Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use
Abstract
1. Introduction
2. Clinical Trials with Melatonin in Heart Diseases
2.1. Acute Coronary Syndrome
2.2. Coronary Artery Disease
2.3. Cardiac Arrhythmias
2.4. Heart Failure
2.5. Hypertension
2.6. Melatonin Receptor Agonists
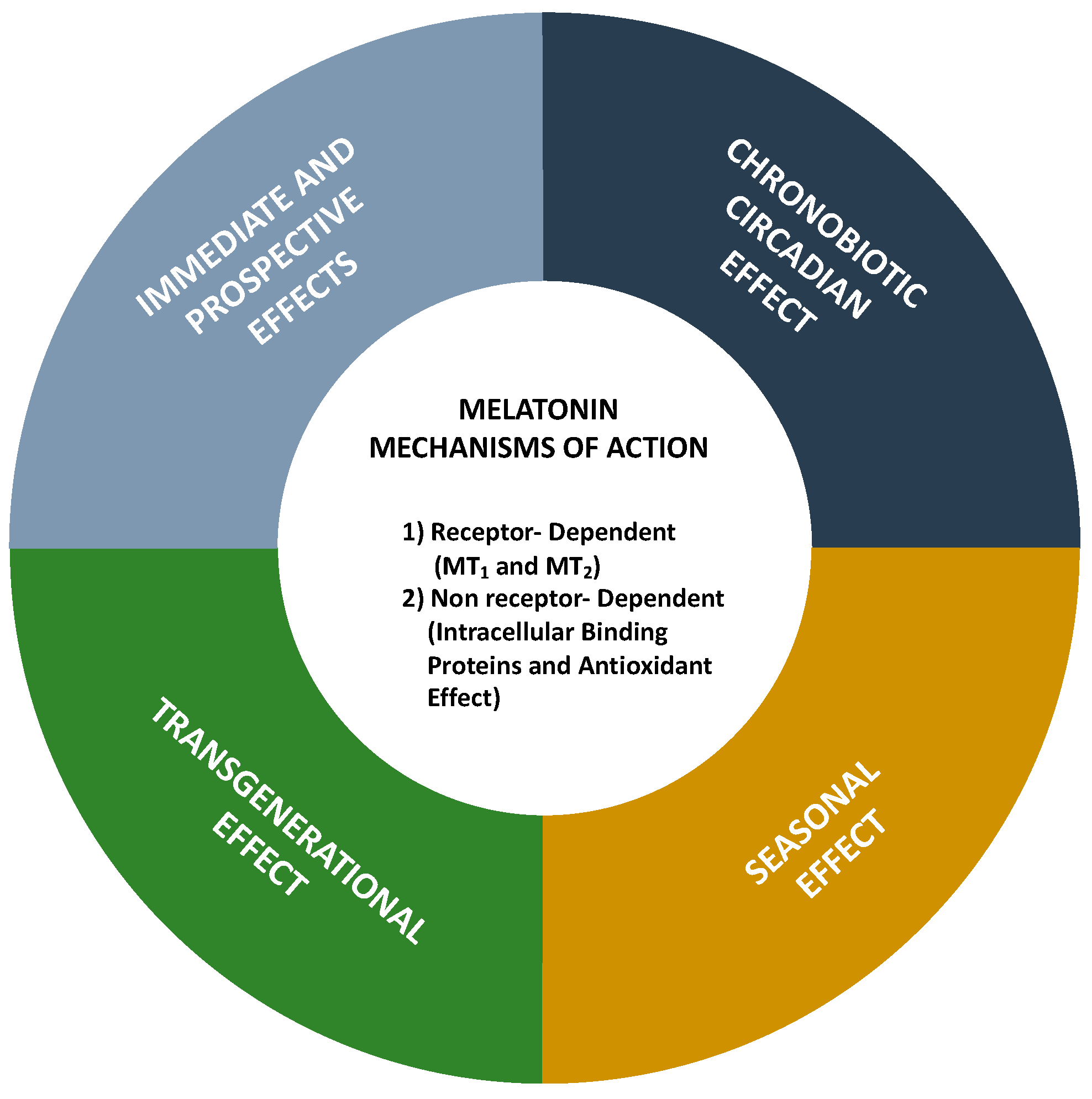
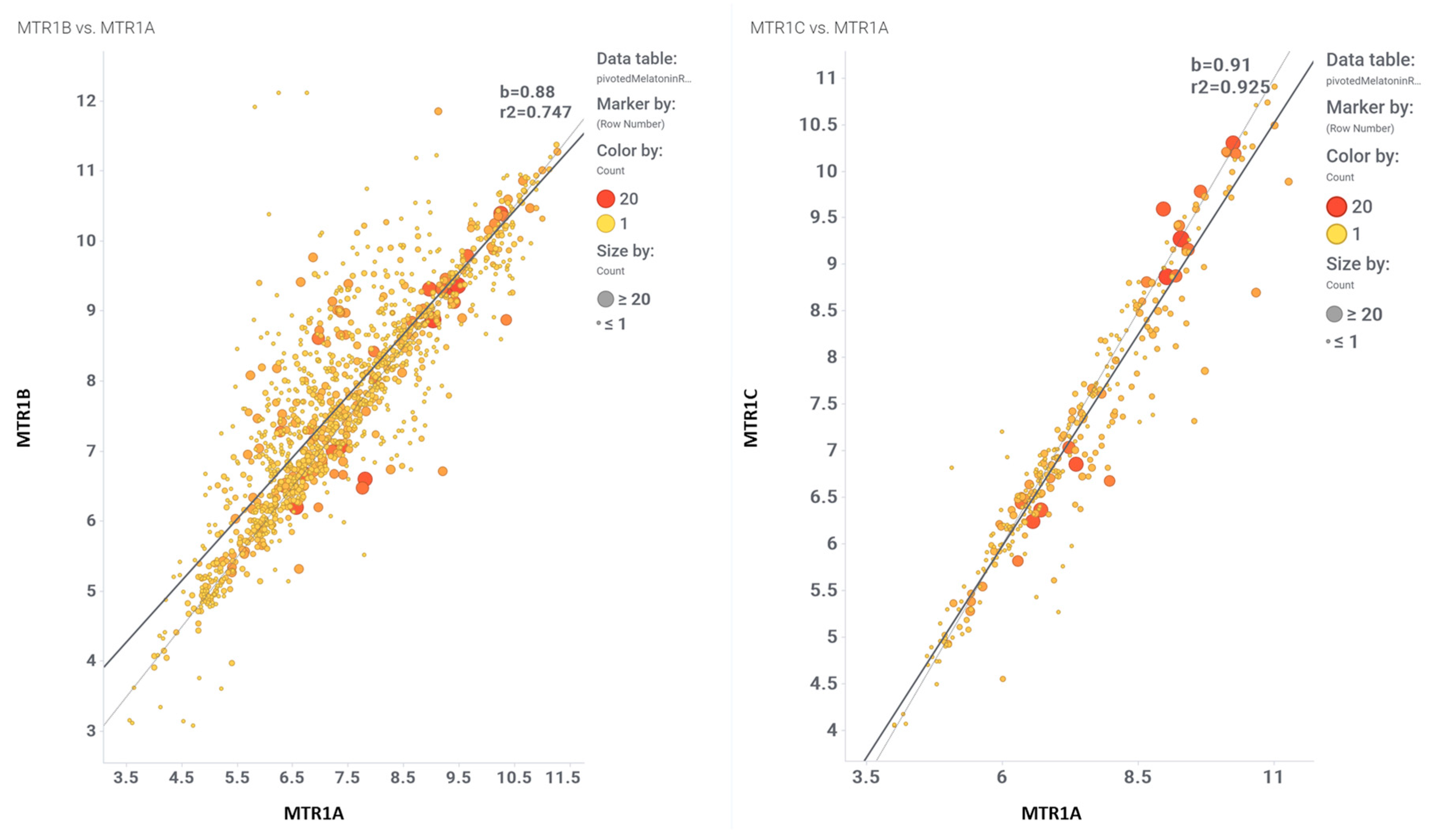
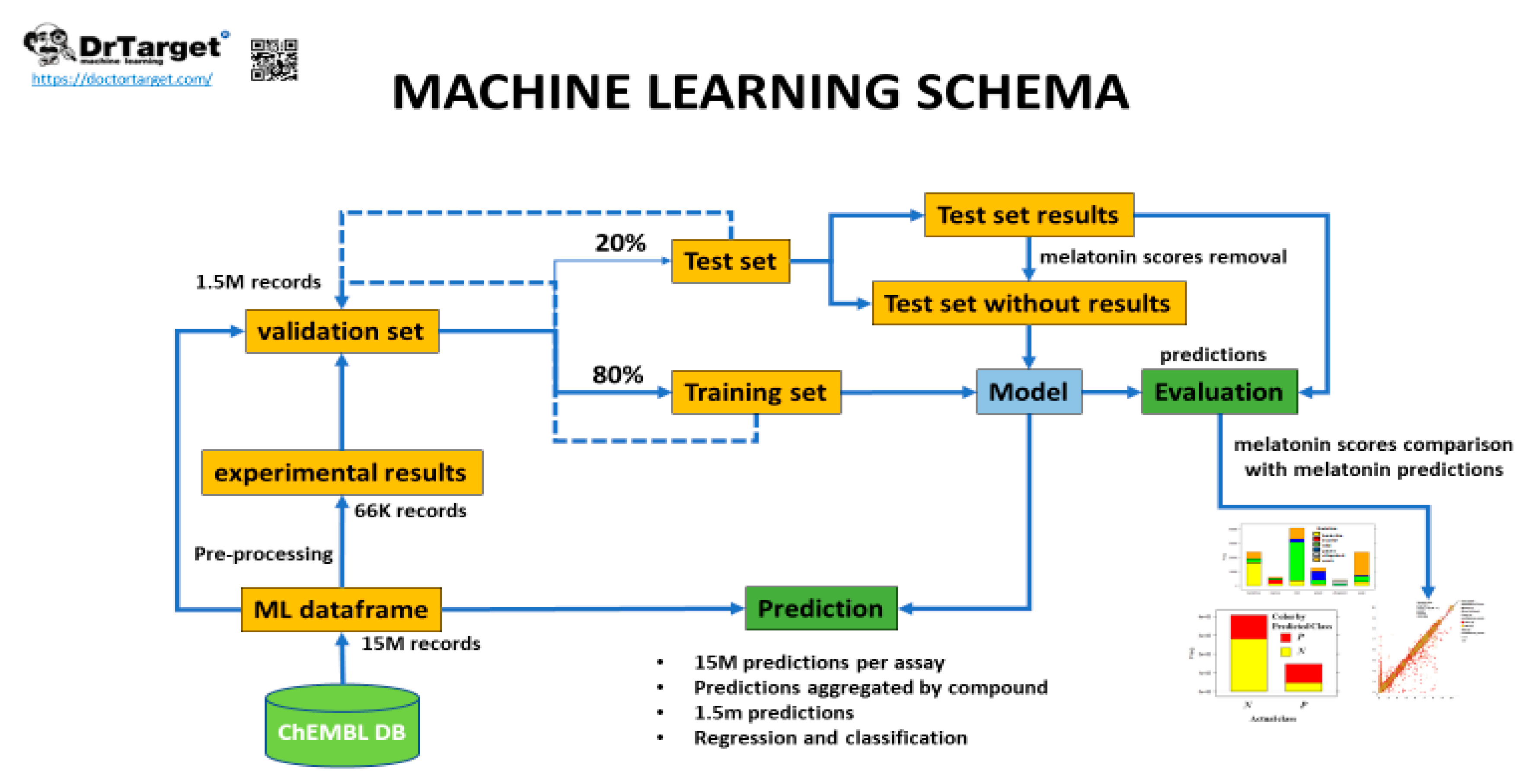
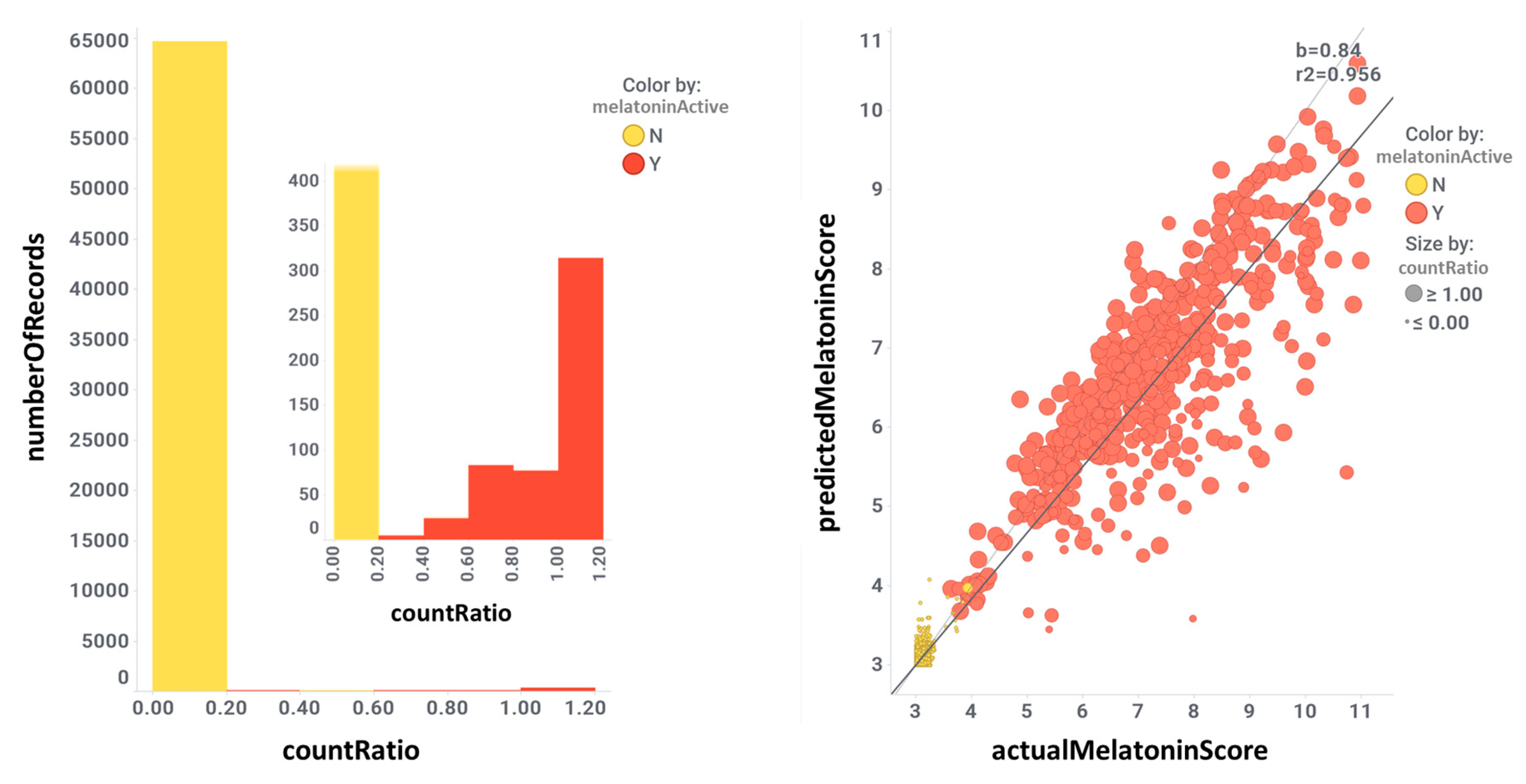
3. Evaluation of Melatonin Cardiovascular Target Tractability and Prediction Using Machine Learning on ChEMBL
4. Conclusions and Further Directions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, F.G.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Bouatia-Naji, N.; Bonnefond, A.; Cavalcanti-Proença, C.; Sparsø, T.; Holmkvist, J.; Marchand, M.; Delplanque, J.; Lobbens, S.; Rocheleau, G.; Durand, E.; et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.; Ljunggren, E.; Bremer, A.; Pedersen, C.; Landén, M.; Thuresson, K.; Giacobini, M.; Melke, J. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med. Genom. 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.A.; Cipolla-Neto, J.; Amaral, F.G.; Michelini, L.C.; Bader, M.; Baltatu, O.C. The Angiotensin-melatonin axis. Int. J. Hypertens 2013, 2013, 521783. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.C.; Amaral, F.G.; Campos, L.A.; Cipolla-Neto, J. Melatonin, mitochondria and hypertension. Cell Mol. Life Sci. 2017, 74, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular benefits of dietary melatonin: A myth or a reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Patiño, P.; Reiter, R.J.; Gil-Martín, E.; López-Muñoz, F.; Romero, A. Melatonin: A hypothesis for Kawasaki disease treatment. Med. Hypotheses 2018, 119, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Avanzas, P. The role of melatonin in acute myocardial infarction. Front. Biosci. 2012, 17, 2433–2441. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Reiter, R.J. Clinical aspects of melatonin in the acute coronary syndrome. Curr. Vasc. Pharm. 2009, 7, 367–373. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc. Res. 2003, 58, 10–19. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; de la Torre-Hernandez, J.M.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Consuegra-Sanchez, L.; Garcia-Saiz, M.D.M.; Aldea-Perona, A.; Virgos-Aller, T.; Azpeitia, A.; et al. MARIA Investigators Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: Results of the Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty trial. J. Pineal. Res. 2017, 62. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; de la Torre-Hernandez, J.M.; Consuegra-Sanchez, L.; Piccolo, R.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Del Mar Garcia-Saiz, M.; Aldea-Perona, A.; Reiter, R.J. MARIA Investigators Usefulness of Early Treatment With Melatonin to Reduce Infarct Size in Patients With ST-Segment Elevation Myocardial Infarction Receiving Percutaneous Coronary Intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated With Angioplasty Trial). Am. J. Cardiol. 2017, 120, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T.; et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef] [PubMed]
