Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations
Abstract
1. Introduction
2. Role of ROS in Normal Cells
3. ROS as Trigger of Oxidative Stress
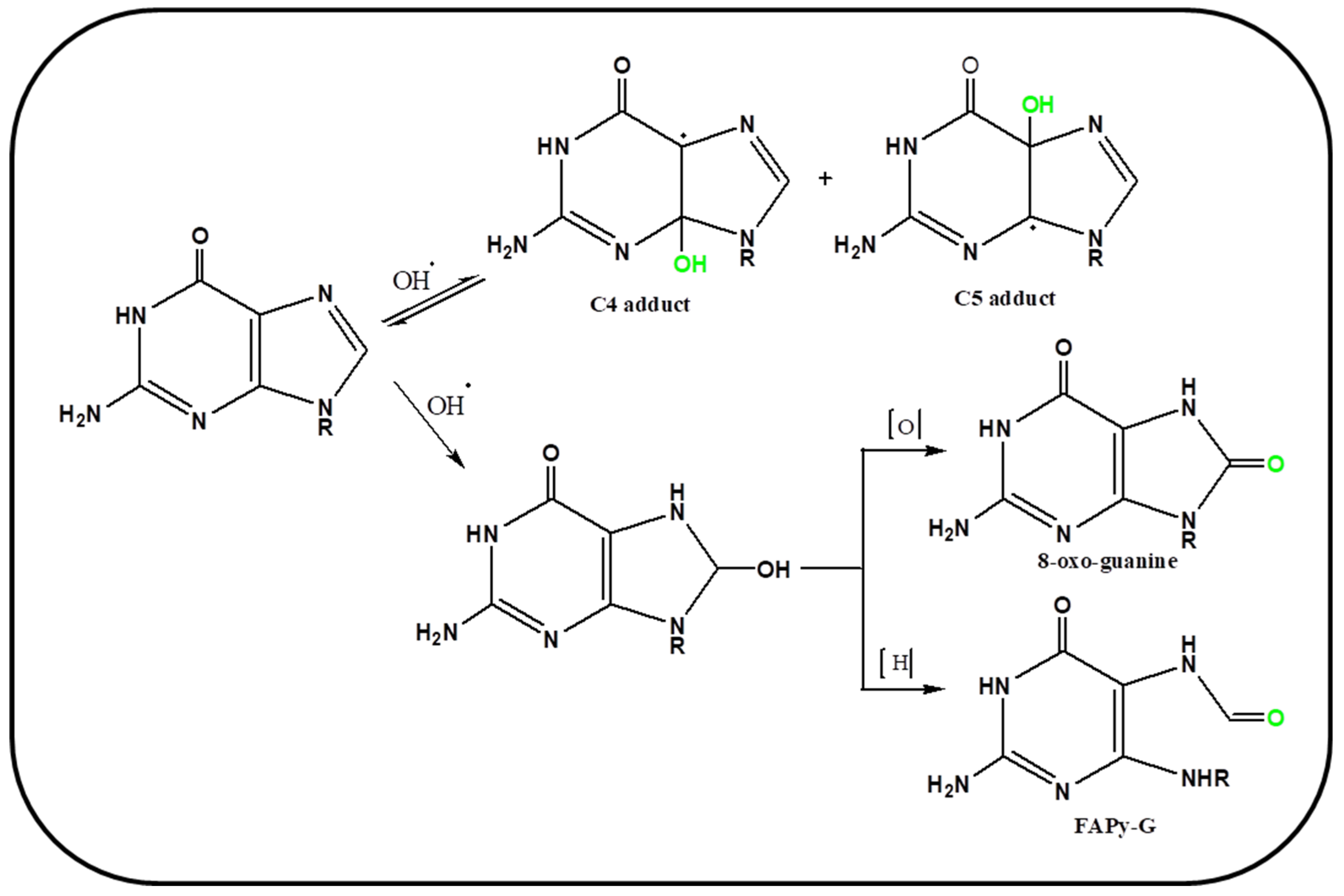
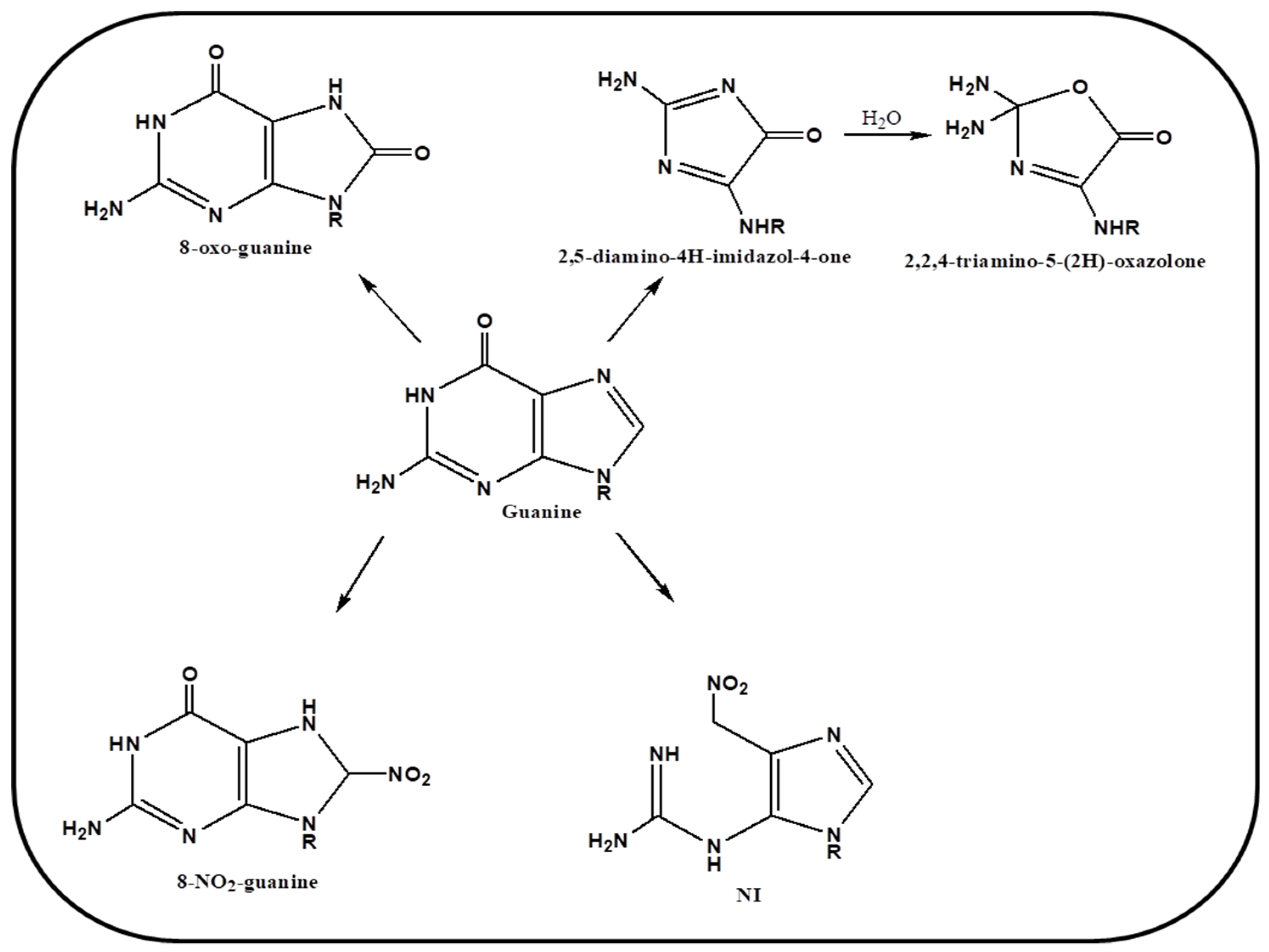
4. Oxidation Mechanism of Guanine Base via ROS
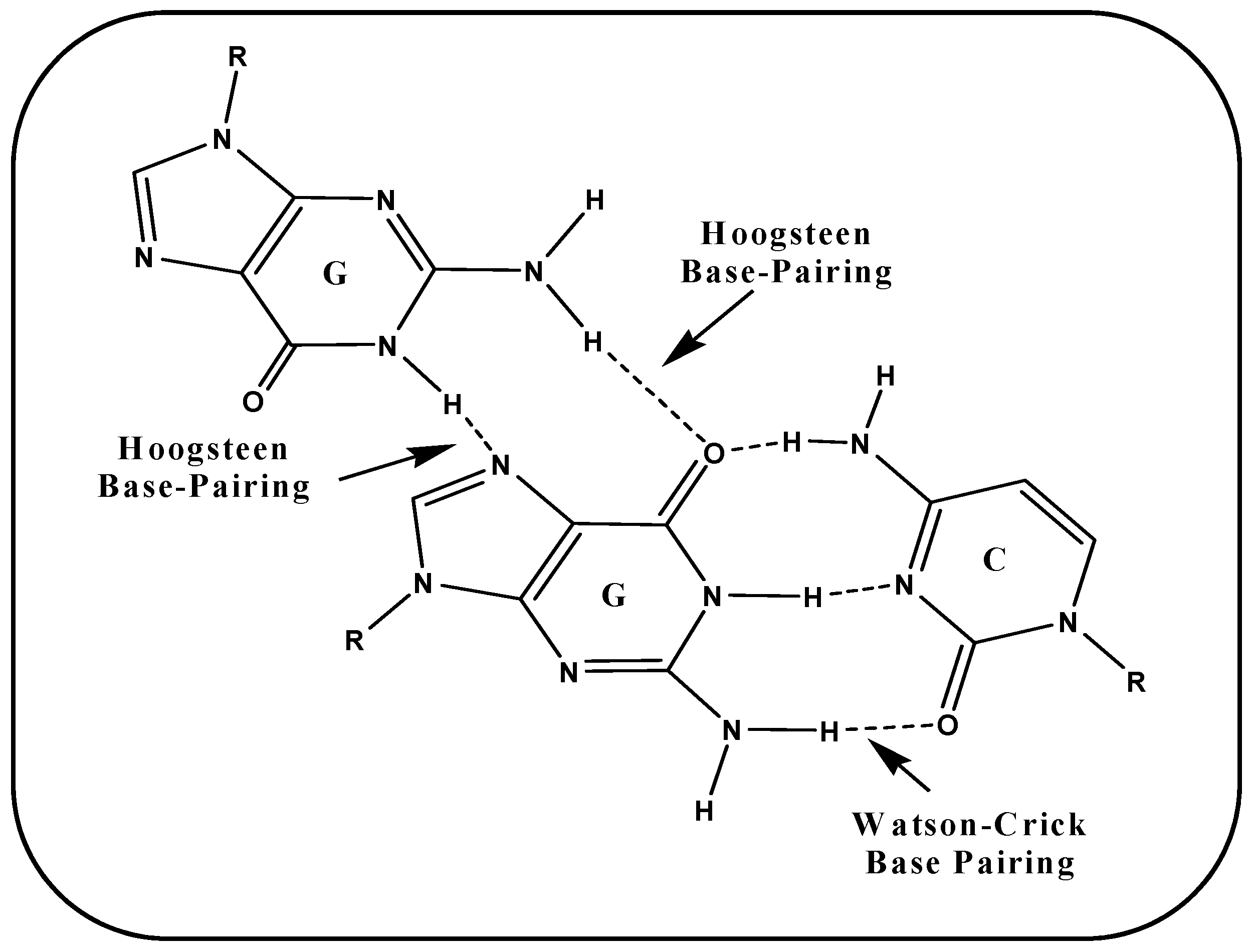
5. G-Quartet Formation and Role of ROS
5.1. (i) Telomeric G-Quadruplex and Role of ROS
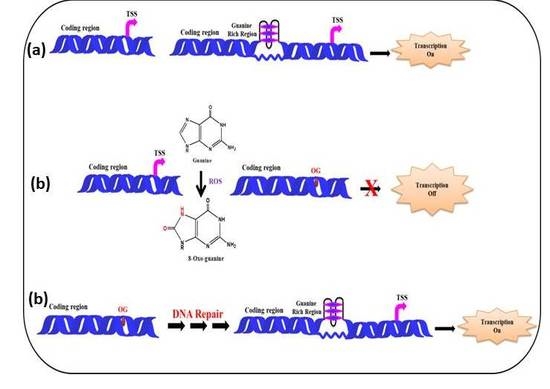
5.2. (ii) G-Quadruplexes in Promoter Region and Effect of ROS
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wallner, S.; Hermetter, A.; Mayer, B.; Wascher, T.C. The alpha-amino group of l-arginine mediates its antioxidant effect. Eur. J. Clin. Investig. 2001, 31, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wang, B.; Gao, G.; Cao, W.; Zhang, Y. L-Arginine supplementation improves antioxidant defenses through L-arginine/nitric oxide pathways in exercised rats. J. Appl. Physiol. 2013, 115, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Melis, J.P.M.; Steeg, H.V.; Luijten, M. Oxidative DNA damage and nucleotide excision repair. Antioxid. Redox Signal. 2013, 18, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Sheinman, M.; Hermsen, R. Effects of DNA oxidation on the evolution of genomes. bioRxiv 2017. [CrossRef]
- Pedro, J.; Angeli, F.; Miyamoto, S.; Schulze, A. Ferroptosis: The Greasy Side of Cell Death. Chem. Res. Toxicol. 2019, 32, 362–369. [Google Scholar]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Prieme, H.; Loft, S. Role of oxidative DNA damage in cancer initiation and promotion. Eur. J. Cancer Prev. 1998, 7, 9–16. [Google Scholar]
- Gabbita, S.P.; Lovell, M.A.; Markesbery, W.R. Increased nuclear DNA oxidation in the brain in Alz-heimer’s disease. J. Neurochem. 1998, 71, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.; Beisiegel, U.; Kontush, A. Lipid per-oxidation in neurodegeneration: New insights into Alz-heimer’s disease. Curr. Opin. Lipidol. 2002, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Boesten, D.M.P.H.J.; de Vos-Houben, J.M.J.; Timmermans, L.; den Hartog, G.J.M.; Bast, A.; Hageman, G.J. Accelerated Aging during Chronic Oxidative Stress: A Role for PARP-1. Oxidative Med. Cell. Longev. 2013, 2013, 680414. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Sasso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Clark, D.W.; Phang, T.; Edwards, M.G.; Geraci, M.W.; Gillespie, M.N. Promoter G-quadruplex sequences are targets for base oxidation and strand cleavage during hypoxia-induced transcription. Free Radic Biol. Med. 2012, 53, 51–59. [Google Scholar] [CrossRef]
- Fedeles, B.I. G-quadruplex–forming promoter sequences enable transcriptional activation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2017, 114, 2788–2790. [Google Scholar] [CrossRef]
