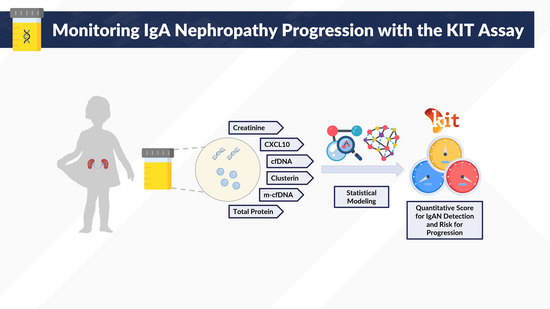
Noninvasive Urinary Monitoring of Progression in IgA Nephropathy
Abstract
1. Introduction
2. Results
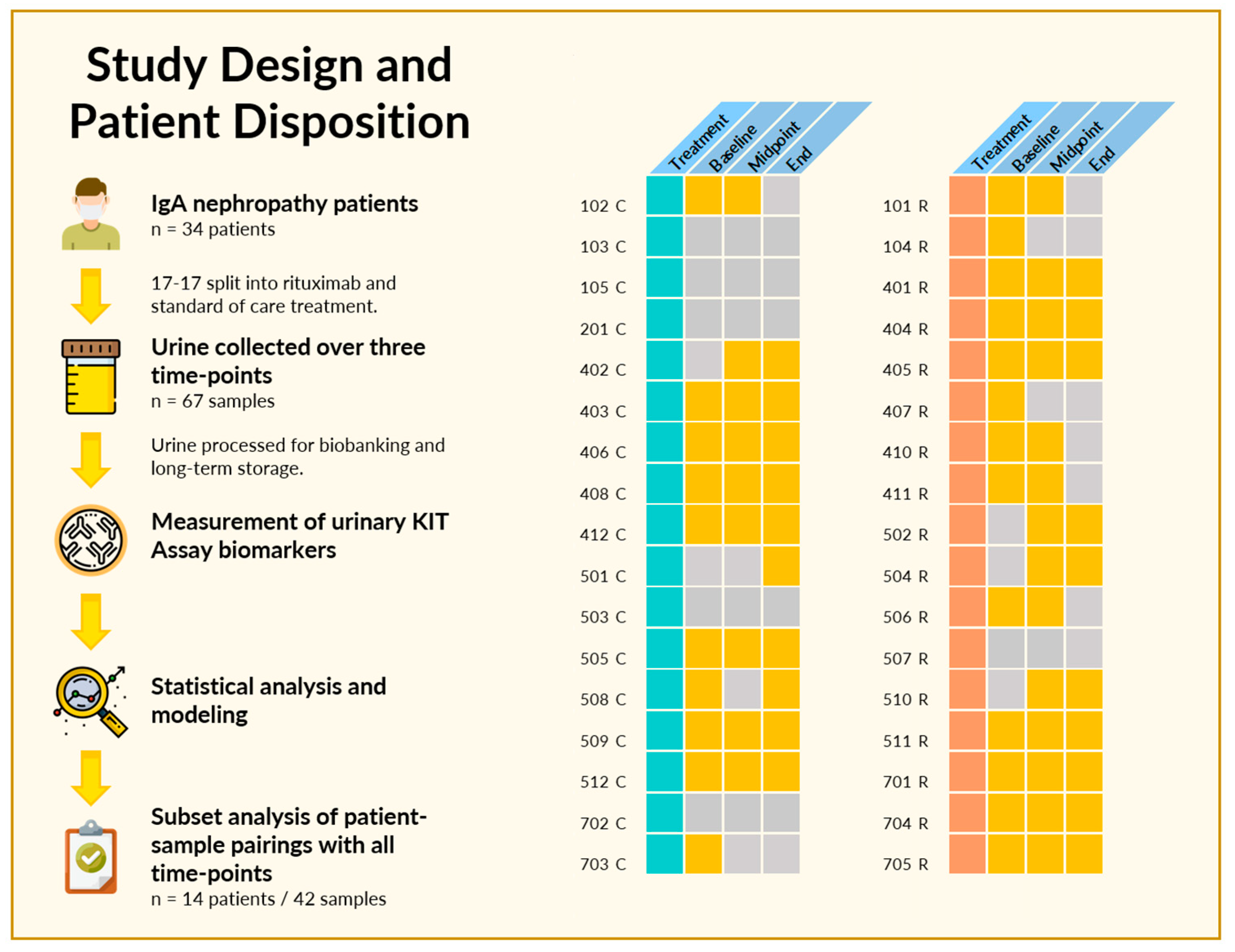
2.1. Study Design and Patient Disposition
2.2. The KIT Assay Biomarkers Can Discriminate Healthy Controls from Patients with IgA Nephropathy
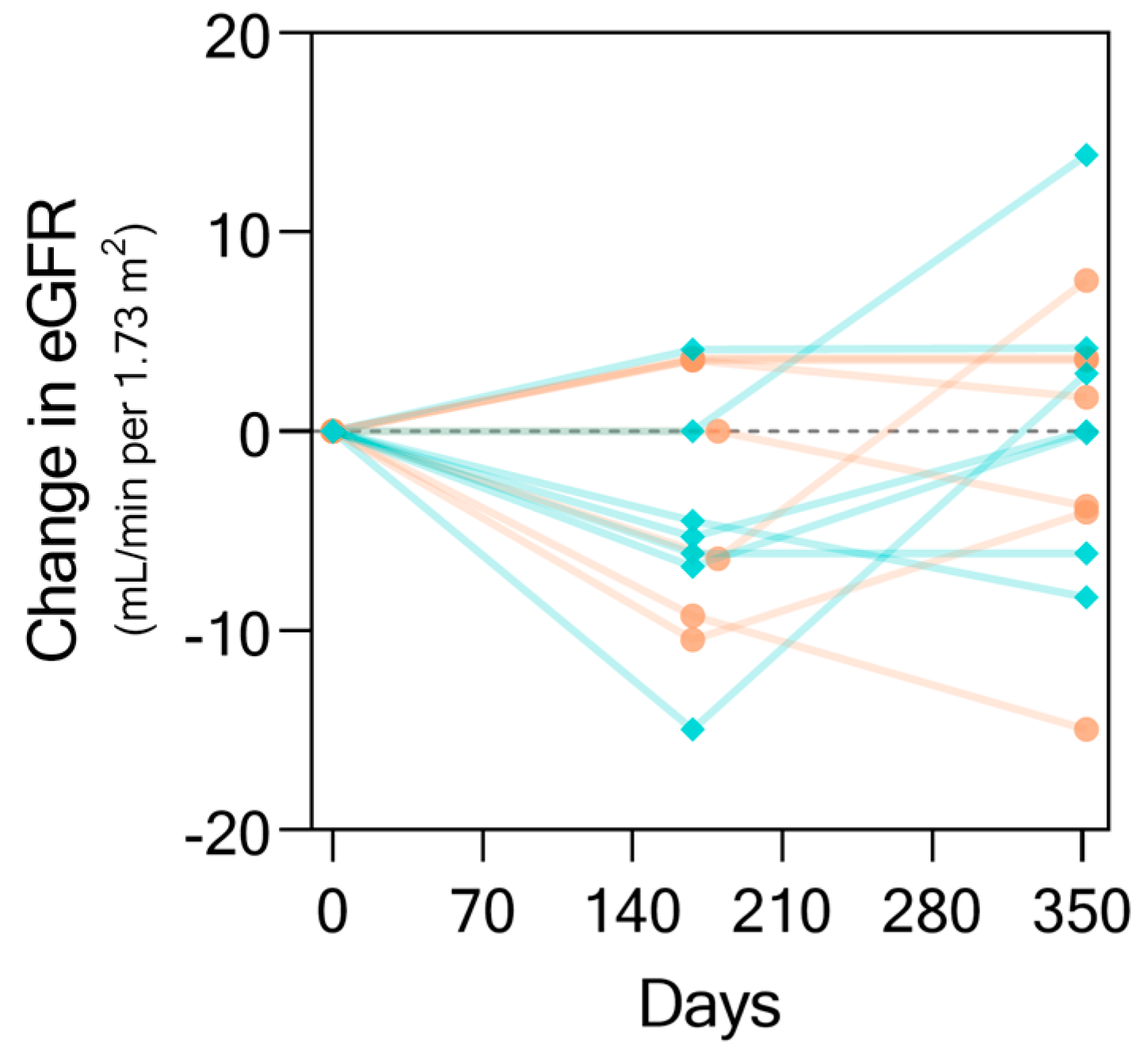
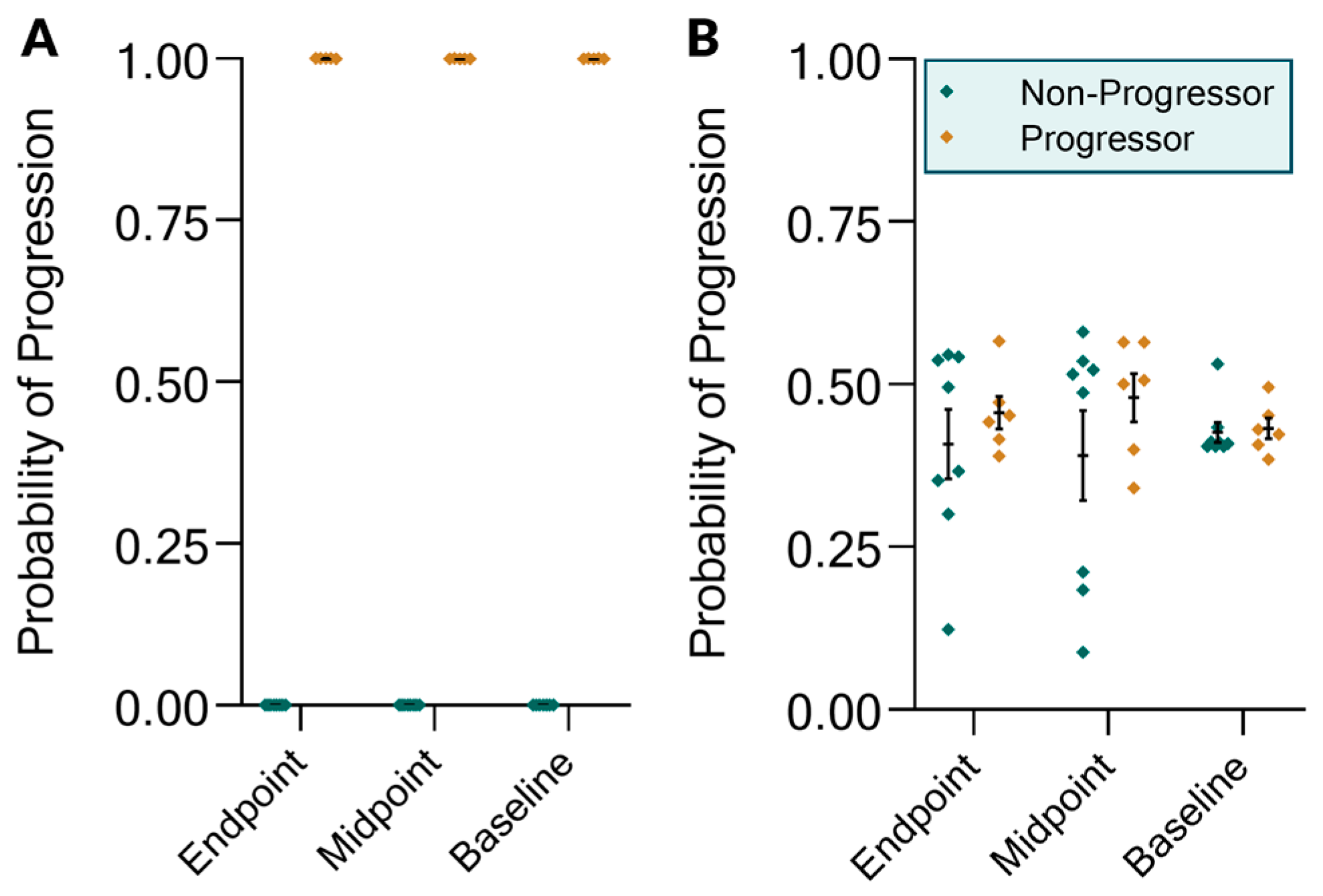
2.3. The KIT Assay Biomarkers Can Discriminate Progressors from Non-Progressors and Predict Progression
3. Materials and Methods
3.1. Patients and Study Characteristics
3.2. Urine Sample Processing
3.3. KIT Assay Biomarkers
3.4. Statistical Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area-under-curve |
| BMI | body mass index |
| cfDNA | cell-free deoxyribonucleic acid |
| DMAIMO | (dimethylamino)iminomethyl-ornithine |
| eGFR | estimated glomerular filtration rate |
| GC–MS | gas chromatography–mass spectrometry |
| HSPN | Henoch–Schönlein purpura nephritis |
| IgAN | immunoglobulin alpha nephropathy |
| KIT | Kidney Injury Test |
| m-cfDNA | methylated cfDNA |
| MAMIMO | (methylamino)(methylimino)methyl-ornithine |
| MDRD | Modification of Diet in Renal Disease |
| RAAS | renin–angiotensin–aldosterone system |
| ROC | Receiver-operator characteristic |
References
- Wyatt, R.J.; Julian, B.A. IgA Nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- D’Amico, G. Clinical features and natural history in adults with IgA nephropathy. Am. J. Kidney Dis. 1988, 12, 353–357. [Google Scholar] [CrossRef]
- Yap, H.K.; Quek, C.M.; Shen, Q.; Joshi, V.; Chia, K.S. Role of urinary screening programmes in children in the prevention of chronic kidney disease. Ann. Acad. Med. Singap. 2005, 34, 3–7. [Google Scholar] [PubMed]