- Ekeloef, S.; Halladin, N.; Fonnes, S.; Jensen, S.E.; Zaremba, T.; Rosenberg, J.; Jonsson, G.; Aarøe, J.; Gasbjerg, L.S.; Rosenkilde, M.M.; et al. Effect of Intracoronary and Intravenous Melatonin on Myocardial Salvage Index in Patients with ST-Elevation Myocardial Infarction: A Randomized Placebo Controlled Trial. J. Cardiovasc. Transl. Res. 2017, 10, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Gögenur, I.; Kücükakin, B.; Panduro Jensen, L.; Reiter, R.J.; Rosenberg, J. Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: A randomized, placebo-controlled, clinical trial. J. Pineal. Res. 2014, 57, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Green, E.A.; Black, B.K.; Biaggioni, I.; Paranjape, S.Y.; Bagai, K.; Shibao, C.; Okoye, M.C.; Dupont, W.D.; Robertson, D.; Raj, S.R. Melatonin reduces tachycardia in postural tachycardia syndrome: A randomized, crossover trial. Cardiovasc. Ther. 2014, 32, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Haghjooy Javanmard, S.; Ziaei, A.; Ziaei, S.; Ziaei, E.; Mirmohammad-Sadeghi, M. The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxid. Med. Cell. Longev. 2013, 2013, 676829. [Google Scholar] [CrossRef]
- Wirtz, P.H.; Spillmann, M.; Bärtschi, C.; Ehlert, U.; von Känel, R. Oral melatonin reduces blood coagulation activity: A placebo-controlled study in healthy young men. J. Pineal Res. 2008, 44, 127–133. [Google Scholar] [CrossRef]
- Madsen, M.T.; Isbrand, A.; Andersen, U.O.; Andersen, L.J.; Taskiran, M.; Simonsen, E.; Gögenur, I. The effect of MElatonin on Depressive symptoms, Anxiety, CIrcadian and Sleep disturbances in patients after acute coronary syndrome (MEDACIS): Study protocol for a randomized controlled trial. Trials 2017, 18, 81. [Google Scholar] [CrossRef]
- Halladin, N.L.; Busch, S.E.; Jensen, S.E.; Hansen, H.S.; Zaremba, T.; Aarøe, J.; Rosenberg, J.; Gögenur, I. Intracoronary and systemic melatonin to patients with acute myocardial infarction: Protocol for the IMPACT trial. Dan. Med. J. 2014, 61, A4773. [Google Scholar] [PubMed]
- Rechciński, T.; Trzos, E.; Wierzbowska-Drabik, K.; Krzemińska-Pakuła, M.; Kurpesa, M. Melatonin for nondippers with coronary artery disease: Assessment of blood pressure profile and heart rate variability. Hypertens Res. 2010, 33, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Kim, S.J.; El-Sokkary, G.H. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: Prevention by melatonin. J. Pineal Res. 1998, 25, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sahna, E.; Olmez, E.; Acet, A. Effects of physiological and pharmacological concentrations of melatonin on ischemia-reperfusion arrhythmias in rats: Can the incidence of sudden cardiac death be reduced? J. Pineal Res. 2002, 32, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Knezl, V.; Viczenczova, C.; Bacova, B.S.; Radosinska, J.; Tribulova, N. Acute anti-fibrillating and defibrillating potential of atorvastatin, melatonin, eicosapentaenoic acid and docosahexaenoic acid demonstrated in isolated heart model. J. Physiol. Pharm. 2015, 66, 83–89. [Google Scholar]