- Lee, S.C.; Zhang, J.; Strom, J.; Yang, D.; Dinh, T.N.; Kappeler, K.; Chen, Q.M. G-Quadruplex in the NRF2 mRNA 5’ Untranslated Region Regulates De Novo NRF2 Protein Translation under Oxidative Stress. Mol. Cell. Biol. 2016, 37, e00122-16. [Google Scholar] [CrossRef]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Chambers, V.S.; Marsico, G.; Boutell, J.M.; Di Antonio, M.; Smith, G.P.; Balasubramanian, S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 2015, 33, 877–881. [Google Scholar] [CrossRef]
- Simonsson, T.; Pecinka, P.; Kubista, M. DNA Tetraplex Formation in the Control Region of C-Myc. Nucleic Acids Res. 1998, 26, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.J.; Hurley, L.H. Direct Evidence for a G-Quadruplex In a Promoter Region and Its Targeting with a Small Molecule to Repress C-MYC Transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef] [PubMed]
- Rankin, S.; Reszka, A.P.; Huppert, J.; Zloh, M.; Parkinson, G.N.; Todd, A.K.; Ladame, S.; Balasubramanian, S.; Neidle, S. Putative DNA Quadruplex Formation within the Human C-Kit Oncogene. J. Am. Chem. Soc. 2005, 127, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, S.; Xodo, L.E. G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription. Nucleic Acids Res. 2006, 34, 2536–2549. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, K.; Rusche, J.J.; Hurley, L.H. Facilitation of a Structural Transition in the Polypurine/Polypyrimidine Tract within the Proximal Promoter Region of the Human VEGF Gene by the Presence of Potassium and G-Quadruplex-Interactive Agents. Nucleic Acids Res. 2005, 33, 6070–6080. [Google Scholar] [CrossRef]
- Sun, D.; Wei-Jun, L.; Guo, K.; Rusche, J.J.; Ebbinghaus, S.; Gokhale, V.; Hurley, L.H. The Proximal Promoter Region of the Human Vascular Endothelial Growth Factor Gene Has A G-Quadruplex Structure that can be targeted by G-Quadruplex–Interactive Agents. Mol. Cancer Ther. 2008, 7, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Agrawal, P.; Brown, R.V.; Hatzakis, E.; Hurley, L.; Yang, D. The Major G-Quadruplex Formed in the Human Platelet-Derived Growth Factor Receptor Β (PDGFR-Β) Promoter Adopts a Novel Broken-Strand Structure in K+ Solution. J. Am. Chem. Soc. 2012, 134, 13220–13223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Lin, C.; Mathad, R.I.; Carver, M.; Yang, D. The major G-quadruplex formed in the human BCL-2 proximal promoter adopts a parallel structure with a 13-nt loop in K+ solution. J. Am. Chem. Soc. 2014, 136, 1750–1753. [Google Scholar] [CrossRef]
- Palumbo, S.L.; Memmott, R.M.; Uribe, D.J.; Krotova-Khan, Y.; Hurley, L.H.; Ebbinghaus, S.W. A Novel G-Quadruplex-Forming GGA Repeat Region in the C-Myb Promoter is a Critical Regulator of Promoter Activity. Nucleic Acids Res. 2008, 36, 1755–1769. [Google Scholar] [CrossRef]
- Tong, X.; Lan, W.; Zhang, X.; Wu, H.; Liu, M.; Cao, C. Solution structure of all parallel G-quadruplex formed by the oncogene RET promoter sequence. Nucleic Acids Res. 2011, 39, 6753–6763. [Google Scholar] [CrossRef]
