Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms
Abstract
1. Introduction
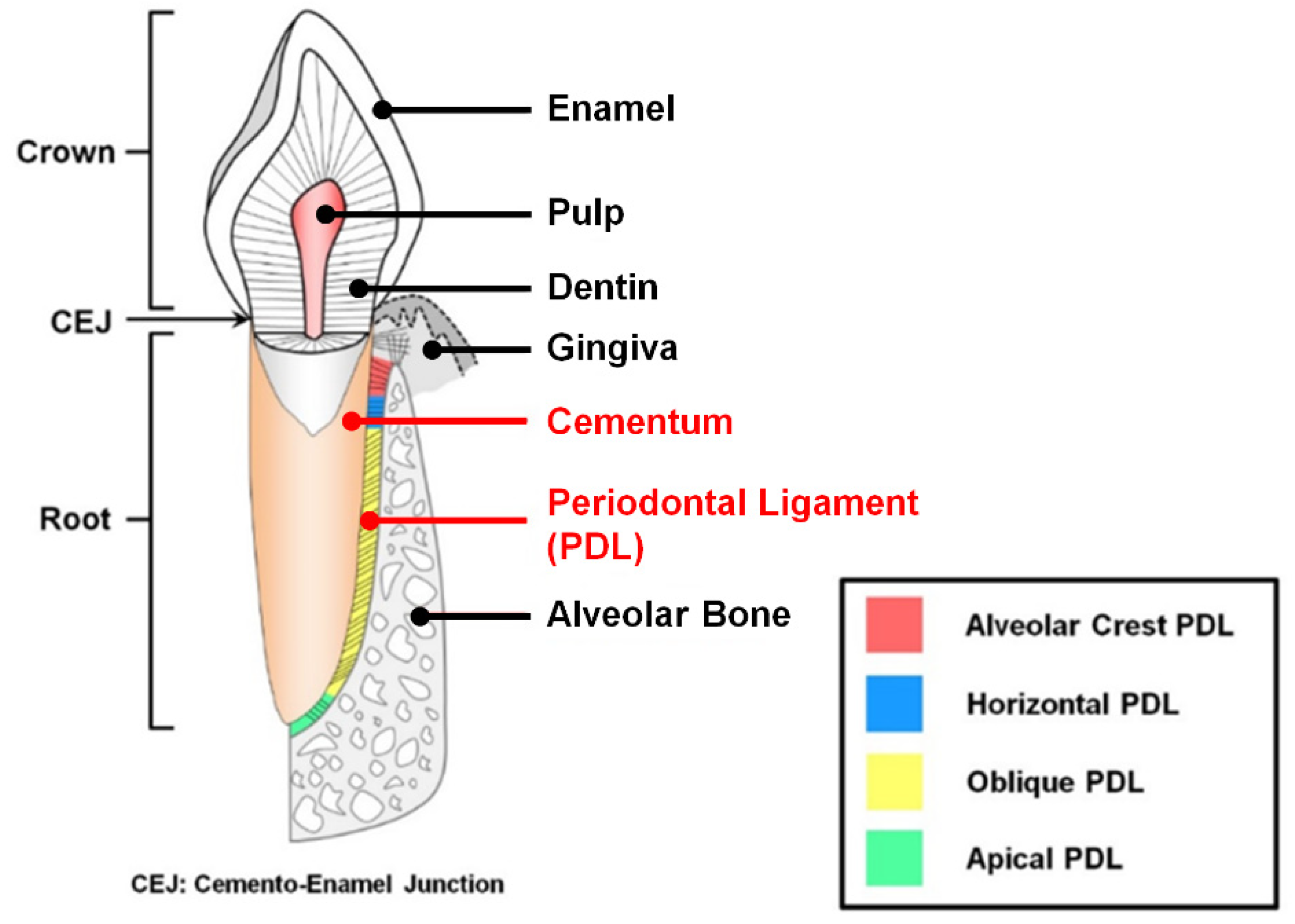
1.1. The Characteristics of Periodontal Ligament and Cementum in the Periodontal Complex
1.2. Periodontal Disease and Treatments
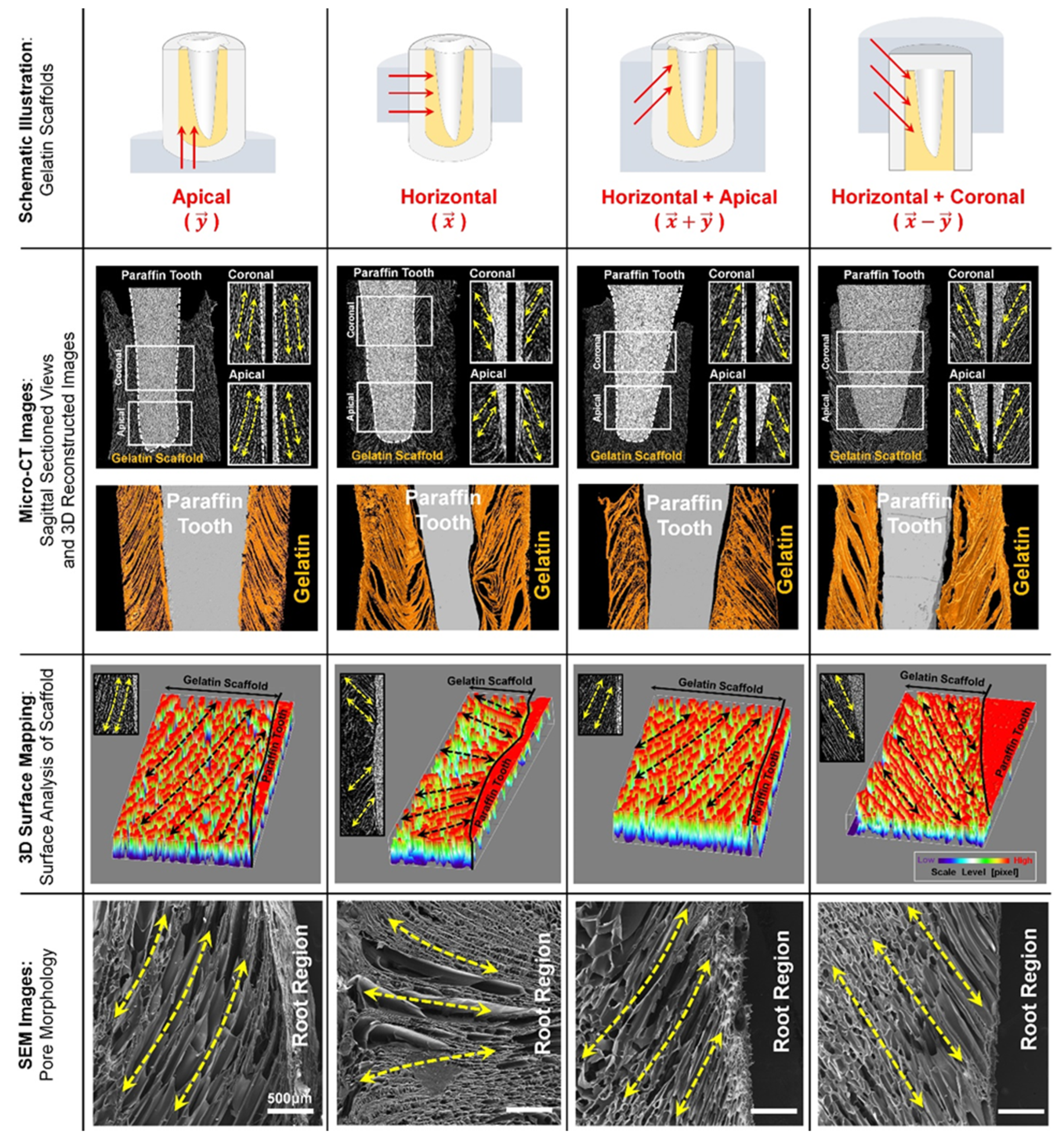
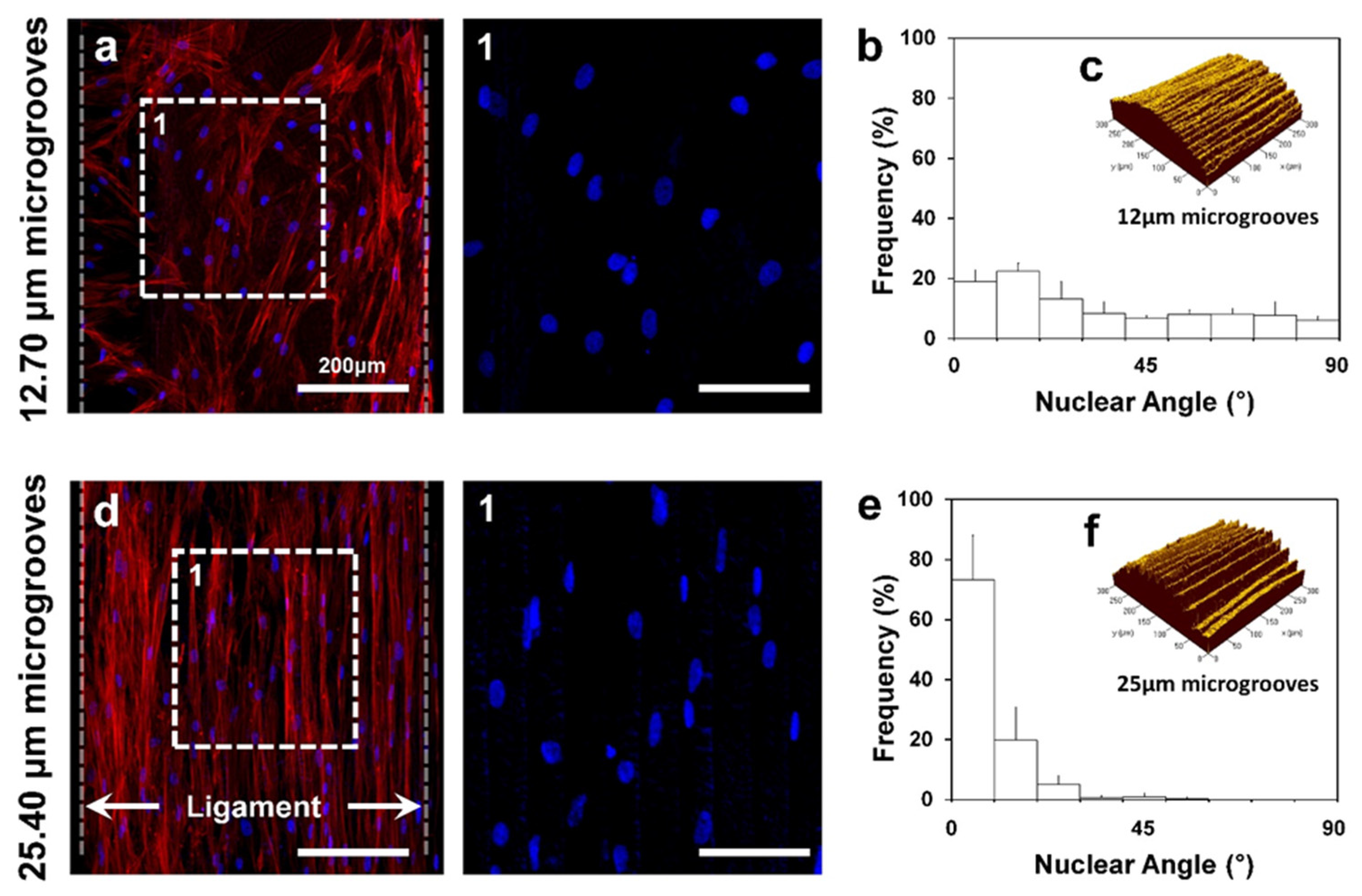
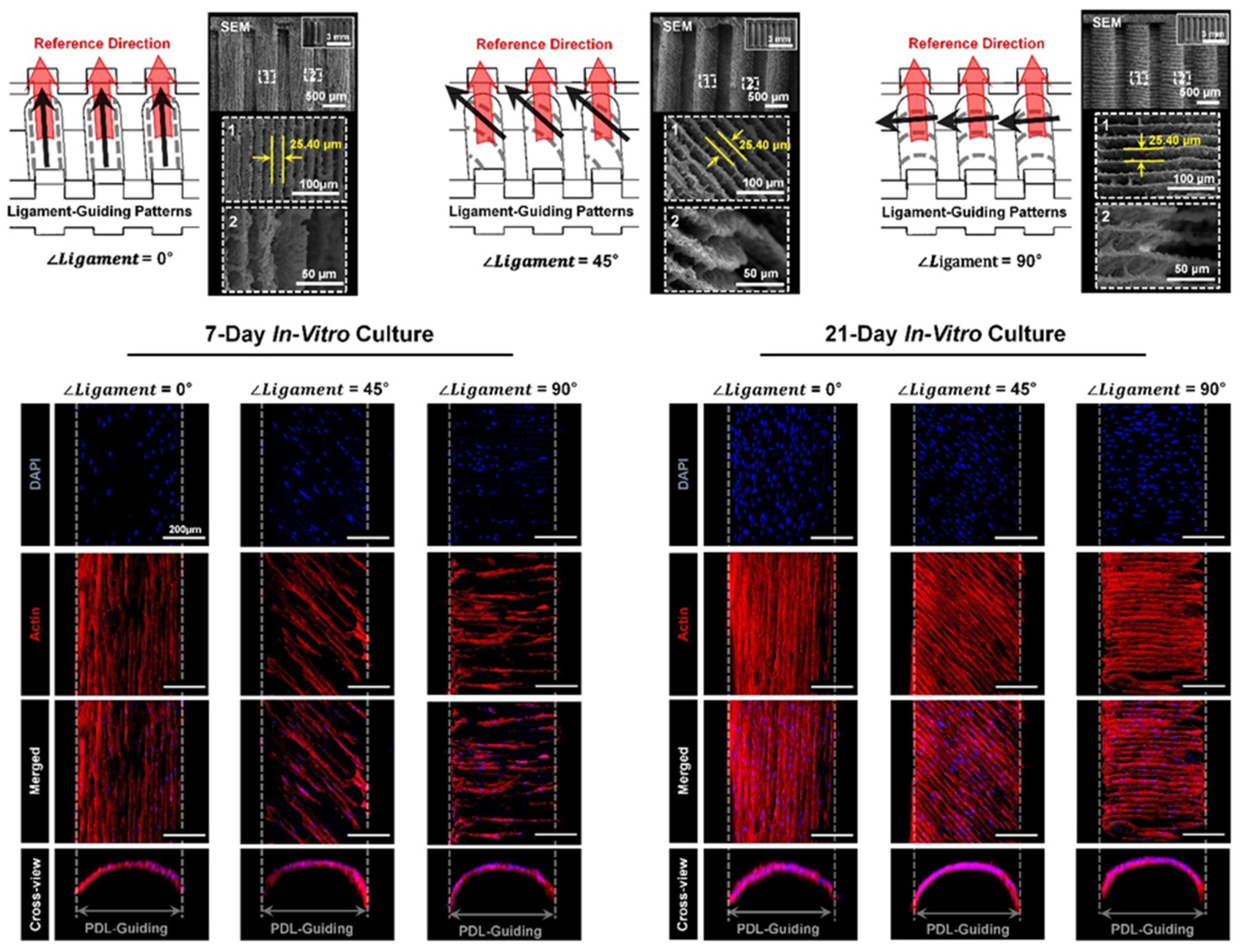
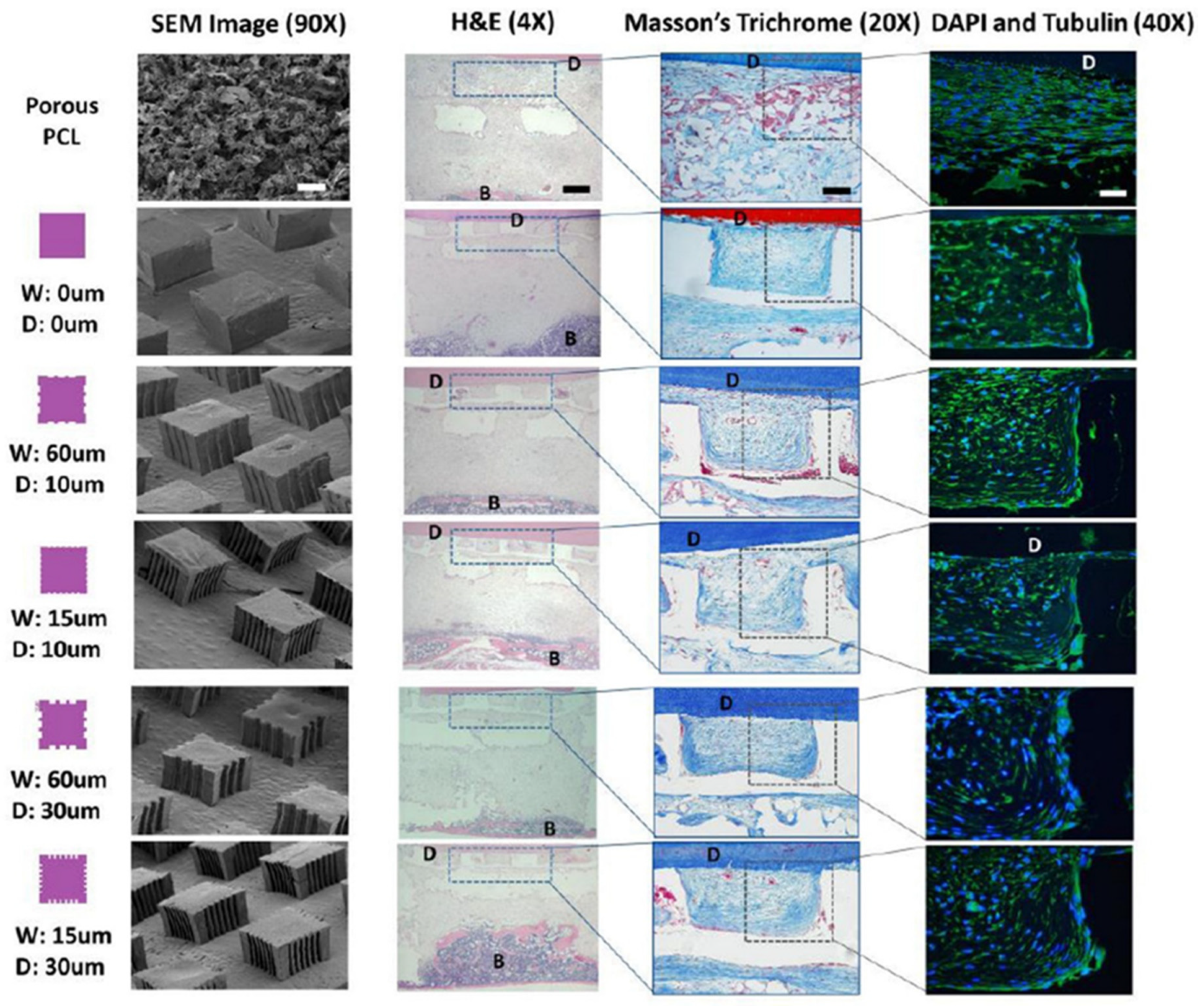
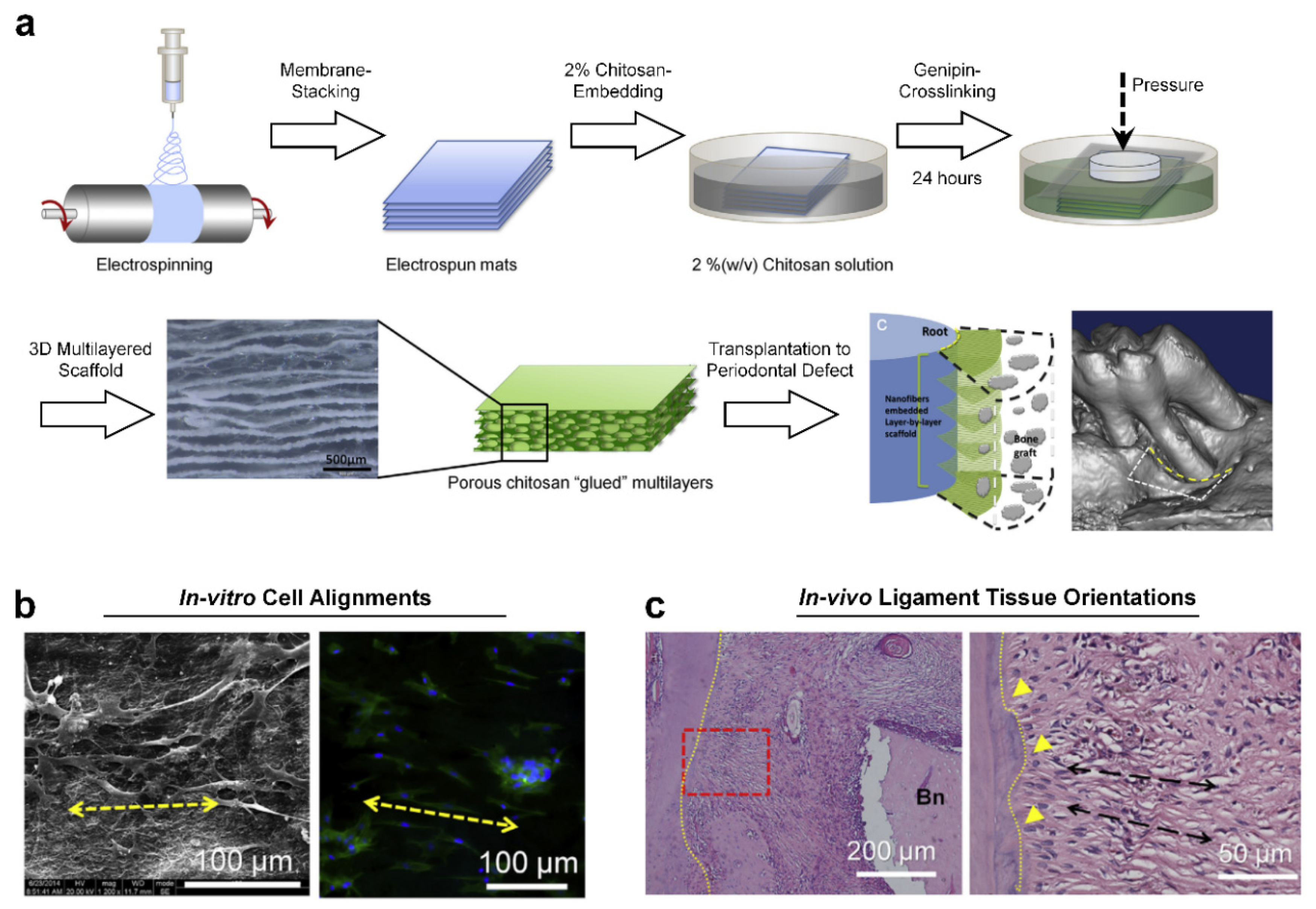
2. PDL Regenerations with Angular Organization Using Topographic Approaches of 3D Platforms
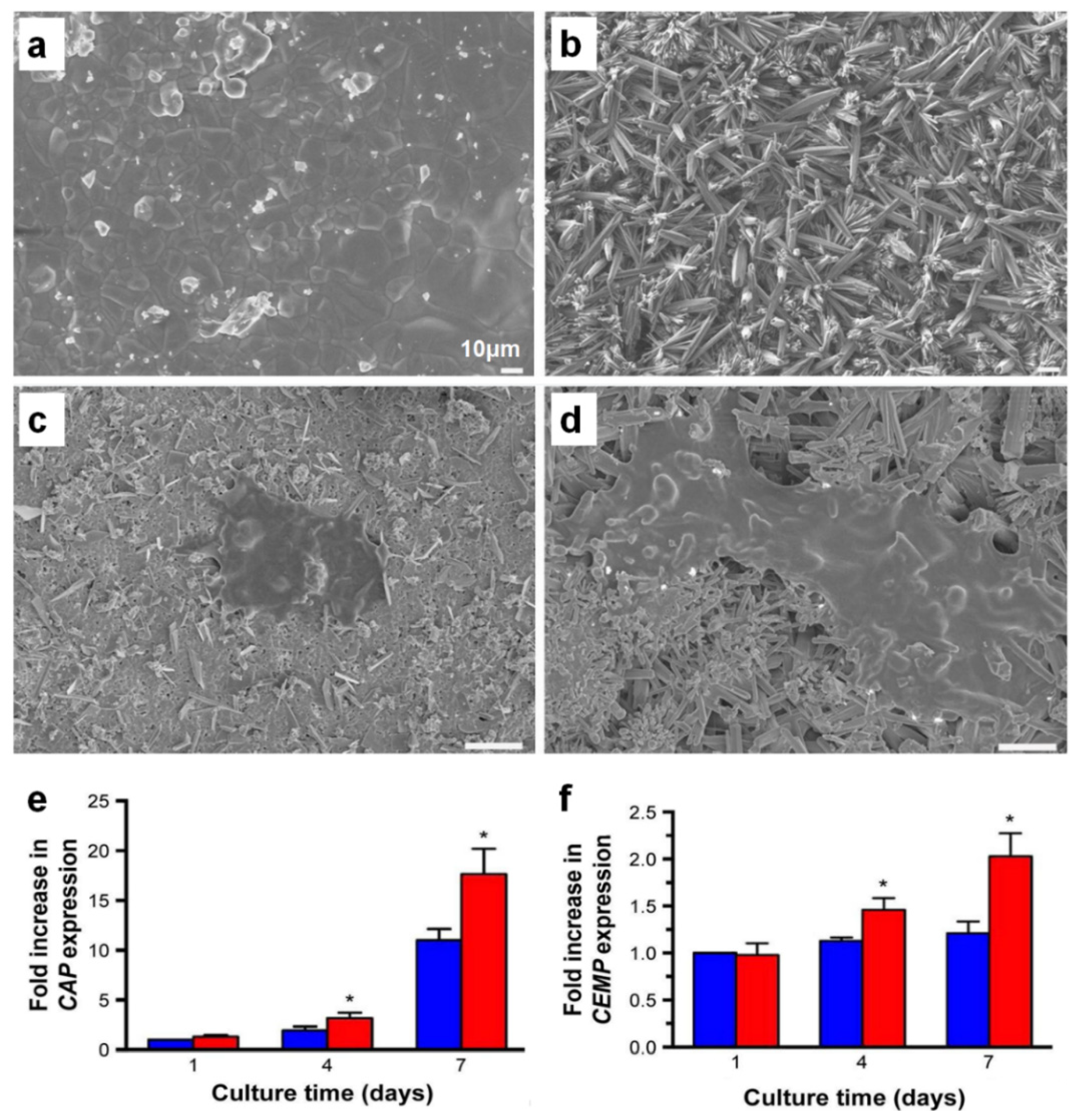
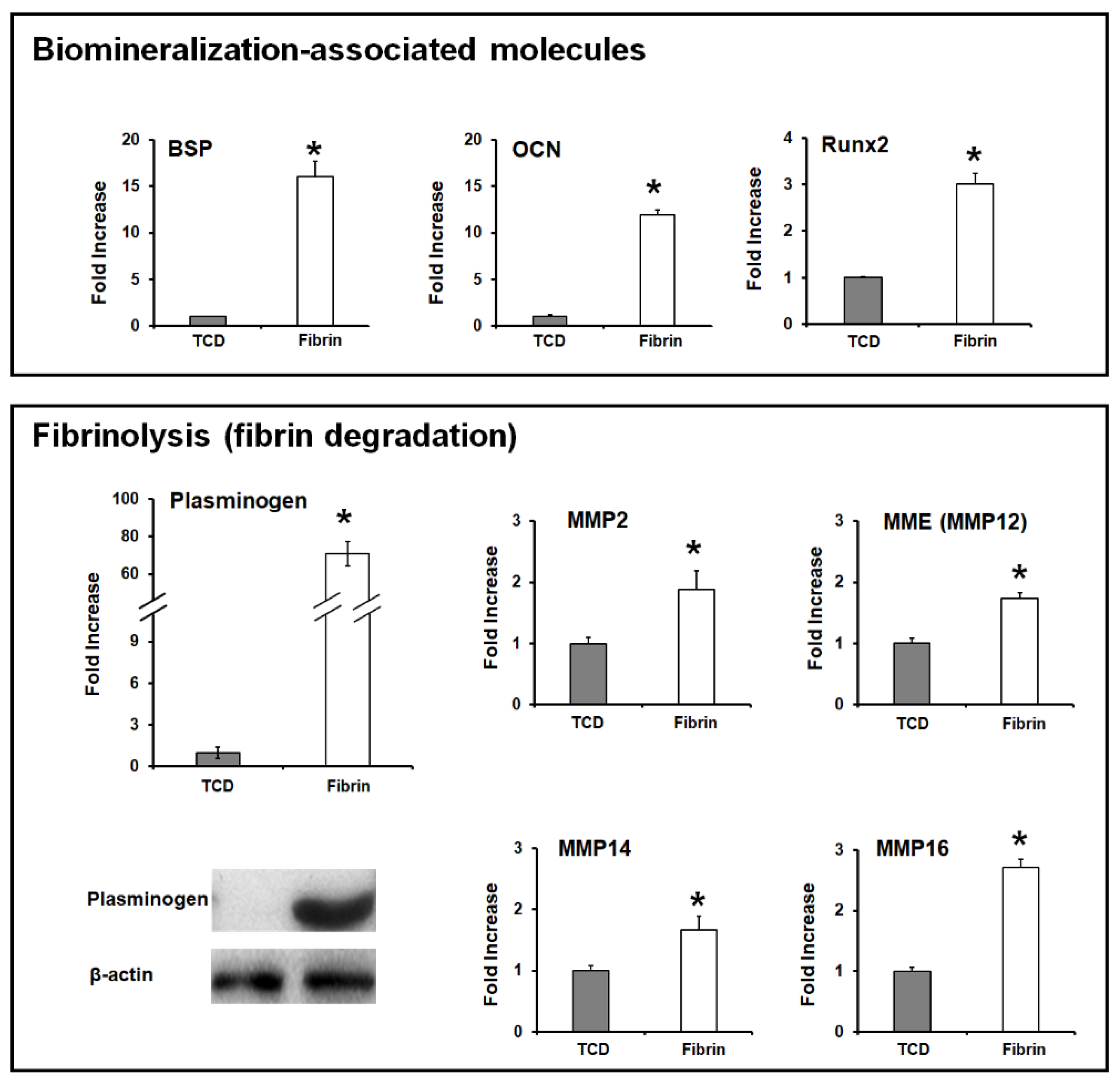
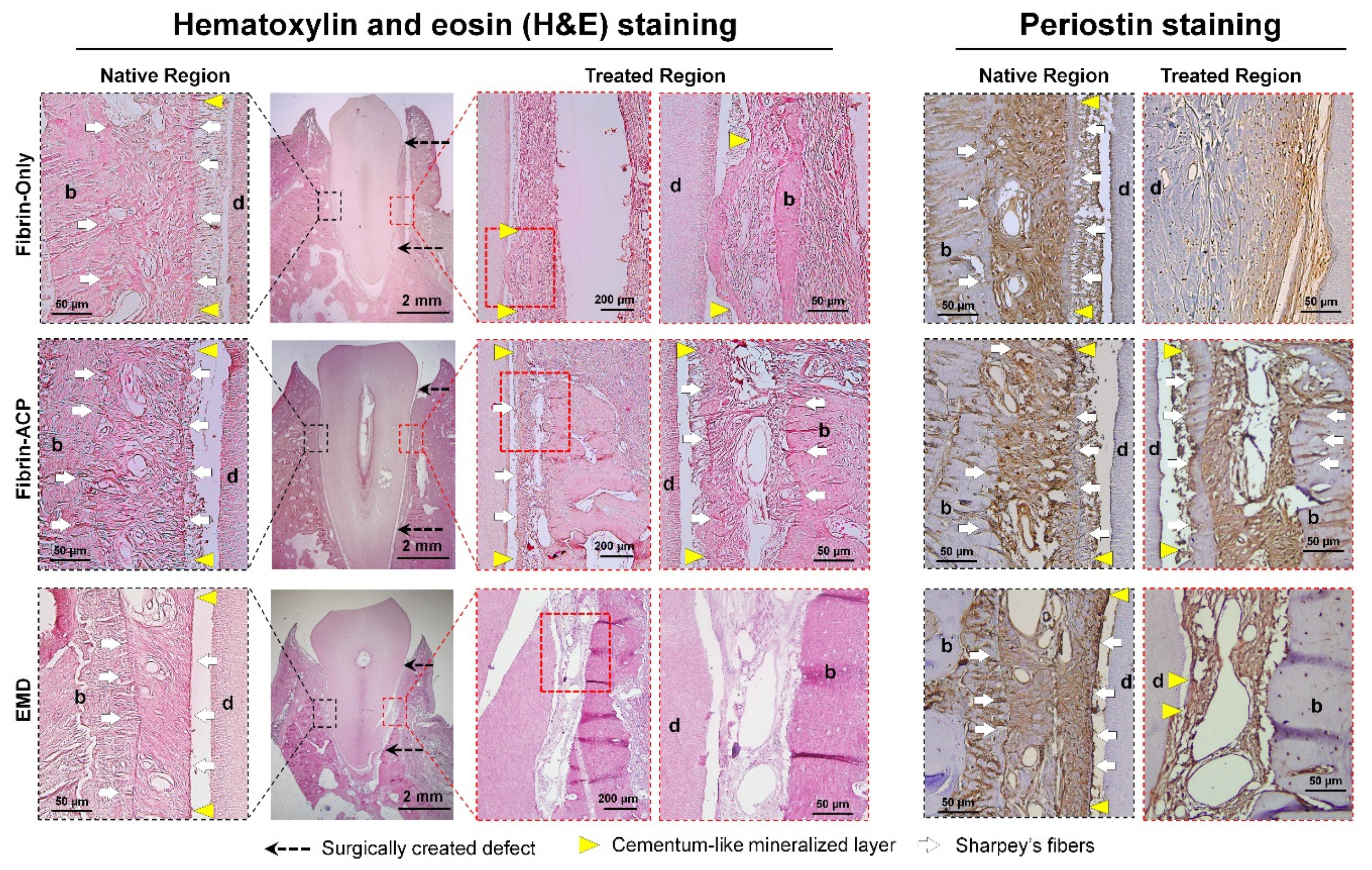
3. Cementum Regeneration on the Tooth-Root Surface Using Biomaterial-Based 3D Engineered Environments
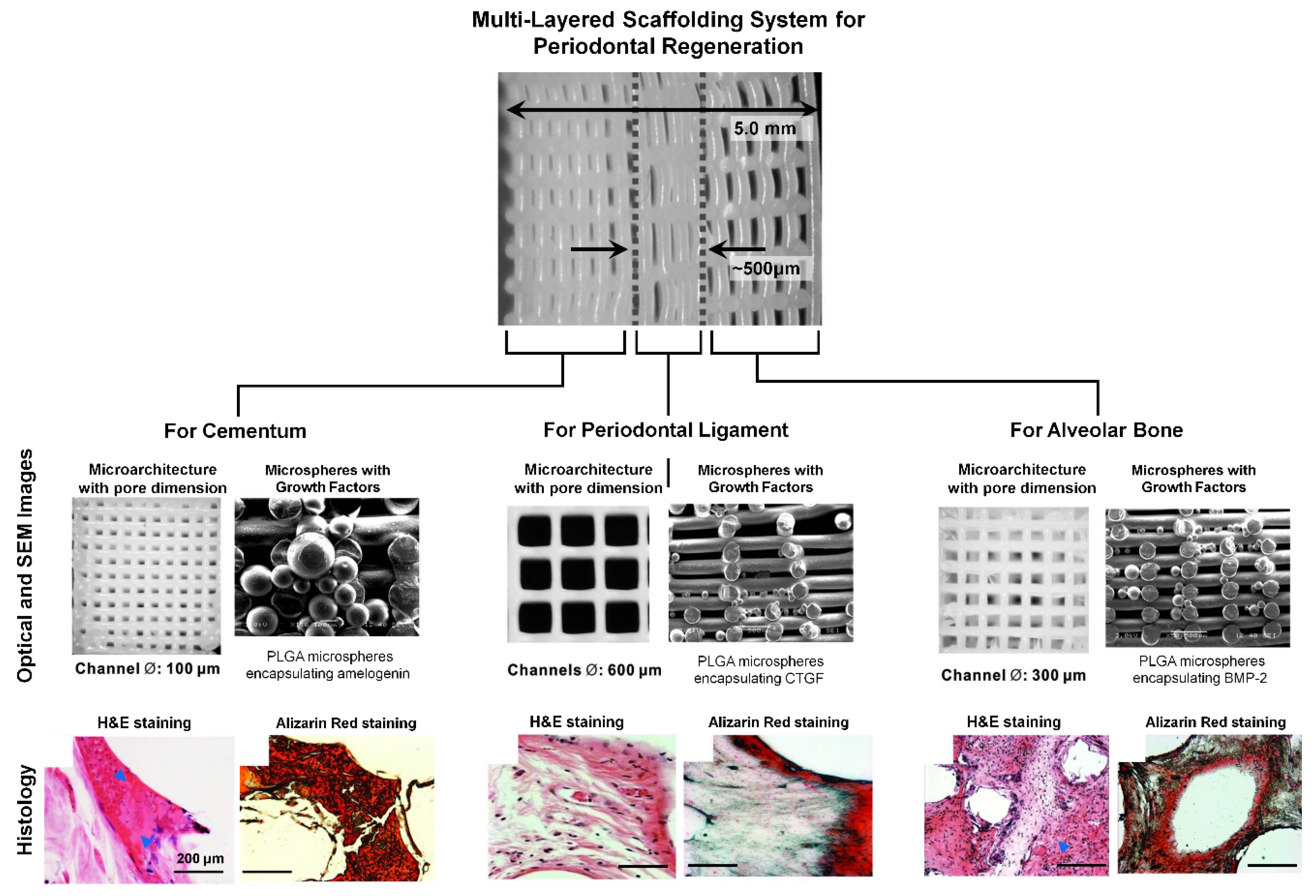
4. Future Prospects for Biomaterial-Based Periodontal Tissue Engineering
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDL | Periodontal ligament |
| CEJ | Cementoenamel junction |
| GTR | Guided tissue regeneration |
| 3D | 3-dimensional |
| Micro-CT | Micro-computed tomography |
| SEM | Scanning electron microscopy |
| PCL | Poly-ε-caprolactone |
| H&E | Hematoxylin and eosin |
| PCE | Poly-ε-caprolactone and polyethylene glycol |
| CVD | Chemical vapor deposition |
| TCD | Tissue culture dish |
| BSP | Bone sialoprotein |
| OCN | Osteocalcin |
| Runx2 | Runx-related gene 2 |
| MMPs | Matrix metalloproteinases |
| EMD | Enamel matrix derivative |
| ACA | ε-aminocaproic acid |
| ACP | ε-aminocaproic acid incorporated with chitosan nanoparticle |
| CaP | Calcium phosphate |
| CAP | Cementum attachment protein |
| CEMP | Cementum protein |
| PLGA | Poly(lactic-co-glycolic acid) |
| CTGF | Connective tissue growth factor |