- Blatt, C.M.; Rabinowitz, S.H.; Lown, B. Central serotonergic agents raise the repetitive extrasystole threshold of the vulnerable period of the canine ventricular myocardium. Circ. Res. 1979, 44, 723–730. [Google Scholar] [CrossRef]
- Diez, E.R.; Prados, L.V.; Carrión, A.; Ponce, Z.A.Z.; Miatello, R.M. A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res. 2009, 46, 155–160. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.J.; Géczy, T.; Szili-Torok, T. Sleep Medications Containing Melatonin can Potentially Induce Ventricular Arrhythmias in Structurally Normal Hearts: A 2-Patient Report. J. Cardiovasc. Pharm. 2017, 70, 267–270. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 2013, 55, 275–286. [Google Scholar] [CrossRef]
- Şehirli, A.Ö.; Koyun, D.; Tetik, Ş.; Özsavcı, D.; Yiğiner, Ö.; Çetinel, Ş.; Tok, O.E.; Kaya, Z.; Akkiprik, M.; Kılıç, E.; et al. Melatonin protects against ischemic heart failure in rats. J. Pineal Res. 2013, 55, 138–148. [Google Scholar] [CrossRef]
- Meneuvonen, P.J.; Karppanen, H. Effects of hydrochlorothiazide, furosemide and ethacrynic acid on pinealectomy-induced hypertension in rats. Ann Med. Exp. Biol. Fenn. 1971, 49, 120–124. [Google Scholar] [PubMed]
- Holmes, S.W.; Sugden, D. Proceedings: The effect of melatonin on pinealectomy-induced hypertension in the rat. Br. J. Pharm. 1976, 56, 360P–361P. [Google Scholar]
- Lusardi, P.; Preti, P.; Savino, S.; Piazza, E.; Zoppi, A.; Fogari, R. Effect of bedtime melatonin ingestion on blood pressure of normotensive subjects. Blood Press. Monit. 1997, 2, 99–103. [Google Scholar] [PubMed]
- Lusardi, P.; Piazza, E.; Fogari, R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: A 24-hour study. Br. J. Clin. Pharm. 2000, 49, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Van Montfrans, G.A.; van Someren, E.J.W.; Mairuhu, G.; Buijs, R.M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Cannoletta, M.; Renzi, A.; Baldassari, F.; Arangino, S.; Volpe, A. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am. J. Hypertens. 2005, 18, 1614–1618. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 2006, 119, 898–902. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Morris, C.J.; Garcia, J.I.; Smales, C.; Kelly, E.E.; Marks, J.; Malhotra, A.; Shea, S.A. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: A randomized controlled trial. Sleep 2012, 35, 1395–1402. [Google Scholar] [CrossRef]