- Mitchell, T.; Ramos-Montoya, A.; Di Antonio, M.; Murat, P.; Ohnmacht, S.; Micco, M.; Jurmeister, S.; Fryer, L.; Balasubramanian, S.; Neidle, S.; et al. Downregulation of androgen receptor transcription by promoter G-quadruplex stabilization as a potential alternative treatment for castrate-resistant prostate cancer. Biochemistry 2013, 52, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.V.; Gaerig, V.C.; Simmons, T.; Brooks, T.A. Helping Eve overcome ADAM: G-quadruplexes in the ADAM-15 promoter as new molecular targets for breast cancer therapeutics. Molecules 2013, 18, 15019–15034. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B.; Trent, J.O.; Gray, R.D.; Dean, W.L.; Buscaglia, R.; Thomas, S.D.; Miller, D.M. An improved model for the hTERT promoter quadruplex. PLoS ONE 2014, 9, e115580. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, D.; Dong, L.; Pan, S.; Hao, F.; Guan, Y. A novel G-quadruplex motif in the Human MET promoter region. Biosci. Rep. 2017, 37, BSR20171128. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Choi, E.S.; Hwang, K.; Kim, J.; Sampath, S.; Venkitaraman, A.R.; Lee, H. The Breast Cancer Susceptibility Gene BRCA2 is Required for the Maintenance of Telomere Homeostasis. J. Biol. Chem. 2012, 287, 5091–5101. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Shumayrikh, N.; Sen, D. G-quadruplex structures formed by expanded hexanucleotide repeat RNA and DNA from the neurodegenerative disease-linked C9orf72 gene efficiently sequester and activate heme. PLoS ONE 2014, 9, e106449. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.; Fratta, P.; Neidle, S.; Parkinson, G.N.; Isaacs, A.M. G-Quadruplexes: Emerging Roles in Neurodegenerative Diseases and the Non-Coding Transcriptome. Fed. Eur. Biochem. Soc. 2015, 589, 1653–1668. [Google Scholar] [CrossRef]
- Zhou, B.; Geng, Y.; Liu, C.; Miao, H.; Ren, Y.; Xu, N.; Shi, X.; You, Y.; Lee, T.; Zhu, G. Characterizations of Distinct Parallel and Antiparallel G-Quadruplexes Formed by Two-Repeat ALS and FTD Related GGGGCC Sequence. Sci. Rep. 2018, 8, 2366. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Jensen, K.B.; Jin, P.; Brown, V.; Warren, S.T.; Darnell, R.B. Fragile X Mental Retardation Protein Targets G Quartet mRNAs Important for Neuronal Function. Cell 2001, 107, 489–499. [Google Scholar] [CrossRef]
- Balkwill, G.D.; Kamila, D.; Garner, T.P.; Hodgman, C.; Flint, A.P.; Searle, M.S. Repression of Translation of Human Estrogen Receptor R by G-Quadruplex Formation. Biochemistry 2009, 48, 11487–11495. [Google Scholar] [CrossRef]
- Bugaut, A.; Balasubramanian, S. 5′-UTR RNA G-quadruplexes: Translation regulation and targeting. Nucleic Acids Res. 2012, 40, 4727–4741. [Google Scholar] [CrossRef] [PubMed]