- Boyd, J.K.; Cheung, C.K.; Molyneux, K.; Feehally, J.; Barratt, J. An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int. 2012, 81, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Reily, C.; Bian, Q.; Neprasova, M.; Novak, J.; Tesar, V. Markers for the progression of IgA nephropathy. J. Nephrol. 2016, 29, 535–541. [Google Scholar] [CrossRef]
- Donadio, J.V.; Joseph, P. Grande IgA Nephropathy. N. Engl. J. Med. 2002, 347, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Julian, B.A.; Wittke, S.; Haubitz, M.; Zürbig, P.; Schiffer, E.; McGuire, B.M.; Wyatt, R.J.; Novak, J. Urinary biomarkers of IgA nephropathy and other IgA-associated renal diseases. World J. Urol. 2007, 25, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; He, Y.; Li, K.; Yang, J.; Li, X.; Lu, R.; Gao, W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol. 2007, 123, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Haubitz, M.; Wittke, S.; Weissinger, E.M.; Walden, M.; Rupprecht, H.D.; Floege, J.; Haller, H.; Mischak, H. Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int. 2005, 67, 2313–2320. [Google Scholar] [CrossRef][Green Version]
- Watson, D.; Yang, J.Y.C.; Sarwal, R.D.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; Louie, V.; Sigdel, S.; Livingstone, D.; Soh, K.; et al. A Novel Multi-Biomarker Assay for Non-Invasive Detection and Quantitative Monitoring of Kidney Injury. J. Clin. Med. 2019, 8, 499. [Google Scholar] [CrossRef]
- Yang, J.Y.C.; Sarwal, R.D.; Ky, K.; Dong, V.; Stoller, M.; Watson, D.; Sarwal, M.M.; Chi, T. Non-Radiologic Assessment of Kidney Stones by KIT, a Spot Urine Assay. Br. J. of Urol. Int. 2019. under review. [Google Scholar]
- Yang, J.Y.C.; Sarwal, R.D.; Sigdel, T.K.; Sarwal, M.M. Predicting Transplant Rejection by a Composite Urinary Injury Score. Am. J. Transpl. 2019, 17, 519. [Google Scholar]