- Rahbari-Oskoui, F.F.; Abramson, J.L.; Bruckman, A.M.; Chapman, A.B.; Cotsonis, G.A.; Johnson, S.A.; Bliwise, D.L. Nighttime administration of high-dose, sustained-release melatonin does not decrease nocturnal blood pressure in African-American patients: Results from a preliminary randomized, crossover trial. Complement Ther. Med. 2019, 43, 157–164. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Zisapel, N. Effect of melatonin on nocturnal blood pressure: Meta-analysis of randomized controlled trials. Vasc. Health Risk Manag. 2011, 7, 577–584. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharm. 2015, 71, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P.T.; McLin, D.E.; Dressman, M.A.; Neubauer, D.N. Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders. Pharmacotherapy 2016, 36, 1028–1041. [Google Scholar] [CrossRef]
- Griesenauer, R.H.; Schillebeeckx, C.; Kinch, M.S. Assessing the public landscape of clinical-stage pharmaceuticals through freely available online databases. Drug Discov. Today 2019, 24, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Mechanism of action of tasimelteon in non-24 sleep-wake syndrome: Treatment for a circadian rhythm disorder in blind patients. CNS Spectr. 2014, 19, 475–478. [Google Scholar] [CrossRef]
- Neubauer, D.N. Tasimelteon for the treatment of non-24-hour sleep-wake disorder. Drugs Today 2015, 51, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, S.M.; Polymeropoulos, M.H.; Fisher, D.M.; Roth, T.; Scott, C.; Birznieks, G.; Klerman, E.B. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: Two randomised controlled multicentre trials. Lancet 2009, 373, 482–491. [Google Scholar] [CrossRef]
- Stroethoff, M.; Behmenburg, F.; Spittler, K.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Mathes, A. Activation of melatonin receptors by ramelteon induces cardioprotection by postconditioning in the rat heart. Anesth. Analg. 2018, 126, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Stroethoff, M.; Christoph, I.; Behmenburg, F.; Raupach, A.; Bunte, S.; Senpolat, S.; Heinen, A.; Hollmann, M.W.; Mathes, A.; Huhn, R. Melatonin Receptor Agonist Ramelteon Reduces Ischemia-Reperfusion Injury Through Activation of Mitochondrial Potassium Channels. J. Cardiovasc. Pharm. 2018, 72, 106–111. [Google Scholar] [CrossRef]
- Kuriyama, A.; Honda, M.; Hayashino, Y. Ramelteon for the treatment of insomnia in adults: A systematic review and meta-analysis. Sleep Med. 2014, 15, 385–392. [Google Scholar] [CrossRef]
- Mahableshwarkar, A.R.; Calabrese, J.R.; Macek, T.A.; Budur, K.; Adefuye, A.; Dong, X.; Hanson, E.; Sachs, G.S. Efficacy and safety of sublingual ramelteon as an adjunctive therapy in the maintenance treatment of bipolar I disorder in adults: A phase 3, randomized controlled trial. J. Affect. Disord. 2017, 221, 275–282. [Google Scholar] [CrossRef]
