffy 2008 [28] | |
| Luminal A; mRNA | RT-PCR | 25 + 0 | No correlation to clinicopathological features. | Decock et al. 2007 [29] | |
| Protein | ELISA | 55 + 0 | No correlation to tumor size or LN metastasis. | Duffy et al. 1995 [30] | |
| Colorectal | Protein | IHC | 548 + 0 | No correlation with clinicopathological features or prognosis. | Koskensalo et al. 2012 [31] |
| Protein | IHC | 5 + 0 | Very little staining (0.1–5%) in cancer cells. | Väyrynen et al. 2012 [32] | |
| Protein | ELISA | 100 + 0 | Correlation with malignancy (p = NR). | Verspaget et al. 1999 [33] | |
| Protein | IHC | 121 + 0 | No expression in cancer cells. | Takeha et al. 1997 [34] | |
| Gastric | Protein | IHC | 276 + 0 | Negative staining associates with stage I cancer (p = 0.022), T1 tumor (p = 0.005), diffuse type (p < 0.001), no LN metastasis (p = 0.016) and age under 67 (p = 0.007). Better prognosis for women with negative staining (p = 0.026). | Laitinen et al. 2018 [35] |
| mRNA | RT-PCR | 34 + 34 | Lower mRNA expression in cancer tissue compared to paired healthy tissue. | Lin et al. 2017 [36] | |
| mRNA | RT-PCR | 17 + 22 | No difference between patients and controls and no correlation to clinicopathological features. | de la Pena et al. 2014 [37] | |
| Protein | ELISA | 81 + 0 | Expression higher (p ≤ 0.001) esp. in well-differentiated (p ≤ 0.002) tumors. No correlation to survival. | Kubben et al. 2006 [38] | |
| Head and neck | OTSCC; protein | IHC | 57 + 0 | High VEGF-C (p = 0.001) and low MMP8 level (p = 0.01) correlate with shorter CSS, combined VEGF-C+/MMP8- status correlate with poor CSS (p < 0.001). No correlation to clinicopathological variables. | Åström et al. 2017 [39] |
| OSCC, CSCC; protein | IHC | 36 OSCC, 25 CSCC + 0 | No correlation to clinicopathological features or overall survival. | Ahmed Haji Omar et al. 2015 [40] | |
| OSCC; protein | IHC | 25 + 0 | 5/25 tumors showed moderate levels. | Lawal et al. 2015 [41] | |
| OTSCC: protein | IHC | 70 + 0 | No correlation to clinicopathological features. | Mäkinen et al. 2012 [42] | |
| Larynx; protein | AG array | 7 + 5 | Levels higher compared to normal mucosa (p = NR). | Korampalli et al. 2011 [43] | |
| OTSCC; protein | IHC | 90 + 0 | Correlation to better prognosis and lower-case fatality (p < 0.05). | Korpi et al. 2008 [44] | |
| SL, SCC; protein, mRNA | IHC, RT-PCR | 32 + 32 | <20% tumors showed expression (mRNA/protein). | Xie et al. 2004 [45] | |
| SG; protein | EIA | 23 + 23 | No differences to normal tissue. | Kayano et al. 2004 [46] | |
| SCC; protein, mRNA | IHC, ISH | 19 + 0 | Low mRNA expression and protein levels in all samples. | Moilanen et al. 2002 [47] | |
| Liver | Protein | IHC | 73 + 0 | Co-overexpression with TGF-β1 predicts poor prognosis in HCC patients (p < 0.025). Correlation to cancer stage (p = 0.038) and metastasis (p = 0.049). | Qin et al. 2016 [48] |
| Lung | Protein | FACS | 22 SCC, 19 AC + 0 | Higher in tumor (p = 0.0084) and SCC vs AC (p = 0.0023). Trend towards positive correlation with recurrence (ns). | Shah et al. 2010 [49] |
| Ovarian | Protein | IHC | 302 + 0 | Correlation to tumor stage (p ≤ 0.01), grade (p ≤ 0.01) and poor prognosis (p = 0.019). | Stadlmann et al. 2003 [50] |