| BMP-2 | Bone morphogenetic protein-2 |
| PDGF-BB | Platelet-derived growth factor-BB |
References
- Menicanin, D.; Hynes, K.; Han, J.; Gronthos, S.; Bartold, P.M. Cementum and Periodontal Ligament Regeneration. Adv. Exp. Med. Biol. 2015, 881, 207–236. [Google Scholar]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014, 93, 1304–1312. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59, 117–130. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, C.H.; Perez, R.A.; Lee, H.Y.; Jang, J.H.; Lee, H.H.; Wall, I.B.; Shi, S.; Kim, H.W. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J. Dent. Res. 2014, 93, 1203–1211. [Google Scholar] [CrossRef]
- Naveh, G.R.; Lev-Tov Chattah, N.; Zaslansky, P.; Shahar, R.; Weiner, S. Tooth-PDL-bone complex: Response to compressive loads encountered during mastication-a review. Arch. Oral. Biol. 2012, 57, 1575–1584. [Google Scholar] [CrossRef]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. 3D Printed, Microgroove Pattern-Driven Generation of Oriented Ligamentous Architectures. Int. J. Mol. Sci. 2017, 18, 1927. [Google Scholar] [CrossRef]
- De Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- LeBlanc, A.R.; Reisz, R.R. Periodontal ligament, cementum, and alveolar bone in the oldest herbivorous tetrapods, and their evolutionary significance. PLoS ONE 2013, 8, e74697. [Google Scholar] [CrossRef]
- Rios, H.F.; Ma, D.; Xie, Y.; Giannobile, W.V.; Bonewald, L.F.; Conway, S.J.; Feng, J.Q. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J. Periodontol 2008, 79, 1480–1490. [Google Scholar] [CrossRef]
- Park, C.H.; Oh, J.H.; Jung, H.M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.I.; Shin, H.I.; Lee, Y.S.; Yu, F.H.; et al. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017, 61, 134–143. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J. Dent. Res. 2005, 84, 390–406. [Google Scholar] [CrossRef]