- Vorlícková, M.; Tomasko, M.; Sagi, A.J.; Bednarova, K.; Sagi, J. 8-Oxoguanine in a quadruplex of the human telomere DNA sequence. FEBS J. 2012, 279, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad, Reactive Oxygen Species (ROS) in Living Cells, Cristiana Filip and Elena Albu; IntechOpen: London, UK, 2017. [Google Scholar]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G. Innate immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Tulard, A.; Hoffschir, F.; de Boisferon, F.H.; Luccioni, C.; Bravard, A. Persistent oxidative stress after ionizing radiation is involved in inherited radiosensitivity. Free Radic. Biol. Med. 2003, 35, 68–77. [Google Scholar] [CrossRef]
- Little, J.B. Ionizing Radiation. In Holland-Frei Cancer Medicine, 5th ed.; Bast, R.C., Jr., Croce, C.M., Hait, W.N., Hong, W.K., Kufe, D.W., Piccart-Gebart, M., Pollock, R.E., Weichselbaum, R.R., Wang, H., Holland, J.F., Eds.; BC Decker: Hamilton, ON, Canada, 2000; Chapter 14. [Google Scholar]
- Klaunig, J.E.; Kamendulis, L.M. The Role of Oxidative Stress in Carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. J. Fed. Am. Soc. Exp. Biol. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Ohtsubo, T.; Ohya, Y.; Nakamura, Y.; Kansui, Y.; Furuichi, M.; Matsumura, K.; Fujii, K.; Iida, M.; Nakabeppu, Y. Accumulation of 8-oxo-deoxyguanosine in Cardiovascular Tissues with the Development of Hypertension. DNA Repair 2007, 6, 760–769. [Google Scholar] [CrossRef]
- Mariarosaria, D.; Eleonora, P.; Eugenia, D. Mechanism of Oxidative DNA Damage Repair and Relevance to Human Pathology. Mutat. Res. 2008, 659, 4–14. [Google Scholar]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base Excision Repair of Oxidative DNA Damage and Association with Cancer and Aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, L.; Bialkowski, K.; Risom, L.; Løhr, M.; Loft, S.; Møller, P. Aging and Defense Against Generation of 8-oxo-7,8-dihydro-2′deoxyguanosine in DNA. Free Radic. Biol. Med. 2009, 47, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.; Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1 [alpha] and adenosine receptors. Nat. Rev. Immunol. 2005, 5, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Krystona, T.B.; Georgieva, A.B.; Pissisb, P.; Georgakilasa, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Eiberger, W.; Volkmer, B.; Amouroux, R.; Dhérin, C.; Radicella, J.P.; Epe, B. Oxidative stress impairs the repair of oxidative DNA base modifications in human skin fibroblasts and melanoma cells. DNA Repair 2008, 7, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition Metals as Catalysts of “autoxidation” Reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.G.; Aust, A.E. Iron mobilization from asbestos by chelators and ascorbic acid. Arch. Biochem. Biophys. 1990, 270, 60–64. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Inoue, S.; Kawanishi, S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987, 47, 6522–6527. [Google Scholar]
- Celander, D.W.; Cech, T.R. Iron (II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: Similar reactivity toward single- and double-stranded forms. Biochemistry 1990, 29, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B. Reactive Metabolites of Oxygen and Nitrogen in Biology and Medicine, Georgetown. Landes 1992, 43, 47–48. [Google Scholar]