| Protein | NR | NR | High MMP8 levels in ovarian cyst fluid are associated with malignancy (p = NR). | Stenman et al. 2003 [51] | |
| Pancreas | Protein | LC-MS | 9 SS, 10 LS + 0 | MMP8 upregulated in SS group (p < 0.05). | Hu et al. 2018 [52] |
| Protein, mRNA | IHC, RT-PCR | 45 + 10 | Expression higher (p = 0.04) in tumor versus healthy tissue. No correlation to clinicopathological features or survival. No mRNA found. | Jones et al. 2004 [53] | |
| Skin | nBCC; protein, mRNA | RT-PCR, WB | 22 + 22 | Higher levels in nBCC tumors (p < 0.0001), but no differences in mRNA expression. | Ciążyńska et al. 2018 [54] |
| SCC; protein | IHC | 31 KA, 15 SCC + 0 | More frequent levels in keratoacanthomas compared to SCCs (ns) | Kuivanen et al. 2006 [55] | |
| SCC; protein | IHC | 9 SCC, 31 leg ulcers + 0 | Cannot distinguish between non-malignant leg ulcers and SCC. | Impola et al. 2005 [56] | |
| Melanoma; protein | IHC | 10 + 0 | Correlates with invasiveness (p = NR). | Giambernardi et al. 2001 [57] | |
| BCC; protein | IHC | 54 +16 | No correlation to collagenolytic activity. | Varani et al. 2000 [58] | |
| Soft tissue | Protein | ICC | 39 + 0 | 6/39 tumors have MMP8 staining. | Roebuck et al. 2005 [59] |
| Thyroid | mRNA | cDNA array | 131 + 0 | Higher expression in malignant neoplasms compared to benign. | Kebebew et al. 2005 [60] |
| Uterine | Protein | EIA, IHC | 53 + 30 | Higher expression (p < 0.05) in patients. No correlation to clinicopathological features. | Ueno et al. 1999 [61] |
| Cancer. | Plasma/Serum | Method | Study Size (Patients + Healthy Controls) | Expression and Prognosis | Authors |
|---|---|---|---|---|---|
| Breast | Serum | Microarray | 11 + 10 | Higher in patients (p = 0.001). | Li et al. 2017 [72] |
| Plasma | ELISA | 208 + 42 | Higher in patients with non-inflammatory breast cancer (p = 0.007). Associated with premenopausal status (p = 0.06), NPI (p = 0.04) and lymph node involvement (pN1-2 p = 0.001). Lower levels in patients with risk of distant metastasis (pN3, p = 0.003). | Decock et al. 2008 [73] | |
| Colorectal | Serum | IFMA | 335 + 47 | Higher in patients with advanced disease (Dukes classification p < 0.001, T status p = 0.004), distant metastasis (p < 0.001), tumor in right side of colon (p = 0.038). Correlation to worse overall survival (p = 0.005) | Böckelman et al. 2018 [74] |
| Serum | IFMA | 271 + 0 | Higher is patients with high mGPS (p < 0.001). Negative correlation with tumor-infiltrating mast cells in invasive margin (p = 0.005) and tumor centre (p = 0.010). Correlation with poor cancer-specific survival (p = 0.009). | Sirniö et al. 2018 [75] | |
| Serum | IFMA | 116 + 83 | Higher in patients (p = 0.0000000015). Correlation with TNM stage (p = 0.00045), T status (p = 0.0035), distant metastasis (p = 0.000054), CLR (p = 0.0057), necrosis (p = 0.0024), high neutrophil and leukocyte cell count (p < 0.05) and peritumoral tumor-destructing inflammatory cell infiltrate (p = 0.041). | Väyrynen et al. 2012 [32] | |
| Esophagus | Serum | Microarray | 10 + 10 | Lower expression in patients (p < 0.01). | Tong et al. 2018 [76] |
