Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions
Abstract
1. Introduction
2. Structure and Regulation of Syntenin
3. Regulation of Cellular Functions by Syntenin
3.1. Regulation of Synapses
3.1.1. The Main Effector Syndecan and Synapse Formation
3.1.2. The Regulator Rheb and Neurodevelopmental Disorders
3.1.3. Association with Other Synaptic Proteins
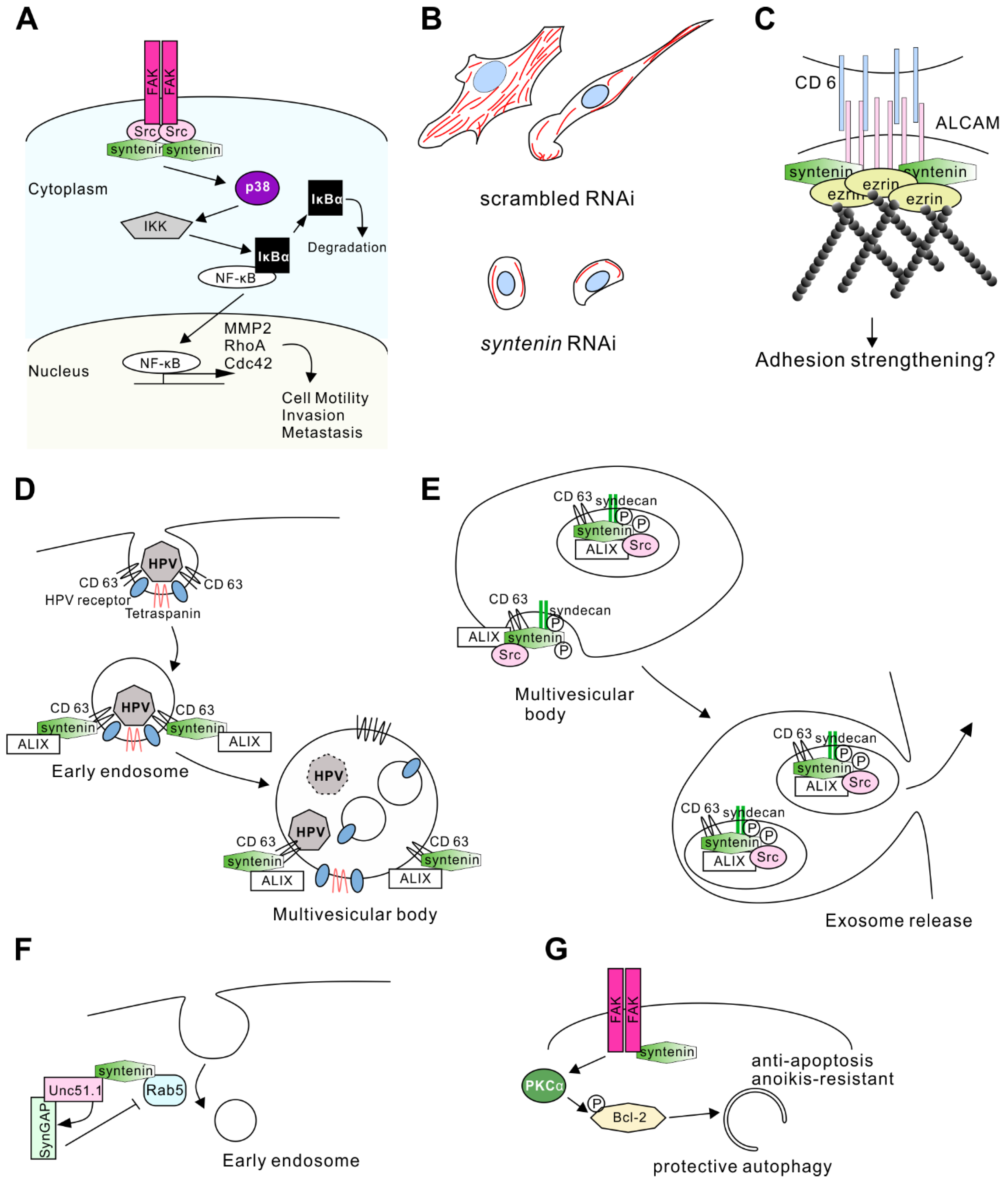
3.2. Tumor Cell Regulation
3.2.1. Tumor Cell Invasion
3.2.2. Mechanism Acting through Focal Adhesion Kinase and c-Src
3.2.3. Another Mechanism Acting through Activated Leukocyte Cell Adhesion Molecule and Merlin
3.2.4. Syntenin as a Target for Anticancer Drugs
3.3. Regulation of Exosome Biogenesis
3.3.1. Interaction with CD63 and ALG-2 interacting protein X
3.3.2. Regulation by c-Src
3.3.3. Role in the Parkinson’s Disease
3.4. Syntenin in other Biological Processes
3.4.1. Neurite Outgrowth
3.4.2. Anoikis-Resistance of Glioma Stem Cells
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALCAM | Activated leukocyte cell adhesion molecule |
| ALIX | ALG-2 interacting protein X |
| CTD | C-terminal domain |
| Dlg | Disks large protein |
| FAK | Focal adhesion kinase |
| GBM | Glioblastoma multiforme |
| GCS | Glioma stem cells |
| GuK | Guanylate kinase-like domain |
| HPV | Human papillomaviruses |
| IKK | IκB kinase |
| IL-5Rα | Interleukin-5 receptor α chain |
| L27 | Domain initially found in LIN2 and LIN7 |
| MMP | Matrix-metalloprotease |
| NF-κB | Nuclear factor-kappa B |
| NTD | N-terminal domain |
| PSD-95 | Postsynaptic density protein 95 |
| PTB | Phosphotyrosine binding domain |
| SH3 | Src homology 3 domain |
| TSC | Tuberous sclerosis complex |
| UCC | Urothelial cell carcinoma |
| ZO-1 | Zonula occludens 1 |
References
- Grootjans, J.J.; Zimmermann, P.; Reekmans, G.; Smets, A.; Degeest, G.; Durr, J.; David, G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA 1997, 94, 13683–13688. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Jiang, H.; Fisher, P.B. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene 1998, 207, 105–110. [Google Scholar] [CrossRef]
- Cho, K.O.; Hunt, C.A.; Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 1992, 9, 929–942. [Google Scholar] [CrossRef]
- Woods, D.F.; Bryant, P.J. ZO-1, DlgA and PSD-95/SAP90: Homologous proteins in tight, septate and synaptic cell junctions. Mech. Dev. 1993, 44, 85–89. [Google Scholar] [CrossRef]
- Beekman, J.M.; Coffer, P.J. The ins and outs of syntenin, a multifunctional intracellular adaptor protein. J. Cell Sci. 2008, 121, 1349–1355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zimmermann, P.; Meerschaert, K.; Reekmans, G.; Leenaerts, I.; Small, J.V.; Vandekerckhove, J.; David, G.; Gettemans, J. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol. Cell 2002, 9, 1215–1225. [Google Scholar] [CrossRef]