- Sigdel, T.K.; Gao, Y.; He, J.; Wang, A.; Nicora, C.D.; Fillmore, T.L.; Shi, T.; Webb-Robertson, B.-J.; Smith, R.D.; Qian, W.-J.; et al. Mining the human urine proteome for monitoring renal transplant injury. Kidney Int. 2016, 89, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.C.; Sigdel, T.K.; Sarwal, M.M. Self-antigens and rejection: A proteomic analysis. Curr. Opin. Organ Transpl. 2016, 21, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.C.; Sarwal, M.M. Transplant genetics and genomics. Nat. Rev. Genet. 2017, 18, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Lafayette, R.A.; Canetta, P.A.; Rovin, B.H.; Appel, G.B.; Novak, J.; Nath, K.A.; Sethi, S.; Tumlin, J.A.; Mehta, K.; Hogan, M.; et al. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J. Am. Soc. Nephrol. 2017, 28, 1306–1313. [Google Scholar] [CrossRef]
- Cattran, D.C.; Coppo, R.; Cook, H.T.; Feehally, J.; Roberts, I.S.D.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 534–545. [Google Scholar] [CrossRef]
- Schena, F.P.; Nistor, I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin. Nephrol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Suzuki, H. Biomarkers for IgA nephropathy on the basis of multi-hit pathogenesis. Clin. Exp. Nephrol. 2019, 23, 26–31. [Google Scholar] [CrossRef]
- Lai, K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012, 8, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Woo, S.H.; Dai, H.; Khatri, P.; Li, L.; Myers, B.; Sarwal, M.M.; Lafayette, R.A. Profiling of Autoantibodies in IgA Nephropathy, an Integrative Antibiomics Approach. J. Am Soc. Nephrol. 2011, 6, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Sigdel, T.K.; Dinh, V.T.; Vu, M.T.; Sarwal, M.M.; Lafayette, R.A. Mapping novel immunogenic epitopes in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2015, 10, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.