| Gastric | Serum | IFMA | 233 + 0 | Higher expression in patients with intestinal cancer (p = 0.044). Patients with intermediate (31–131 ng/mL) serum MMP8 levels had better prognosis (p = 0.002). | Laitinen et al. 2018 [35] |
| Head and neck | Serum | IFMA | 33 SCC, 175 benign + 0 | Higher in tonsillar SCC patients compared to patients with benign tonsillar disease (p = NR). | Ilmarinen et al. 2017 [66] |
| Plasma | IFMA | 198 + 0 | Trend towards favorable outcome (ns.). | Nurmenniemi et al. 2012 [77] | |
| Plasma | IFMA | 136 + 0 | Does not correlate with survival or lymph node involvement. | Pradhan-Palikhe et al. 2010 [78] | |
| Serum | EIA | 59 + 0 | Higher than healthy persons (p < 0.05). Correlation with tumor stage (p = NR). | Kuropkat et al. 2004 [79] | |
| Serum | NR | 73 + 74 | Higher than healthy persons, correlation with overall TNM status (p = NR). | Kuropkat et al. 2002 [80] | |
| Kidney | Serum | ELISA | 43+ 10 | Surgery lowers MMP-8 levels (p = NR). | Kołomecki et al. 2001 [81] |
| Liver | Serum | IFMA | 134 + 0 | Worse overall survival (p = 0.013). Correlation to BCLC criteria (p < 0.0001) and tumor size (p < 0.0001). | Lempinen et al. 2013 [82] |
| Melanoma | Serum | IFMA | 117 + 0 | Elevated in vascular-invading (p = 0.001), ulcerating (p = 0.003) and bleeding (p = 0.033) melanomas. Correlation to worse outcome (p = 0.023). | Vihinen et al. 2008 [83] |
| Nerves | Plasma | NR | 14 + 0 | Elevated in carotid body cancer patients (p = NR). | Serra et al. 2014 [84] |
| Pancreas | Serum | MAP kit | 109 + 40 | Expression higher in cancer patients (p = 0.0001). | Park et al. 2012 [85] |
| Thyroid | Plasma | ELISA | 22 + 0 | No correlation with tumor presence (p = NR). | Komorowski et al. 2002 [86] |
| Urothelial | Plasma | FACS | 135 + 0 | No correlation to clinical stage or cancer specific mortality in high-grade tumors. | Svatek et al. 2010 [87] |
| Cancer | SNPs | Study Size (Patients + Healthy Controls) | Effect on Patient (p-Value) | Authors |
|---|---|---|---|---|
| Bladder | rs11225395, rs35866072, rs34009635 | 375 + 375 | No effect on bladder cancer risk. | Tsai et al. 2018 [90] |
| rs11225395 | 200 + 200 | Reduced risk of bladder cancer (p = 0.006). | Srivastava et al. 2013 [91] | |
| rs1940475 | 243 invasive, 315 superficial | Protects from invasive phenotype in former smokers (p = 0.05). | Kader et al. 2007 [92] | |
| rs1940475 | 560 + 560 | Trend to increased risk of invasive bladder cancer in never smokers (ns.). | Kader et al. 2006 [93] | |
| Breast | rs11225395, rs35866072, rs34009635 | 1232 + 1232 | No effect on breast cancer risk. | Hsiao et al. 2018 [94] |
| rs11225395 | 6307 + 0 | Better overall survival (p = 0.021). | Beeghly-Fadiel et al. 2012 [95] | |
| rs11225395 | 300 + 300 | Not associated with breast cancer risk. | Dȩbniak et al. 2011 [96] | |
| rs1940475, rs1320632, rs11225395, rs17099436, rs10895353, rs7943404, rs1892886, rs2508383, rs1276284 | 4470 + 4560 | rs1892886: Associated with breast cancer (p = 0.0097). | Mavaddat et al. 2009 [97] | |