- De Berardis, D.; Di Iorio, G.; Acciavatti, T.; Conti, C.; Serroni, N.; Olivieri, L.; Cavuto, M.; Martinotti, G.; Janiri, L.; Moschetta, F.S.; et al. The emerging role of melatonin agonists in the treatment of major depression: Focus on agomelatine. CNS Neurol. Disord. Drug Targets 2011, 10, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Flaugh, M.E.; Crowell, T.A.; Clemens, J.A.; Sawyer, B.D. Synthesis and evaluation of the antiovulatory activity of a variety of melatonin analogues. J. Med. Chem. 1979, 22, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.I.; Witt-Enderby, P.A.; Julius, J. Synthesis and pharmacological evaluation of pentacyclic 6a,7-dihydrodiindole and 2,3-dihydrodiindole derivatives as novel melatoninergic ligands. Bioorg. Med. Chem. 2008, 16, 7654–7661. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, G.; Stankov, B.; Duranti, A.; Biella, G.; Lucini, V.; Salvatori, A.; Fraschini, F. 2-Substituted 5-methoxy-N-acyltryptamines: Synthesis, binding affinity for the melatonin receptor, and evaluation of the biological activity. J. Med. Chem. 1993, 36, 4069–4074. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, G.; Diamantini, G.; Di Giacomo, B.; Spadoni, G.; Esposti, D.; Nonno, R.; Lucini, V.; Pannacci, M.; Fraschini, F.; Stankov, B.M. 1-(2-Alkanamidoethyl)-6-methoxyindole derivatives: A new class of potent indole melatonin analogues. J. Med. Chem. 1997, 40, 2003–2010. [Google Scholar] [CrossRef]
- Mor, M.; Rivara, S.; Silva, C.; Bordi, F.; Plazzi, P.V.; Spadoni, G.; Diamantini, G.; Balsamini, C.; Tarzia, G.; Fraschini, F.; et al. Melatonin receptor ligands: Synthesis of new melatonin derivatives and comprehensive comparative molecular field analysis (CoMFA) study. J. Med. Chem. 1998, 41, 3831–3844. [Google Scholar] [CrossRef] [PubMed]
- Rivara, S.; Mor, M.; Silva, C.; Zuliani, V.; Vacondio, F.; Spadoni, G.; Bedini, A.; Tarzia, G.; Lucini, V.; Pannacci, M.; et al. Three-dimensional quantitative structure-activity relationship studies on selected MT1 and MT2 melatonin receptor ligands: Requirements for subtype selectivity and intrinsic activity modulation. J. Med. Chem. 2003, 46, 1429–1439. [Google Scholar] [CrossRef]
- Spadoni, G.; Balsamini, C.; Diamantini, G.; Di Giacomo, B.; Tarzia, G.; Mor, M.; Plazzi, P.V.; Rivara, S.; Lucini, V.; Nonno, R.; et al. Conformationally restrained melatonin analogues: Synthesis, binding affinity for the melatonin receptor, evaluation of the biological activity, and molecular modeling study. J. Med. Chem. 1997, 40, 1990–2002. [Google Scholar] [CrossRef]
- Zlotos, D.P.; Jockers, R.; Cecon, E.; Rivara, S.; Witt-Enderby, P.A. MT1 and MT2 melatonin receptors: Ligands, models, oligomers, and therapeutic potential. J. Med. Chem. 2014, 57, 3161–3185. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; McCorvy, J.D.; Patel, N.; Han, G.W.; Huang, X.-P.; Gati, C.; Batyuk, A.; Slocum, S.T.; Ishchenko, A.; et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature 2019, 569, 284–288. [Google Scholar] [CrossRef]
- Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Han, G.W.; Patel, N.; Huang, X.-P.; Batyuk, A.; Gati, C.; Slocum, S.T.; Li, C.; et al. XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity. Nature 2019, 569, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, R.; Miyao, T.; Jasial, S.; Vogt, M.; Bajorath, J. Prediction of compound profiling matrices using machine learning. ACS Omega 2018, 3, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Nowotka, M.; Papadatos, G.; Dedman, N.; Gaulton, A.; Atkinson, F.; Bellis, L.; Overington, J.P. ChEMBL web services: Streamlining access to drug discovery data and utilities. Nucleic Acids Res. 2015, 43, W612–W620. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; De Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Mervin, L.H.; Afzal, A.M.; Drakakis, G.; Lewis, R.; Engkvist, O.; Bender, A. Target prediction utilising negative bioactivity data covering large chemical space. J. Cheminform. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Varnek, A.; Baskin, I. Machine learning methods for property prediction in chemoinformatics: Quo Vadis? J. Chem. Inf. Model. 2012, 52, 1413–1437. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Vickery, B.; Kouzi, S.; Patel, K. Melatonin use in an inpatient academic medical center: Factors affecting provider documentation of patients’ sleep quality. J. Am. Pharm. Assoc. 2019, 59, 533–538. [Google Scholar] [CrossRef] [PubMed]
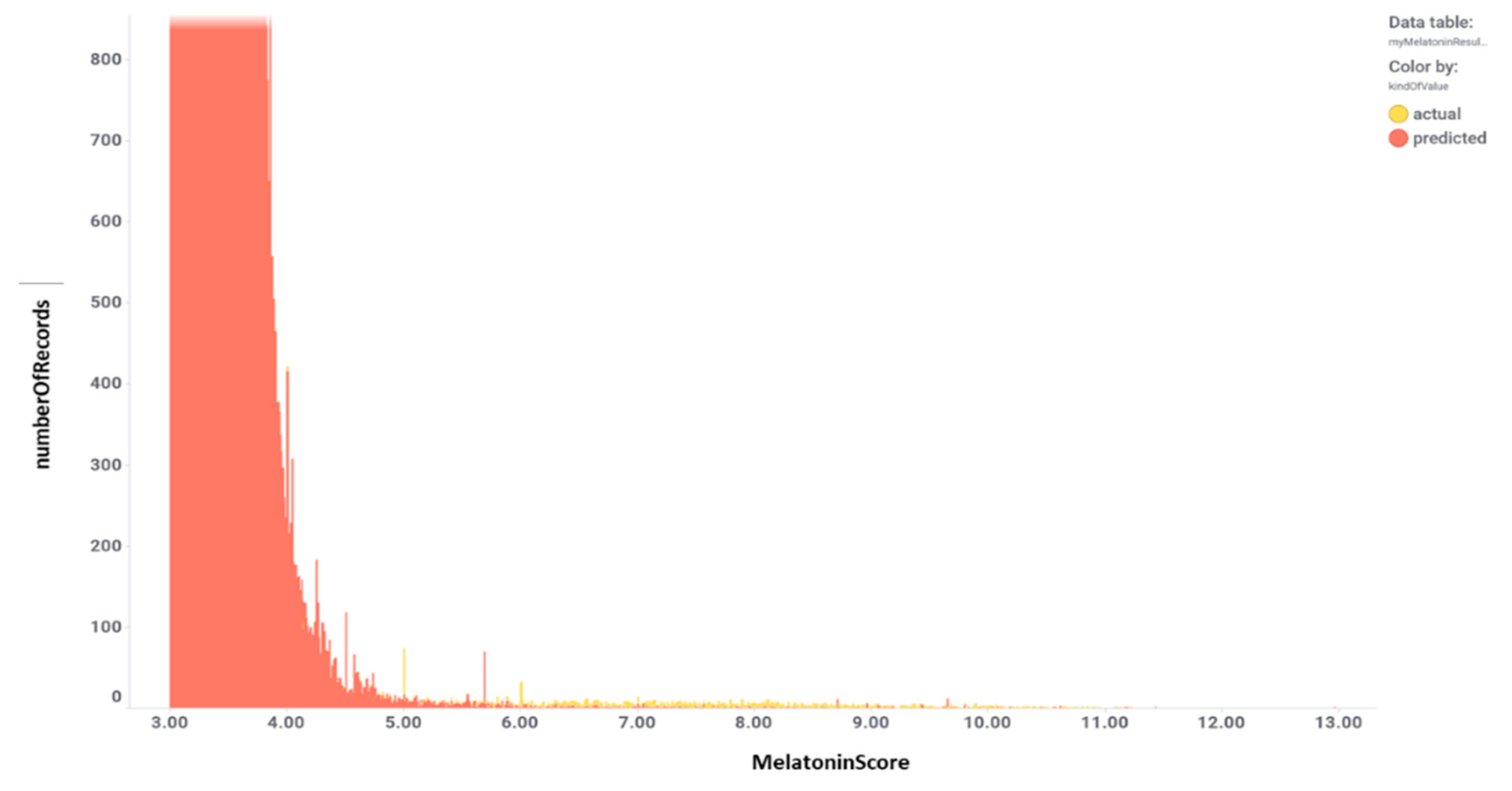
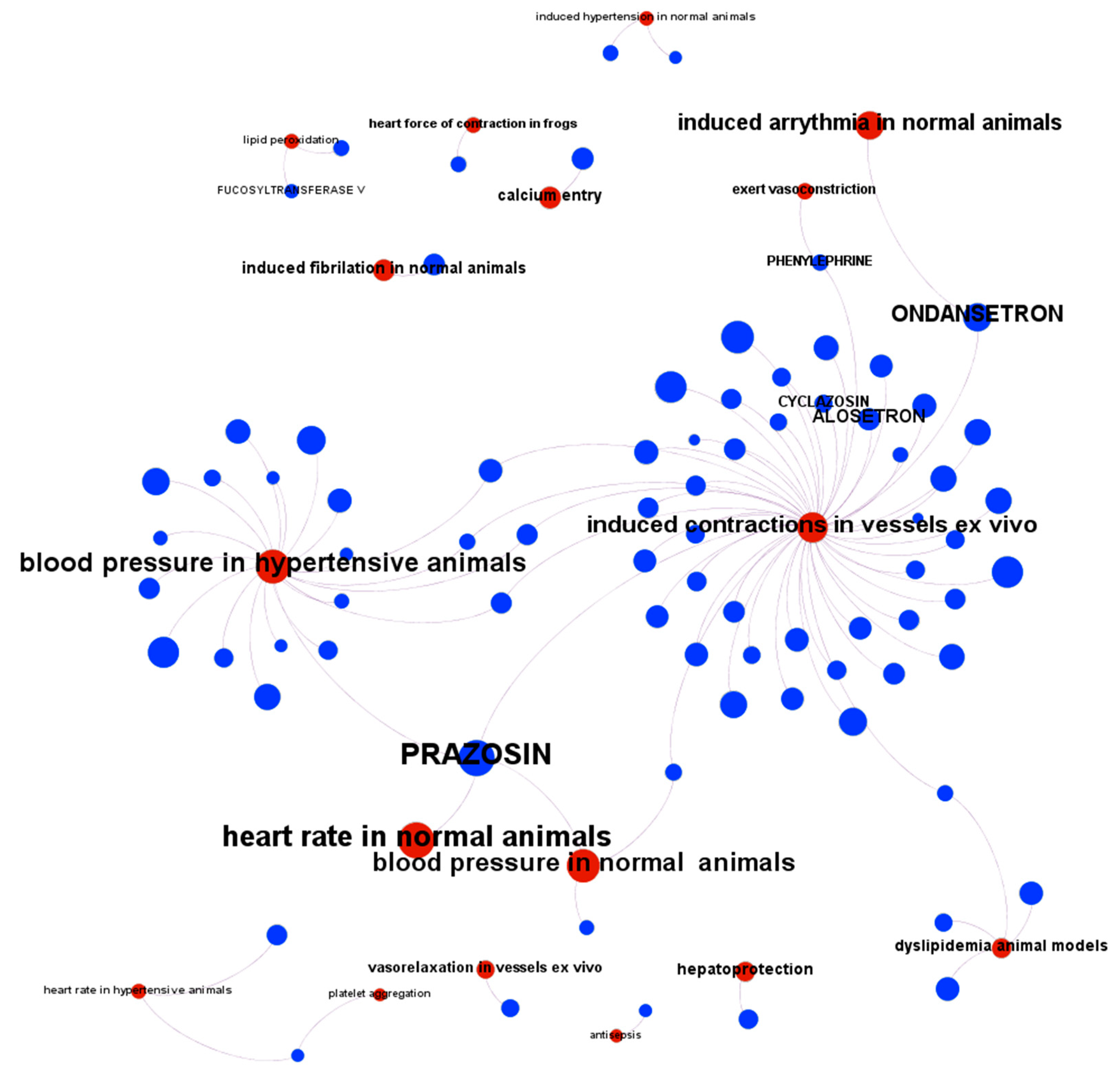
| Target Disease, Reference | Objective | Study Phase | Study Design | Melatonin dosing | Outcomes |
|---|---|---|---|---|---|
| Myocardial Infarction [12] | Melatonin Adjunct in the Acute myocaRdial Infarction Treated With Angioplasty (MARIA)/NCT00640094 | 2 | randomized, double-blind, placebo controlled trial | Intravenous and intracoronary melatonin during primary percutaneous coronary intervention (PPCI). | Negative: melatonin in patients with STEMI was not associated with a reduction in infarct size and has an unfavourable effect on the ventricular volumes and LVEF evolution |
| ST-Elevation Myocardial Infarction (STEMI) [13] | Post hoc of MARIA study/NCT00640094 | 2 | randomized, double-blind, placebo controlled trial | Positive: melatonin in patients with STEMI who presented early after symptom onset was associated with a significant reduction in the infarct size after pPCI | |
| Coronary artery bypass grafting [14] | Efficacy of melatonin in reducing early reperfusion injury and acute oxidative stress in patients undergoing coronary artery bypass grafting (CABG). | 2 | randomized, open-label, placebo-controlled trial | Positive: melatonin significantly reduced CABG related cardiac injury and oxidative stress. | |
| ST-Elevation Myocardial Infarction (STEMI) [15] | To study whether the administration of melatonin during acute myocardial reperfusion improves myocardial salvage assessed by cardiac magnetic resonance imaging (CMR) in patients with STEMI | 2 | randomized, double-blinded, placebo controlled trial | intracoronary or intravenous melatonin (total 50 mg) | Negative: melatonin did not improve the myocardial salvage index. |