- Jang, A.T.; Lin, J.D.; Choi, R.M.; Choi, E.M.; Seto, M.L.; Ryder, M.I.; Gansky, S.A.; Curtis, D.A.; Ho, S.P. Adaptive properties of human cementum and cementum dentin junction with age. J. Mech. Behav. Biomed. Mater. 2014, 39, 184–196. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Selvig, K.A. Dental cementum: The dynamic tissue covering of the root. Periodontol 2000 1997, 13, 41–75. [Google Scholar] [CrossRef]
- Foster, B.L. On the discovery of cementum. J. Periodontal Res. 2017, 52, 666–685. [Google Scholar] [CrossRef]
- Chen, F.M.; Jin, Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Tribble, G.D.; Lamont, R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000 2010, 52, 68–83. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y.S.; Choi, Y. Bacterial invasion and persistence: Critical events in the pathogenesis of periodontitis? J. Periodontal Res. 2015, 50, 570–585. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, K.H.; Lee, Y.M.; Seol, Y.J. Advanced Engineering Strategies for Periodontal Complex Regeneration. Materials 2016, 9, 57. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration–a materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Florjanski, W.; Orzeszek, S.; Olchowy, A.; Grychowska, N.; Wieckiewicz, W.; Malysa, A.; Smardz, J.; Wieckiewicz, M. Modifications of Polymeric Membranes Used in Guided Tissue and Bone Regeneration. Polymers 2019, 11, 782. [Google Scholar] [CrossRef]
- Liao, S.; Wang, W.; Uo, M.; Ohkawa, S.; Akasaka, T.; Tamura, K.; Cui, F.; Watari, F. A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration. Biomaterials 2005, 26, 7564–7571. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem. Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef]