- Ohno, K.; Koroll, M.; El Far, O.; Scholze, P.; Gomeza, J.; Betz, H. The neuronal glycine transporter 2 interacts with the PDZ domain protein syntenin-1. Mol. Cell. Neurosci. 2004, 26, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Hirbec, H.; Martin, S.; Henley, J.M. Syntenin is involved in the developmental regulation of neuronal membrane architecture. Mol. Cell. Neurosci. 2005, 28, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ro, Y.T.; Jang, B.K.; Shin, C.Y.; Park, E.U.; Kim, C.G.; Yang, S.I. Akt regulates the expression of MafK, synaptotagmin I, and syntenin-1, which play roles in neuronal function. J. Biomed. Sci. 2010, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Yasuda, S.; Katsurabayashi, S.; Kawano, H.; Endo, K.; Takasaki, K.; Iwasaki, K.; Ichikawa, M.; Kobayashi, T.; Hino, O.; et al. Rheb activation disrupts spine synapse formation through accumulation of syntenin in tuberous sclerosis complex. Nat. Commun. 2015, 6, 6842. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, T.; Kim, J.H.; Zhan, C.; Hatten, M.E. Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev. 2004, 18, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Das, S.K.; Pradhan, A.K.; Emdad, L.; Shen, X.N.; Windle, J.J.; Sarkar, D.; Fisher, P.B. Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells. Oncotarget 2016, 7, 54102–54119. [Google Scholar] [CrossRef] [PubMed]
- Kegelman, T.P.; Das, S.K.; Hu, B.; Bacolod, M.D.; Fuller, C.E.; Menezes, M.E.; Emdad, L.; Dasgupta, S.; Baldwin, A.S.; Bruce, J.N.; et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014, 16, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; te Riet, J.; Eich, C.; Harkes, R.; Smisdom, N.; Bouhuijzen Wenger, J.; Ameloot, M.; Holt, M.; Kanger, J.S.; Figdor, C.G.; et al. Syntenin-1 and ezrin proteins link activated leukocyte cell adhesion molecule to the actin cytoskeleton. J. Biol. Chem. 2014, 289, 13445–13460. [Google Scholar] [CrossRef] [PubMed]
- Koroll, M.; Rathjen, F.G.; Volkmer, H. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J. Biol. Chem. 2001, 276, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Bhutia, S.K.; Kegelman, T.P.; Peachy, L.; Oyesanya, R.A.; Dasgupta, S.; Sokhi, U.K.; Azab, B.; Dash, R.; Quinn, B.A.; et al. MDA-9/syntenin: A positive gatekeeper of melanoma metastasis. Front. Biosci. (Landmark Ed.) 2012, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Cooper, D.R.; Devedjiev, Y.; Derewenda, U.; Derewenda, Z.S. Molecular roots of degenerate specificity in syntenin’s PDZ2 domain: Reassessment of the PDZ recognition paradigm. Structure 2003, 11, 845–853. [Google Scholar] [CrossRef]
- Wawrzyniak, A.M.; Vermeiren, E.; Zimmermann, P.; Ivarsson, Y. Extensions of PSD-95/discs large/ZO-1 (PDZ) domains influence lipid binding and membrane targeting of syntenin-1. FEBS Lett. 2012, 586, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Cierpicki, T.; Bushweller, J.H.; Derewenda, Z.S. Probing the supramodular architecture of a multidomain protein: The structure of syntenin in solution. Structure 2005, 13, 319–327. [Google Scholar] [CrossRef]