| Elective abdominal aortic aneurism repair [16] | To study the effect of perioperative melatonin treatment on clinical cardiac morbidity and markers of myocardial ischemia in patients undergoing elective surgery for abdominal aortic aneurism | randomized, placebo-controlled, clinical trial | infusion over a 2-hr period either, 50 mg melatonin or placebo intra-operatively, and 10 mg melatonin or placebo orally, the first three nights after surgery. | Positive: melatonin decreased clinical cardiac morbidity and the occurrence of myocardial ischemia after abdominal aortic aneurism repair. | |
| Postural tachycardia syndrome (POTS) [17] | Tested the hypothesis that melatonin will attenuate the tachycardia and improve symptom burden in patients with POTS. NCT00262470 | 2 | randomized, single-blinded, crossover trial | melatonin 3 mg orally and placebo, on separate mornings, in a randomized crossover design | Negative: There was no significant difference in the reduction of systolic blood pressure between melatonin and placebo, either with standing or while seated. The symptom burden was not improved with melatonin compared with placebo. |
| Coronary artery bypass grafting surgery (CABG) [18] | To investigate the effects of Melatoninon nuclear erythroid 2-related factor 2(Nrf2) activity in patients undergoing CABG surgery | 2 | randomized triple-blind placebo-controlled trial | 10 mg oral melatonin (Melatonin group, n = 15) or placebo (placebo group, n = 15) before sleeping for 1 month before surgery | Positive: Increase by melatonin of nuclear erythroid 2-related factor 2(Nrf2) activity in patients undergoing CABG surgery. |
| Blood coagulation activity [19] | To investigate if oral administration of melatonin is associated with decreased plasma levels of procoagulant hemostatic measures | 2 | randomized, placebo-controlled, single-blinded trial | 3 mg of oral melatonin or placebo, and one hour thereafter, levels of melatonin, fibrinogen, and D-dimer as well as activities of coagulation factor VII (FVII:C) and VIII (FVIII:C) were measured in plasma | Positive: lower levels of the coagulation measures FVIII:C and fibrinogen one hour after oral intake of a single dose of 3 mg of melatonin compared to placebo medication. Suggested potential implications for the use of melatonin as a theapeutic agent in patients at-risk of atherothrombotic events such as patients with CAD or systemic hypertension |
| ChEMBL ID | Name | Synonyms | Max Phase | Molecular Weight | QED Weighted | Mesh_Heading |
|---|---|---|---|---|---|---|
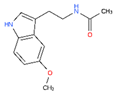 CHEMBL45 | MELATONIN | Circadin, General Nutrit, Health Aid, Heidadouppi, Icenia, Life Ext, Melapure, Melatonin, N-(2-(5-Methoxy-1H-Indol-3-Yl)Ethyl)Acetamide, N-Acetyl-5-Methoxytryptamine, Natrol, Nature’s Blend, Natures Bounty, Quality Health, S.Gard, Travelag, Vespro, Vytalonin | 4 | 232.28 | 0.84 | Anxiety, Aortic Aneurysm, Atrial Fibrillation, Attention Deficit Disorder with Hyperactivity, Autistic Disorder, Barrett Esophagus, Brain Ischemia, Breast Neoplasms, Carcinoma, Non-Small-Cell Lung, Child