- Halliwell, B.; Aruoma, O.I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991, 281, 9–19. [Google Scholar] [CrossRef]
- Goldberg, I.H. Free radical mechanism in neocarzinostatin-induced DNA damage. Free Radic Biol. Med. 1987, 3, 41–54. [Google Scholar] [CrossRef]
- Aust, A.E.; Eveleigh, J.F. Mechanism of DNA Oxidation (44449). Exp. Biol. Med. 1999, 222, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ohshima, H. DNA Damage Induced by Peroxynitrite: Subsequent Biological Effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: Structures and mechanisms of product formation. Nitric Oxide 2006, 14, 109–121. [Google Scholar] [CrossRef]
- Tretyakova, N.Y.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-Induced Secondary Oxidative Lesions at Guanine Nucleobases: Chemical Stability and Recognition by the Fpg DNA Repair Enzyme. Chem. Res. Toxicol. 2000, 13, 658–664. [Google Scholar] [CrossRef]
- Kaushik, M.; Kaushik, S.; Bansal, A.; Saxena, S.; Kukreti, S. Structural diversity and specific recognition of four stranded G-quadruplex DNA. Curr. Mol. Med. 2011, 11, 744–769. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, S. A triple stranded G-quadruplex formation in the promoter region of human myosin β (Myh7) gene. J. Biomol. Struct. Dyn. 2018, 36, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Arachchilage, G.M.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Siebenmorgen, T.; Zacharias, M. Origin of Ion Specificity of Telomeric DNA G-Quadruplexes Investigated by Free-Energy Simulations. Biophys. J. 2017, 112, 2280–2290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiziria, E.; Gorgoshidze, M.; Gogichaishvili, S.; Sokhadze, V.; Khachidze, D.; Kiladze, M.; Lomidze, E.; Barbakadze, S.; Tvauri, G.; Monaselidze, J. Influence of K+ Ions on Thermodynamic Stability of DNA G-Quadruplex. Bull. Georgian Natl. Acad. Sci. 2017, 11, 42–46. [Google Scholar]
- Rhodes, D.; Lipps, H.J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef]
- Bedrat, A.; Lacroix, L.; Mergny, J.L. Re-evaluation of G-quadruplex propensity with G4Hunter. Nucleic Acids Res. 2016, 44, 1746–1759. [Google Scholar] [CrossRef]
- Kouzine, F.; Wojtowicz, D.; Baranello, L.; Yamane, A.; Nelson, S.; Resch, W.; Kieffer-Kwon, K.R.; Benham, C.J.; Casellas, R.; Przytycka, T.M.; et al. Permanganate/S1 nuclease footprinting reveals non-B DNA structures with regulatory potential across a mammalian genome. Cell Syst. 2017, 4, 344–356. [Google Scholar] [CrossRef]
- Rigo, R.; Palumbo, M.; Sissi, C. G-quadruplexes in human promoters: A challenge for therapeutic applications. Biochim. Biophys. Acta 2017, 1861, 1399–1413. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Buket, O.; Clement, L.; DanZhou, Y. DNA G-quadruplex and its potential as anticancer drug target. Sci. China Chem. 2014, 57, 1605–1614. [Google Scholar] [CrossRef]