- Choi, Y.; Yun, J.H.; Yoo, J.; Lee, I.; Kim, H.; Son, H.N.; Kim, I.S.; Yoon, H.S.; Zimmermann, P.; Couchman, J.R.; et al. New structural insight of C-terminal region of Syntenin-1, enhancing the molecular dimerization and inhibitory function related on Syndecan-4 signaling. Sci. Rep. 2016, 6, 36818. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Cooper, D.R.; Jelen, F.; Devedjiev, Y.; Derewenda, U.; Dauter, Z.; Otlewski, J.; Derewenda, Z.S. PDZ tandem of human syntenin: Crystal structure and functional properties. Structure 2003, 11, 459–468. [Google Scholar] [CrossRef]
- Grembecka, J.; Cierpicki, T.; Devedjiev, Y.; Derewenda, U.; Kang, B.S.; Bushweller, J.H.; Derewenda, Z.S. The binding of the PDZ tandem of syntenin to target proteins. Biochemistry 2006, 45, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Latysheva, N.; Muratov, G.; Rajesh, S.; Padgett, M.; Hotchin, N.A.; Overduin, M.; Berditchevski, F. Syntenin-1 is a new component of tetraspanin-enriched microdomains: Mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol. Cell. Biol. 2006, 26, 7707–7718. [Google Scholar] [CrossRef] [PubMed]
- McClelland, A.C.; Sheffler-Collins, S.I.; Kayser, M.S.; Dalva, M.B. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc. Natl. Acad. Sci. 2009, 106, 20487–20492. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.J.; Reekmans, G.; Ceulemans, H.; David, G. Syntenin-Syndecan Binding Requires Syndecan-Synteny and the Co-operation of Both PDZ Domains of Syntenin. J. Biol. Chem. 2000, 275, 19933–19941. [Google Scholar] [CrossRef]
- Boukerche, H.; Aissaoui, H.; Prevost, C.; Hirbec, H.; Das, S.K.; Su, Z.Z.; Sarkar, D.; Fisher, P.B. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene 2010, 29, 3054–3066. [Google Scholar] [CrossRef]
- Hung, A.Y.; Sheng, M. PDZ domains: Structural modules for protein complex assembly. J. Biol. Chem. 2002, 277, 5699–5702. [Google Scholar] [CrossRef]
- Chao, H.W.; Hong, C.J.; Huang, T.N.; Lin, Y.L.; Hsueh, Y.P. SUMOylation of the MAGUK protein CASK regulates dendritic spinogenesis. J. Cell Biol. 2008, 182, 141–155. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Yang, F.C.; Kharazia, V.; Naisbitt, S.; Cohen, A.R.; Weinberg, R.J.; Sheng, M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J. Cell Biol. 1998, 142, 139–151. [Google Scholar] [CrossRef]
- Sheng, M. PDZs and receptor/channel clustering: Rounding up the latest suspects. Neuron 1996, 17, 575–578. [Google Scholar] [CrossRef]
- Helmke, B.M.; Polychronidis, M.; Benner, A.; Thome, M.; Arribas, J.; Deichmann, M. Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol. Rep. 2004, 12, 221–228. [Google Scholar] [CrossRef]
- Qian, X.L.; Li, Y.Q.; Yu, B.; Gu, F.; Liu, F.F.; Li, W.D.; Zhang, X.M.; Fu, L. Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation. PLoS ONE 2013, 8, e60046. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Q.; Shi, P.; Liu, Z.; Luo, J.; Shao, Z. Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res. 2013, 15, R50. [Google Scholar] [CrossRef]
- Filla, M.S.; Dam, P.; Rapraeger, A.C. The cell surface proteoglycan syndecan-1 mediates fibroblast growth factor-2 binding and activity. J. Cell. Physiol. 1998, 174, 310–321. [Google Scholar] [CrossRef]