- Sculean, A.; Chapple, I.L.; Giannobile, W.V. Wound models for periodontal and bone regeneration: The role of biologic research. Periodontol 2000 2015, 68, 7–20. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontol 2000 2012, 59, 185–202. [Google Scholar] [CrossRef]
- Grandfield, K.; Herber, R.P.; Chen, L.; Djomehri, S.; Tam, C.; Lee, J.H.; Brown, E.; Woolwine, W.R., 3rd; Curtis, D.; Ryder, M.; et al. Strain-guided mineralization in the bone-PDL-cementum complex of a rat periodontium. Bone Rep. 2015, 3, 20–31. [Google Scholar] [CrossRef]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem. Cells Dev. 2011, 20, 1659–1668. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Taut, A.D.; Padial-Molina, M.; Flanagan, C.L.; Pilipchuk, S.P.; Hollister, S.J.; Giannobile, W.V. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng. Part C Methods 2014, 20, 533–542. [Google Scholar] [CrossRef]
- McKee, M.D.; Hoac, B.; Addison, W.N.; Barros, N.M.; Millan, J.L.; Chaussain, C. Extracellular matrix mineralization in periodontal tissues: Noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol 2000 2013, 63, 102–122. [Google Scholar] [CrossRef]
- Lui, H.; Bindra, R.; Baldwin, J.; Ivanovski, S.; Vaquette, C. Additively Manufactured Multiphasic Bone-Ligament-Bone Scaffold for Scapholunate Interosseous Ligament Reconstruction. Adv. Healthc. Mater. 2019, 8, 1900133. [Google Scholar] [CrossRef]
- Abdal-Ha, A.; Hamlet, S.; Ivanovski, S. Fabrication of a thick three-dimensional scaffold with an open cellular-like structure using airbrushing and thermal cross-linking of molded short nanofibers. Biofabrication 2018, 11, 015006. [Google Scholar] [CrossRef]