- Yang, F.; Sun, X.; Wang, L.; Li, Q.; Guan, A.; Shen, G.; Tang, Y. Selective recognition of c-myc promoter G-quadruplex and down-regulation of oncogene c-myc transcription in human cancer cells by 3,8 a-disubstituted indolizinone. RSC Adv. 2017, 7, 51965–51969. [Google Scholar] [CrossRef]
- Brázda, V.; Hároníková, L.; Liao, J.C.C.; Fojta, M. DNA and RNA Quadruplex-Binding Proteins. Int. J. Mol. Sci. 2014, 15, 17493–17517. [Google Scholar] [CrossRef] [PubMed]
- Heddi, B.; Cheong, V.V.; Martadinata, H.; Phan, A.T. Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: Solution structure of a peptide–quadruplex complex. Proc. Natl. Acad. Sci. USA 2015, 112, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- McAninch, D.S.; Heinaman, A.M.; Lang, C.N.; Moss, K.R.; Bassell, G.J.; Mihailescua, M.R.; Evans, T.L. Fragile X mental retardation protein recognizes a G quadruplex structure within the survival motor neuron domain containing 1 mRNA 5′-UTR. Mol. BioSyst. 2017, 13, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hoshiyama, H.; Shay, J.W.; Wright, W.E. Quantitative telomeric overhang determination using a double-strand specific nuclease. Nucleic Acids Res. 2008, 36, e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.-Q.; Chen, Z.; Zheng, K.-W.; Chen, C.-Y.; Hao, Y.-H.; Tan, Z. G-quadruplex formation at the 30 end of telomere DNA inhibits its extension by telomerase, polymerase and unwinding by helicase. Nucleic Acids Res. 2011, 39, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Barton, J.K. Charge Transport in DNA Duplex/Quadruplex Conjugates. Biochemistry 2003, 42, 14159–14165. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Petersen, S.; Saretzki, G.; von Zglinicki, T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998, 239, 152–160. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef]
- Zglinicki, T.V. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Stewart, S.A.; Ben-Porath, I.; Carey, V.J.; O’Connor, B.F.; Hahn, W.C.; Weinberg, R.A. Erosion of the telomeric single-strand overhang at replicative senescence. Nat. Genet. 2003, 33, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. NY Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.F.; Saretzki, G.; Zglinicki, T.V. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007, 35, 7505–7513. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative Stress Induces Persistent Telomeric DNA Damage Responsible for Nuclear Morphology Change in Mammalian Cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Graham, M.K.; Meeker, A. Telomeres and telomerase in prostate cancer development and therapy. Nat. Rev. Urol. 2017, 14, 607–619. [Google Scholar] [CrossRef]
- Opresko, P.L.; Fan, J.; Danzy, S.; Wilson, D.M.; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef]
- Lee, H.-T.; Bose, A.; Lee, C.-Y.; Opresko, P.L.; Myong, S. Molecular mechanisms by which oxidative DNA damage promotes telomerase activity. Nucleic Acids Res. 2017, 45, 11752–11765. [Google Scholar] [CrossRef]