| rs10895353, rs7943404, rs11225395, rs1320632, rs1940475, rs1892886, rs17099436, rs2508383, rs1276284 | 1333 + 0 | rs11225395, rs1940475, rs1892886 and rs1276284: Less metastasis (p = 0.02, p = 0.03, p = 0.03, p = 0.03, respectively).rs11225395: Higher overall (p = 0.02) and disease-specific survival (p = 0.02) and less relapse (p = 0.04) in early stage patients. | Decock et al. 2007 [98] | |
| Gastric | rs1940475 | 254 + 0 | Higher risk for recurrence (p = 0.005) and lower overall survival (p = 0.001), recurrence-free survival (p = 0.005) and disease-free survival (p = 0.011). | Lin et al. 2017 [36] |
| rs11225395, rs2155052 | 79 + 169 | No correlation to risk or clinicopathological parameters. | Kubben et al. 2006 [99] | |
| Head and neck | rs11225395, rs35866072, rs34009635 | 788 + 956 | No effect on oral cancer risk. | Hung et al. 2017 [100] |
| rs11225395 | 198 + 0 | No significant effect on overall survival in NPC. | Liu et al. 2013 [101] | |
| rs11225395 | 136 + 0 | No correlation to survival or LNM in HNSCC patients. | Pradhan-Palikhe et al. 2010 [78] | |
| Leukemia | rs11225395, rs35866072, rs34009635 | 266 + 266 | No effect on childhood leukemia risk. | Pei et al. 2017 [102] |
| Liver | rs11225395 | 434 + 480 | Increased risk of HCC in non-HBV-carriers (p = 0.03). | Qiu et al. 2008 [103] |
| Lung | rs11225395, rs35866072, rs34009635 | 358 + 716 | No effect on lung cancer risk. | Shen et al. 2017 [104] |
| rs2155052 | 501 + 510 | Reduced risk for lung cancer (p = 0.019), especially in males (p = 0.021), ever smokers (p = 0.034) and patients with family history of lung cancer (p = 0.011). Reduced risk for small cell carcinoma (p = 0.023) and squamous cell carcinoma (p = 0.008). | González-Arriaga et al. 2008 [105] | |
| Ovarian | rs17099462 | 417 + 417 | Reduced overall survival (p = 0.0257). | Wang et al. 2015 [106] |
| rs11225395, rs2155052 | 35 malignant, 51 benign + 37 | rs11225395: Increased risk for ovarian cancer (p = 0.02) and tendency towards worse overall survival (ns.). | Arechavaleta-Velasco et al. 2014 [107] | |
| Skin | rs1940475 | 285 SCC, 300 BCC, 218 melanoma + 870 | Reduced risk for BCC (p = 0.04), no effect in SCC or melanoma. | Nan et al. 2008 [108] |
| rs11225395 | 300 melanoma + 300 | Increased risk for melanoma (p = 0.017). | Dȩbniak et al. 2011 [96] | |
| Thyroid | rs1940475 | 31 PTC, 19 FTC and 9 ATC + 0 | Present in 80.6% of PTC, 73.6% of FTC and 88.8% of ATC tumors. | Murugan et al. 2011 [109] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juurikka, K.; Butler, G.S.; Salo, T.; Nyberg, P.; Åström, P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 4506. https://doi.org/10.3390/ijms20184506
Juurikka K, Butler GS, Salo T, Nyberg P, Åström P. The Role of MMP8 in Cancer: A Systematic Review. International Journal of Molecular Sciences. 2019; 20(18):4506. https://doi.org/10.3390/ijms20184506
Chicago/Turabian StyleJuurikka, Krista, Georgina S. Butler, Tuula Salo, Pia Nyberg, and Pirjo Åström. 2019. "The Role of MMP8 in Cancer: A Systematic Review" International Journal of Molecular Sciences 20, no. 18: 4506. https://doi.org/10.3390/ijms20184506
APA StyleJuurikka, K., Butler, G. S., Salo, T., Nyberg, P., & Åström, P. (2019). The Role of MMP8 in Cancer: A Systematic Review. International Journal of Molecular Sciences, 20(18), 4506. https://doi.org/10.3390/ijms20184506