- Tsumanuma, Y.; Iwata, T.; Kinoshita, A.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Yamato, M.; Okano, T.; Izumi, Y. Allogeneic Transplantation of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets in Canine Critical-Size Supra-Alveolar Periodontal Defect Model. Biores. Open Access 2016, 5, 22–36. [Google Scholar] [CrossRef]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef]
- Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Liu, X.; Sun, Y.; Fan, Y. Influence of Micropatterning on Human Periodontal Ligament Cells’ Behavior. Biophys. J. 2018, 114, 1988–2000. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, S.Y.; Lee, J.H.; Kim, D.H.; Yun, J.H. Evaluation of the periodontal regenerative properties of patterned human periodontal ligament stem cell sheets. J. Periodontal Implant Sci. 2017, 47, 402–415. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- Shi, X.; Fujie, T.; Saito, A.; Takeoka, S.; Hou, Y.; Shu, Y.; Chen, M.; Wu, H.; Khademhosseini, A. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv. Mater. 2014, 26, 3290–3296. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Itoga, K.; Yamato, M.; Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 2011, 12, 1414–1418. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Fretwurst, T.; Yu, N.; Larsson, L.; Kavanagh, N.M.; Asa’ad, F.; Cheng, K.C.K.; Lahann, J.; Giannobile, W.V. Micropatterned Scaffolds with Immobilized Growth Factor Genes Regenerate Bone and Periodontal Ligament-Like Tissues. Adv. Healthc. Mater. 2018, 7, e1800750. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.S.; Eltohamy, M.; Kim, T.H.; Kim, H.W. Dynamic Mechanical and Nanofibrous Topological Combinatory Cues Designed for Periodontal Ligament Engineering. PLoS ONE 2016, 11, e0149967. [Google Scholar] [CrossRef]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef]