- Grollman, A.P.; Moriya, M. Mutagenesis by 8-oxoguanine: An enemy within. Trends Genet. 1993, 9, 246–249. [Google Scholar] [CrossRef]
- Bielskutė, S.; Plavec, J.; Podbevšek, P. Impact of Oxidative Lesions on the Human Telomeric G-Quadruplex. J. Am. Chem. Soc. 2019, 141, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Bozkus, F. Could serum levels of telomerase be considered as an oxidative stress marker in COPD? Telomere Telomerase 2016, 3, e1258. [Google Scholar] [CrossRef][Green Version]
- Aeby, E.; Ahmed, W.; Redon, S.; Simanis, V.; Lingner, J. Peroxiredoxin 1 Protects Telomeres from Oxidative Damage and Preserves Telomeric DNA for Extension by Telomerase. Cell Rep. 2016, 17, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, A.; Esposito, V.; Mayol, L.; Giancola, C.; Petraccone, L.; Galeone, A. The oxidative damage to the human telomere: Effects of 5-hydroxymethyl-2′-deoxyuridine on telomeric G-quadruplex structures. Org. Biomol. Chem. 2015, 13, 7421–7429. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Lormand, J.; Bose, A.; Lee, H.-T.; Kim, G.S.; Li, J.; Sobol, R.W.; Freudenthal, B.D.; Myong, S.; Opresko, P.L. Oxidative guanine base damage regulates human telomerase activity. Nat. Struct. Mol. Biol. 2016, 23, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Liu, Y. The origin of oxidized guanine resolves the puzzle of oxidation induced telomere-length alterations. Nat. Struct. Mol. Biol. 2016, 23, 1070–1071. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef]
- Zhou, W.; Suntharalingam, K.; Brand, N.J.; Barton, P.J.R.; Vilar, R.; Ying, L. Possible Regulatory Roles of Promoter G-Quadruplexes in Cardiac Function-Related Genes—Human TnIc as a Model. PLoS ONE 2013, 8, e53137. [Google Scholar] [CrossRef]
- Yuan, L.; Tian, T.; Chen, Y.; Yan, S.; Xing, X.; Zhang, Z.; Zhai, Q.; Xu, L.; Wang, S.; Weng, X.; et al. Existence of G-quadruplex structures in promoter region of oncogenes confirmed by G-quadruplex DNA cross-linking strategy. Sci. Rep. 2013, 3, 01811. [Google Scholar] [CrossRef]
- Fleming, A.M.; Zhou, J.; Wallace, S.S.; Burrows, C.J. A Role for the Fifth G-Track in G-Quadruplex Forming Oncogene Promoter Sequences during Oxidative Stress: Do These “Spare Tires” Have an Evolved Function? ACS Cent. Sci. 2015, 1, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Dinga, Y.; Burrowsa, C.J. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. USA 2017, 114, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.K.; Zybailov, B.L.; Maddukuri, L.; Gao, J.; Marecki, J.C.; Jaiswal, M.; Bell, M.R.; Griffin, W.C.; Reed, M.R.; Chib, S.; et al. Evidence that G-quadruplex DNA Accumulates in the Cytoplasm and Participates in Stress Granule Assembly in Response to Oxidative Stress. J. Biol. Chem. 2016, 291, 18041–18057. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, M.; Li, H.; Wang, M.; Luo, X.; Huang, Y.; Wang, H.-H.; Nie, Z.; Yao, S. Simultaneous Monitoring of Cell-surface Receptor and Tumor-targeted Photodynamic Therapy via TdT-initiated Poly-G-Quadruplexes. Sci. Rep. 2018, 8, 5551. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, S.; Ferino, A.; Miglietta, G.; Pedersen, E.B.; Xodo, L.E. The regulatory G4 motif of the Kirsten ras (KRAS) gene is sensitive to guanine oxidation: Implications on transcription. Nucleic Acids Res. 2018, 46, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 Diabetes as a redox disease. Lancet 2014, 383, 841–843. [Google Scholar] [CrossRef]