-Q.; Fatima, H.; Julian, B.A.; Novak, J. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Specific for Galactose-Deficient IgA1. J. Am. Soc. Nephrol. 2019, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Eitner, F. Current Therapy for IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.C.; Verleden, S.E.; Zarinsefat, A.; Vanaudenaerde, B.M.; Vos, R.; Verleden, G.M.; Sarwal, R.D.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; et al. Cell-Free DNA and CXCL10 Derived from Bronchoalveolar Lavage Predict Lung Transplant Survival. J. Clin. Med. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Sacreas, A.; Yang, J.Y.C.; Vanaudenaerde, B.M.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; Verleden, G.M.; Vos, R.; Verleden, E.; Sarwal, M.M. The common rejection module in chronic rejection post lung transplantation. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Sigdel, T.K.; Yang, J.Y.C.; Bestard, O.; Schroeder, A.; Hsieh, S.-C.; Liberto, J.M.; Damm, I.; Geraedts, A.C.M.; Sarwal, M.M. A urinary Common Rejection Module (uCRM) score for non-invasive kidney transplant monitoring. PLoS ONE 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Takano, H.; Kang, D.; Nagahama, K.; Aoki, M.; Morita, M.; Kaneko, T.; Tsuruoka, S.; Shimizu, A. Endothelial cell injury in acute and chronic glomerular lesions in patients with IgA nephropathy. Hum. Pathol. 2016, 49, 135–144. [Google Scholar] [CrossRef]
- Reich, H.N.; Troyanov, S.; Scholey, J.W.; Cattran, D.C. Remission of Proteinuria Improves Prognosis in IgA Nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3177–3183. [Google Scholar] [CrossRef]