- Shang, S.; Yang, F.; Cheng, X.; Walboomers, X.F.; Jansen, J.A. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur. Cell Mater. 2010, 19, 180–192. [Google Scholar] [CrossRef]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef]
- Kim, J.J.; Bae, W.J.; Kim, J.M.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J. Biomater. Appl. 2014, 28, 1069–1078. [Google Scholar] [CrossRef]
- Bae, W.J.; Min, K.S.; Kim, J.J.; Kim, J.J.; Kim, H.W.; Kim, E.C. Odontogenic responses of human dental pulp cells to collagen/nanobioactive glass nanocomposites. Dent. Mater. 2012, 28, 1271–1279. [Google Scholar] [CrossRef]
- Kobayashi, M.; Khalil, H.A.; Lei, N.Y.; Wang, Q.; Wang, K.; Wu, B.M.; Dunn, J.C.Y. Bioengineering functional smooth muscle with spontaneous rhythmic contraction in vitro. Sci. Rep. 2018, 8, 13544. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Sun, Z.; Su, W.; Pan, G.; Zhao, S.; Zhao, J.; Cui, W. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 2017, 61, 204–216. [Google Scholar] [CrossRef]
- Yang, M.; Gao, X.; Shen, Z.; Shi, X.; Lin, Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019, 9, 507–518. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Anusaksathien, O.; Jin, Q.; Zhao, M.; Somerman, M.J.; Giannobile, W.V. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J. Periodontol 2004, 75, 429–440. [Google Scholar] [CrossRef]
- Jin, Q.M.; Zhao, M.; Webb, S.A.; Berry, J.E.; Somerman, M.J.; Giannobile, W.V. Cementum engineering with three-dimensional polymer scaffolds. J. Biomed. Mater. Res. A 2003, 67, 54–60. [Google Scholar] [CrossRef]