- Zimmermann, P.; Zhang, Z.; Degeest, G.; Mortier, E.; Leenaerts, I.; Coomans, C.; Schulz, J.; N’Kuli, F.; Courtoy, P.J.; David, G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 2005, 9, 377–388. [Google Scholar] [CrossRef]
- Ethell, I.M.; Yamaguchi, Y. Cell Surface Heparan Sulfate Proteoglycan Syndecan-2 Induces the Maturation of Dendritic Spines in Rat Hippocampal Neurons. J. Cell Biol. 1999, 144, 575–586. [Google Scholar] [CrossRef]
- Sulka, B.; Lortat-Jacob, H.; Terreux, R.; Letourneur, F.; Rousselle, P. Tyrosine dephosphorylation of the syndecan-1 PDZ binding domain regulates syntenin-1 recruitment. J. Biol. Chem. 2009, 284, 10659–10671. [Google Scholar] [CrossRef]
- Koo, B.K.; Jung, Y.S.; Shin, J.; Han, I.; Mortier, E.; Zimmermann, P.; Whiteford, J.R.; Couchman, J.R.; Oh, E.S.; Lee, W. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 2006, 355, 651–663. [Google Scholar] [CrossRef]
- Yamagata, K.; Sanders, L.K.; Kaufmann, W.E.; Yee, W.; Barnes, C.A.; Nathans, D.; Worley, P.F. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J. Biol. Chem. 1994, 269, 16333–16339. [Google Scholar]
- Zhang, Y.; Gao, X.; Saucedo, L.J.; Ru, B.; Edgar, B.A.; Pan, D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003, 5, 578–581. [Google Scholar] [CrossRef]
- Yasuda, S.; Sugiura, H.; Katsurabayashi, S.; Shimada, T.; Tanaka, H.; Takasaki, K.; Iwasaki, K.; Kobayashi, T.; Hino, O.; Yamagata, K. Activation of Rheb, but not of mTORC1, impairs spine synapse morphogenesis in tuberous sclerosis complex. Sci. Rep. 2014, 4, 5155. [Google Scholar] [CrossRef]
- Torres, R.; Firestein, B.L.; Dong, H.; Staudinger, J.; Olson, E.N.; Huganir, R.L.; Bredt, D.S.; Gale, N.W.; Yancopoulos, G.D. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 1998, 21, 1453–1463. [Google Scholar] [CrossRef]
- Xu, N.J.; Sun, S.; Gibson, J.R.; Henkemeyer, M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat. Neurosci. 2011, 14, 1421–1429. [Google Scholar] [CrossRef]
- Saitsu, H.; Kato, M.; Osaka, H.; Moriyama, N.; Horita, H.; Nishiyama, K.; Yoneda, Y.; Kondo, Y.; Tsurusaki, Y.; Doi, H.; et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia 2012, 53, 1441–1449. [Google Scholar] [CrossRef]
- Ohtahara, S.; Yamatogi, Y. Epileptic encephalopathies in early infancy with suppression-burst. J. Clin. Neurophysiol. 2003, 20, 398–407. [Google Scholar] [CrossRef]
- Enz, R. The trick of the tail: Protein-protein interactions of metabotropic glutamate receptors. Bioessays 2007, 29, 60–73. [Google Scholar] [CrossRef]
- Enz, R.; Croci, C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem. J. 2003, 372, 183–191. [Google Scholar] [CrossRef]
- Hirbec, H.; Perestenko, O.; Nishimune, A.; Meyer, G.; Nakanishi, S.; Henley, J.M.; Dev, K.K. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J. Biol. Chem. 2002, 277, 15221–15224. [Google Scholar] [CrossRef]
- Hirbec, H.; Francis, J.C.; Lauri, S.E.; Braithwaite, S.P.; Coussen, F.; Mulle, C.; Dev, K.K.; Coutinho, V.; Meyer, G.; Isaac, J.T.; et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 2003, 37, 625–638. [Google Scholar] [CrossRef]