- Bartas, M.; Brazda, V.; Karlicky, V.; Cerven, J.; Pecinka, P. Bioinformatics analyses and in vitro evidence for five and six stacked G-quadruplex forming sequences. Biochimie 2018, 150, 70–75. [Google Scholar] [CrossRef]
- Dvorkin, S.A.; Karslslotis, A.I.; da Silva, M.W. Encoding canonical DNA quadruplex structure. Sci. Adv. 2018, 4, eaat3007. [Google Scholar] [CrossRef]
- Kaushik, M.; Kaushik, S.; Roy, K.; Singh, A.; Mahendru, S.; Kumar, M.; Chaudhary, S.; Ahmed, S.; Kukreti, S. A bouquet of DNA structures: Emerging diversity. Biochem. Biophys. Rep. 2016, 5, 388–395. [Google Scholar] [CrossRef][Green Version]


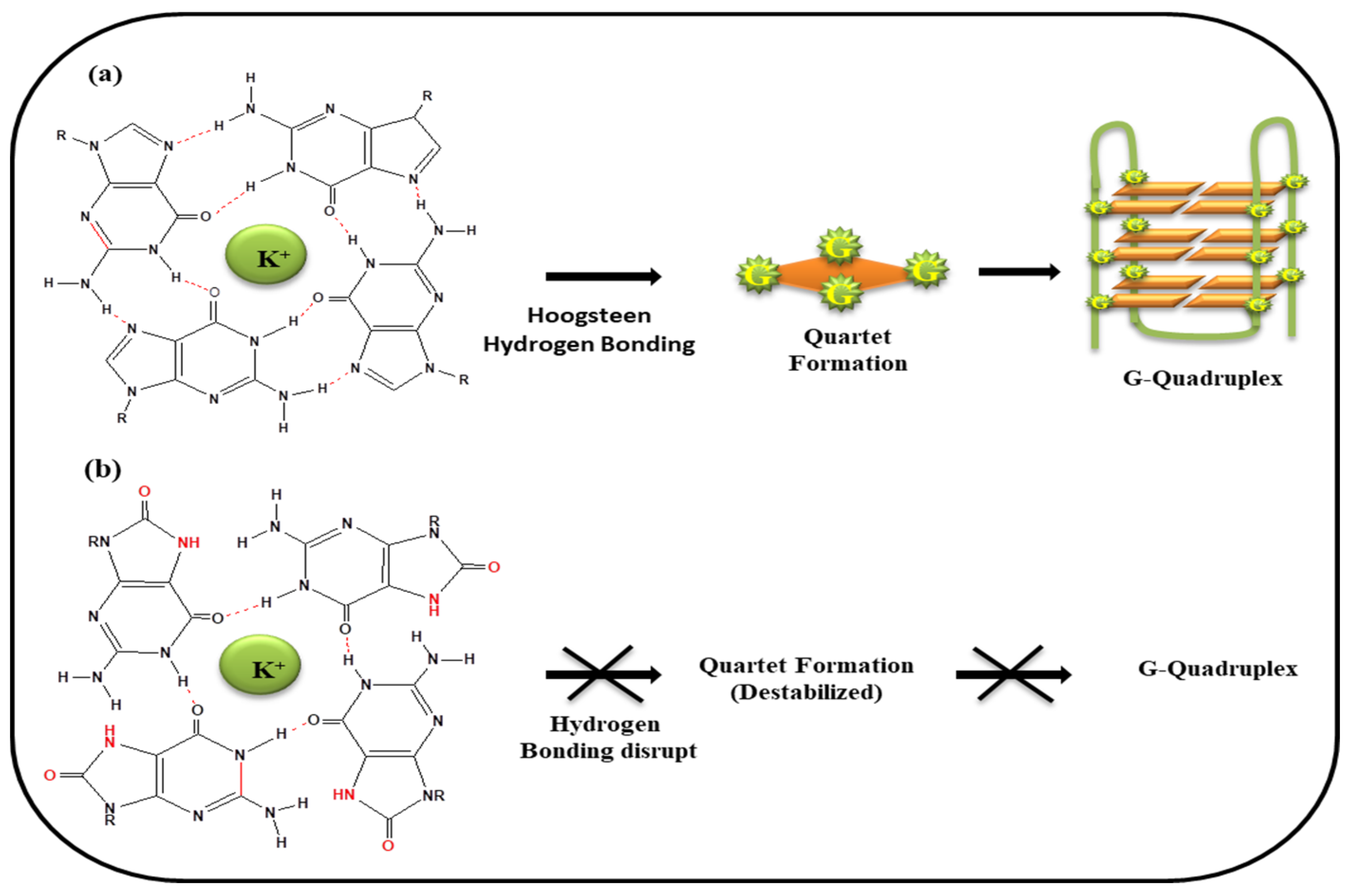
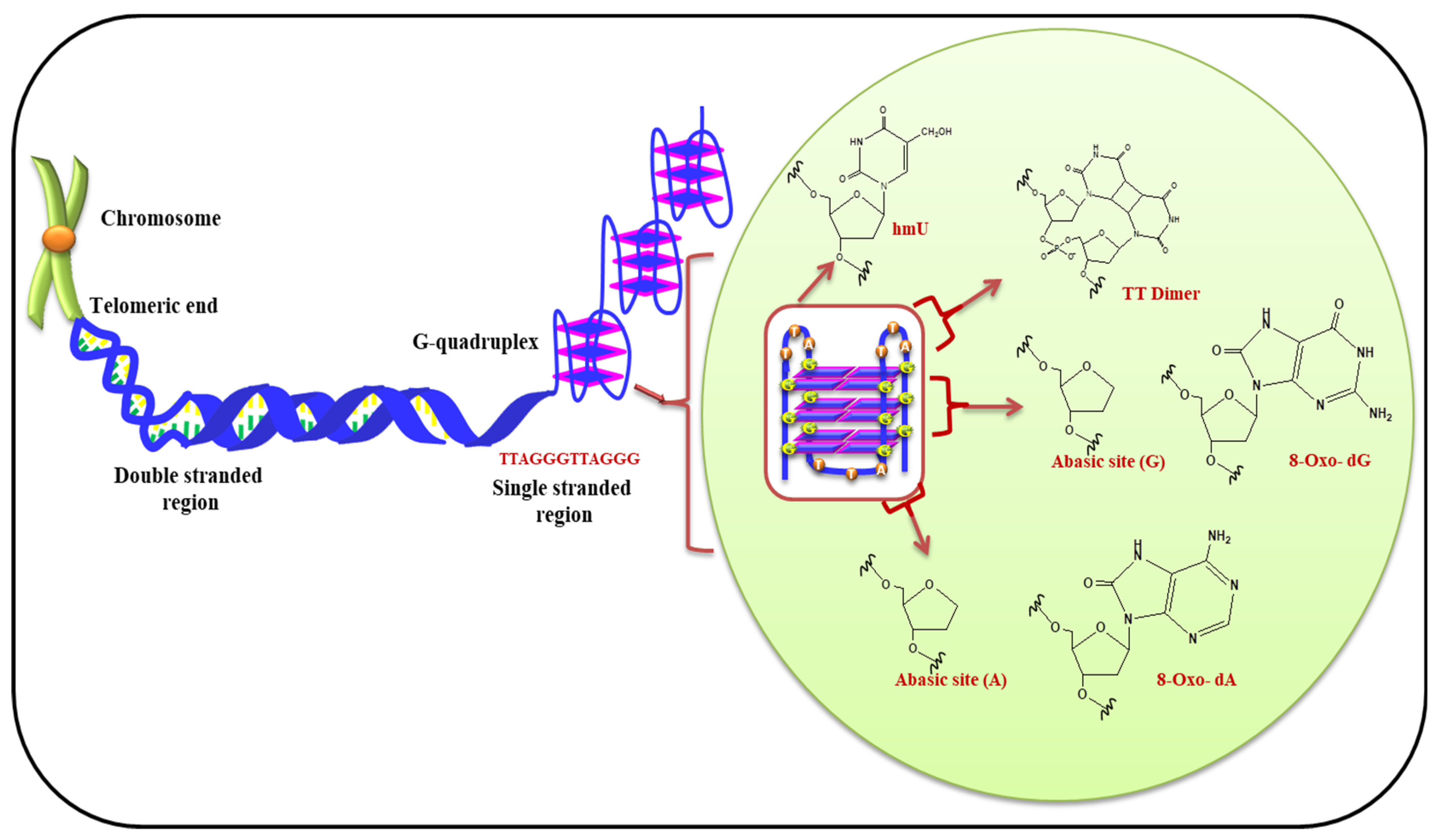
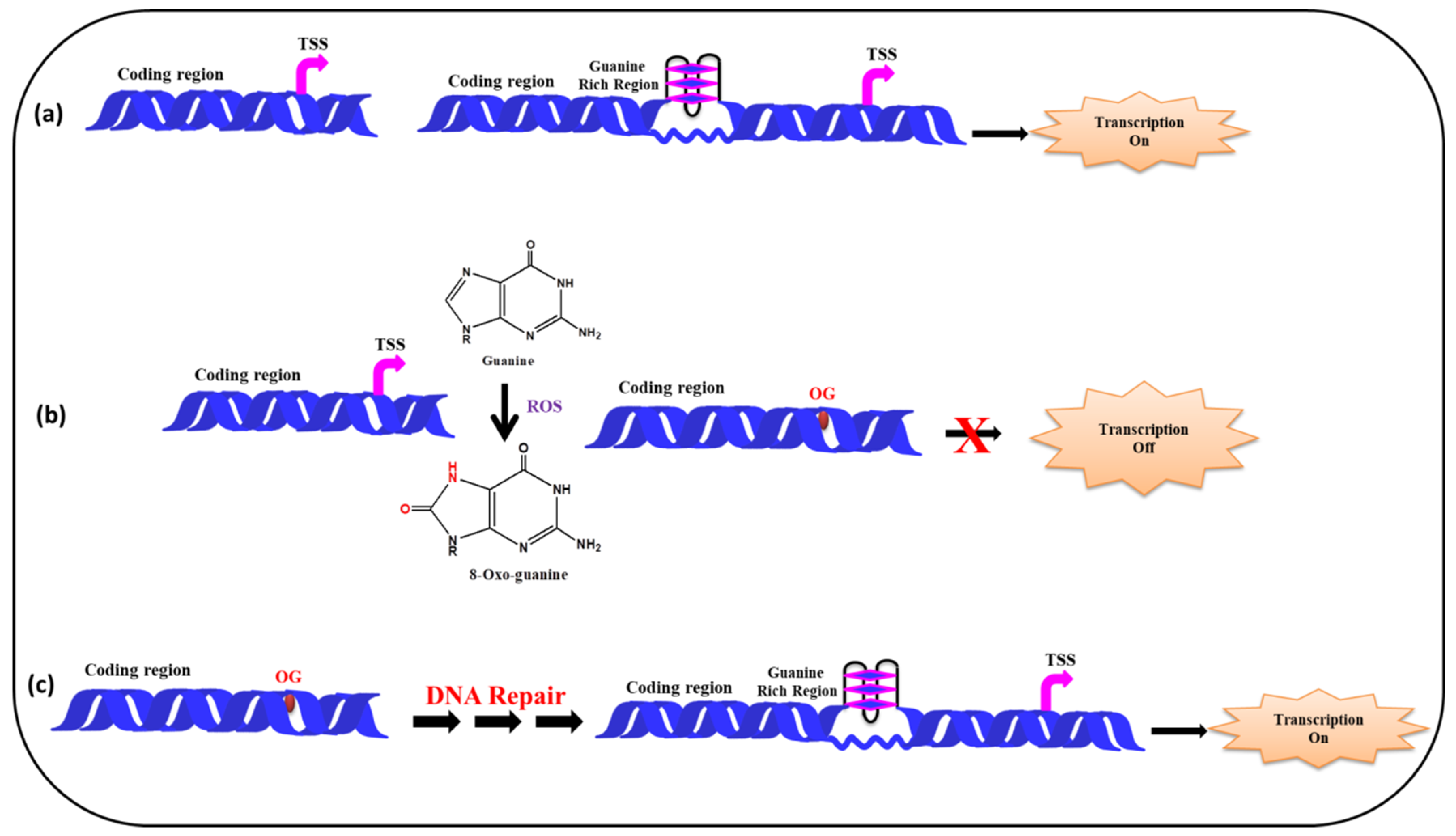
| S. No. | Gene | Disease | References |
|---|---|---|---|
| 1 | c-Myc | Gastrointestinal, ovarian and breast cancer tumors | [21,22] |
| 2 | c-Kit | Gastrointestinal stromal tumors (GIST) | [23] |
| 3 | KRAS | Pancreatic carcinoma | [24] |
| 4 | VEGF | Tumor angiogenesis | [25,26] |
| 5 | PDGF | Cancers and fibrotic disorders | [27] |
| 6 | BCL-2 | B-cell and T-cell lymphomas and breast prostate cervical Colorectal and non-small cell lung carcinomas | [28] |
| 7 | C-Myb | Leukemias | [29] |
| 8 | RET | Thyroid cancers | [30] |
| 9 | AR | Castrate-resistant prostate cancer | [31] |
| 10 | ADAM | Breast cancer | [32] |
| 11 | hTERT | Limitless replication and cancer | [33] |
| 12 | MET | Cancers of kidney, liver, stomach, breast, and brain | [34] |
| 13 | BRCA2 | Familial breast/ovarian cancer, telomere homeostasis | [35] |
| 14 | C9orf72 Gene | Amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD) | [36,37,38] |
| 15 | FMR1 Gene | Fragile X syndrome | [39] |
| 16 | ESR1 | Cancer and neoplasia | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. Int. J. Mol. Sci. 2019, 20, 4258. https://doi.org/10.3390/ijms20174258
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. International Journal of Molecular Sciences. 2019; 20(17):4258. https://doi.org/10.3390/ijms20174258
Chicago/Turabian StyleSingh, Anju, Ritushree Kukreti, Luciano Saso, and Shrikant Kukreti. 2019. "Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations" International Journal of Molecular Sciences 20, no. 17: 4258. https://doi.org/10.3390/ijms20174258
APA StyleSingh, A., Kukreti, R., Saso, L., & Kukreti, S. (2019). Oxidative Stress: Role and Response of Short Guanine Tracts at Genomic Locations. International Journal of Molecular Sciences, 20(17), 4258. https://doi.org/10.3390/ijms20174258