- Monteiro, N.; Yelick, P.C. Advances and perspectives in tooth tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2443–2461. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Wikesjo, U.M.; Sorensen, R.G.; Kinoshita, A.; Jian Li, X.; Wozney, J.M. Periodontal repair in dogs: Effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J. Clin. Periodontol 2004, 31, 662–670. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010, 20, 8730–8746. [Google Scholar] [CrossRef]
- Lemaitre, M.; Monsarrat, P.; Blasco-Baque, V.; Loubieres, P.; Burcelin, R.; Casteilla, L.; Planat-Benard, V.; Kemoun, P. Periodontal Tissue Regeneration Using Syngeneic Adipose-Derived Stromal Cells in a Mouse Model. Stem. Cells Transl. Med. 2017, 6, 656–665. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem. Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomedicine 2015, 10, 7031–7044. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem. Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef]
- Gronthos, S.; Mrozik, K.; Shi, S.; Bartold, P.M. Ovine periodontal ligament stem cells: Isolation, characterization, and differentiation potential. Calcif. Tissue Int. 2006, 79, 310–317. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef]
- Ji, K.; Liu, Y.; Lu, W.; Yang, F.; Yu, J.; Wang, X.; Ma, Q.; Yang, Z.; Wen, L.; Xuan, K. Periodontal tissue engineering with stem cells from the periodontal ligament of human retained deciduous teeth. J. Periodontal Res. 2013, 48, 105–116. [Google Scholar] [CrossRef]
- Maeda, K.; Takahashi, N.; Kobayashi, Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J. Mol. Med. (Berl.) 2013, 91, 15–23. [Google Scholar] [CrossRef]