- Gutiérrez, E. IgA nephropathy: Is a new approach beyond proteinuria necessary? Pediatr. Nephrol. 2019, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Burnham, P.; Dadhania, D.; Heyang, M.; Chen, F.; Westblade, L.F.; Suthanthiran, M.; Lee, J.R.; De Vlaminck, I. Urinary cell-free DNA is a versatile analyte for monitoring infections of the urinary tract. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Vlková, B.; Lauková, L.; Bábíčková, J.; Boor, P. Cell-free DNA: The role in pathophysiology and as a biomarker in kidney diseases. Expert Rev. Mol. Med. 2018, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | IgA Cohort (n = 34) 1 |
|---|---|
| Age, years | 40 (21–63) |
| Sex | |
| ● Female | 9 |
| ● Male | 25 |
| Race | |
| ● Caucasian | 24 |
| ● Asian/Pacific Islander | 6 |
| ● Hispanic/Latino | 3 |
| ● African American | 1 |
| Weight, kg | 89.7 (57–120) |
| eGFR, mL/min per 1.73 m2 | 49 (30–122) |
| Treatment | |
| ● Rituximab | 17 |
| ● Standard of Care | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.Y.C.; Sarwal, R.D.; Fervenza, F.C.; Sarwal, M.M.; Lafayette, R.A. Noninvasive Urinary Monitoring of Progression in IgA Nephropathy. Int. J. Mol. Sci. 2019, 20, 4463. https://doi.org/10.3390/ijms20184463
Yang JYC, Sarwal RD, Fervenza FC, Sarwal MM, Lafayette RA. Noninvasive Urinary Monitoring of Progression in IgA Nephropathy. International Journal of Molecular Sciences. 2019; 20(18):4463. https://doi.org/10.3390/ijms20184463
Chicago/Turabian StyleYang, Joshua Y. C., Reuben D. Sarwal, Fernando C. Fervenza, Minnie M. Sarwal, and Richard A. Lafayette. 2019. "Noninvasive Urinary Monitoring of Progression in IgA Nephropathy" International Journal of Molecular Sciences 20, no. 18: 4463. https://doi.org/10.3390/ijms20184463
APA StyleYang, J. Y. C., Sarwal, R. D., Fervenza, F. C., Sarwal, M. M., & Lafayette, R. A. (2019). Noninvasive Urinary Monitoring of Progression in IgA Nephropathy. International Journal of Molecular Sciences, 20(18), 4463. https://doi.org/10.3390/ijms20184463