Development Disorders, Pervasive, Cognitive Dysfunction, Colitis, Ulcerative, Dementia, Depressive Disorder, Dermatitis, Atopic, Diabetes Mellitus, Epilepsy, Fatigue, Fibromyalgia, Gastroesophageal Reflux, Head and Neck Neoplasms, Hemorrhage, Hypertension, Melanoma, Metabolic Diseases, Metabolic Syndrome, Migraine Disorders, Multiple Sclerosis, Relapsing-Remitting, Myocardial Infarction, Neonatal Sepsis, Neoplasms, Nocturnal Enuresis, Pain, Premature Birth, Reperfusion Injury, Schizophrenia, Sepsis, Skin Diseases, Sleep Initiation and Maintenance Disorders, Stomatitis, Substance Withdrawal Syndrome, Sunburn |
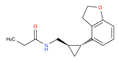 CHEMBL2103822 | TASIMELTEON | BMS-214778, Hetlioz, Tasimelteon, VEC-162 | 4 | 245.32 | 0.88 | Depressive Disorder, Liver Diseases, Sleep Initiation and Maintenance Disorders, Smith-Magenis Syndrome |
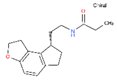 CHEMBL1218 | RAMELTEON | Ramelteon, Rozerem, TAK-375 | 4 | 259.35 | 0.9 | Bipolar Disorder, Depressive Disorder, Marijuana Abuse, Migraine with Aura, Migraine without Aura, Pulmonary Disease, Chronic Obstructive, Sleep Apnea, Obstructive, Sleep Initiation and Maintenance Disorders, Substance-Related Disorders, Tobacco Use Disorder |
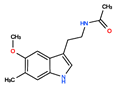 CHEMBL3230568 | No Data | 0 | 246.31 | 0.87 | ||
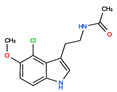 CHEMBL3230569 | No Data | 0 | 266.73 | 0.89 | ||
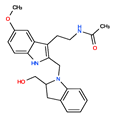 CHEMBL498494 | No Data | 0 | 393.49 | 0.58 | ||
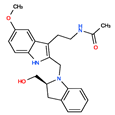 CHEMBL498493 | No Data | 0 | 393.49 | 0.58 | ||
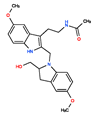 CHEMBL525374 | No Data | 0 | 423.51 | 0.52 | ||
 CHEMBL15060 | No Data | 0 | 308.38 | 0.76 | ||
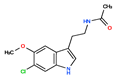 CHEMBL34730 | No Data | 6-Chloromelatonin | 0 | 266.73 | 0.89 | |
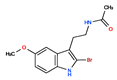 CHEMBL33415 | No Data | 0 | 311.18 | 0.91 | ||
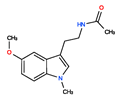 CHEMBL33700 | No Data | 0 | 246.31 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltatu, O.C.; Senar, S.; Campos, L.A.; Cipolla-Neto, J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. Int. J. Mol. Sci. 2019, 20, 4342. https://doi.org/10.3390/ijms20184342
Baltatu OC, Senar S, Campos LA, Cipolla-Neto J. Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. International Journal of Molecular Sciences. 2019; 20(18):4342. https://doi.org/10.3390/ijms20184342
Chicago/Turabian StyleBaltatu, Ovidiu Constantin, Sergio Senar, Luciana Aparecida Campos, and José Cipolla-Neto. 2019. "Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use" International Journal of Molecular Sciences 20, no. 18: 4342. https://doi.org/10.3390/ijms20184342
APA StyleBaltatu, O. C., Senar, S., Campos, L. A., & Cipolla-Neto, J. (2019). Cardioprotective Melatonin: Translating from Proof-of-Concept Studies to Therapeutic Use. International Journal of Molecular Sciences, 20(18), 4342. https://doi.org/10.3390/ijms20184342