- Han, P.; Ivanovski, S.; Crawford, R.; Xiao, Y. Activation of the Canonical Wnt Signaling Pathway Induces Cementum Regeneration. J. Bone Miner. Res. 2015, 30, 1160–1174. [Google Scholar] [CrossRef]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.M.; Piccolo, S.; Schmidt-Ullrich, R.; et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef]
- Scheller, E.L.; Chang, J.; Wang, C.Y. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J. Dent. Res. 2008, 87, 126–130. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, G.; Zheng, Y.; Zhu, X.; Chen, Z.; Zhang, Z.; Chen, Y. The non-canonical BMP and Wnt/beta-catenin signaling pathways orchestrate early tooth development. Development 2015, 142, 128–139. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, G.; Chen, M.; Wang, C.; He, L.; Xiang, L.; Chen, D.; Ling, J.; Mao, J.J. Lhx8 mediated Wnt and TGFbeta pathways in tooth development and regeneration. Biomaterials 2015, 63, 35–46. [Google Scholar] [CrossRef]
- Rahman, S.U.; Park, C.H.; Baek, J.H.; Ryoo, H.M.; Woo, K.M. Fibrin-Enhanced Canonical Wnt Signaling Directs Plasminogen Expression in Cementoblasts. Int. J. Mol. Sci. 2017, 18, 2380. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. https://doi.org/10.3390/ijms20184364
Park CH. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. International Journal of Molecular Sciences. 2019; 20(18):4364. https://doi.org/10.3390/ijms20184364
Chicago/Turabian StylePark, Chan Ho. 2019. "Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms" International Journal of Molecular Sciences 20, no. 18: 4364. https://doi.org/10.3390/ijms20184364
APA StylePark, C. H. (2019). Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. International Journal of Molecular Sciences, 20(18), 4364. https://doi.org/10.3390/ijms20184364