- Talukdar, G.; Inoue, R.; Yoshida, T.; Mori, H. Impairment in extinction of cued fear memory in syntenin-1 knockout mice. Neurobiol. Learn. Mem. 2018, 149, 58–67. [Google Scholar] [CrossRef]
- Boukerche, H.; Su, Z.Z.; Emdad, L.; Baril, P.; Balme, B.; Thomas, L.; Randolph, A.; Valerie, K.; Sarkar, D.; Fisher, P.B. mda-9/Syntenin: A positive regulator of melanoma metastasis. Cancer Res. 2005, 65, 10901–10911. [Google Scholar] [CrossRef]
- Boukerche, H.; Su, Z.Z.; Prevot, C.; Sarkar, D.; Fisher, P.B. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc. Natl. Acad. Sci. USA 2008, 105, 15914–15919. [Google Scholar] [CrossRef]
- Dasgupta, S.; Menezes, M.E.; Das, S.K.; Emdad, L.; Janjic, A.; Bhatia, S.; Mukhopadhyay, N.D.; Shao, C.; Sarkar, D.; Fisher, P.B. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin. Cancer Res. 2013, 19, 4621–4633. [Google Scholar] [CrossRef]
- Koo, T.H.; Lee, J.J.; Kim, E.M.; Kim, K.W.; Kim, H.D.; Lee, J.H. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene 2002, 21, 4080–4088. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.Y.; Jeon, Y.K.; Chung, D.H.; Kim, Y.G.; Kim, C.W. Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1. Exp. Mol. Med. 2014, 46, e90. [Google Scholar] [CrossRef]
- Zhong, D.; Ran, J.H.; Tang, W.Y.; Zhang, X.D.; Tan, Y.; Chen, G.J.; Li, X.S.; Yan, Y. Mda-9/syntenin promotes human brain glioma migration through focal adhesion kinase (FAK)-JNK and FAK-AKT signaling. Asian Pac. J. Cancer Prev. 2012, 13, 2897–2901. [Google Scholar] [CrossRef]
- Kegelman, T.P.; Das, S.K.; Emdad, L.; Hu, B.; Menezes, M.E.; Bhoopathi, P.; Wang, X.Y.; Pellecchia, M.; Sarkar, D.; Fisher, P.B. Targeting tumor invasion: The roles of MDA-9/Syntenin. Expert Opin. Ther. Targets 2015, 19, 97–112. [Google Scholar] [CrossRef]
- Oyesanya, R.A.; Bhatia, S.; Menezes, M.E.; Dumur, C.I.; Singh, K.P.; Bae, S.; Troyer, D.A.; Wells, R.B.; Sauter, E.R.; Sidransky, D.; et al. MDA-9/Syntenin regulates differentiation and angiogenesis programs in head and neck squamous cell carcinoma. Oncoscience 2014, 1, 725–737. [Google Scholar] [CrossRef]
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor beta1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 2016, 7, 80175–80189. [Google Scholar] [CrossRef]
- Storz, P.; Toker, A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003, 22, 109–120. [Google Scholar] [CrossRef]
- Ishizawar, R.; Parsons, S.J. c-Src and cooperating partners in human cancer. Cancer Cell 2004, 6, 209–214. [Google Scholar] [CrossRef]
- Irby, R.B.; Yeatman, T.J. Role of Src expression and activation in human cancer. Oncogene 2000, 19, 5636–5642. [Google Scholar] [CrossRef]
- Joo, N.E.; Watanabe, T.; Chen, C.; Chekenya, M.; Stallcup, W.B.; Kapila, Y.L. NG2, a novel proapoptotic receptor, opposes integrin alpha4 to mediate anoikis through PKCalpha-dependent suppression of FAK phosphorylation. Cell Death Differ. 2008, 15, 899–907. [Google Scholar] [CrossRef]
- Dai, B.; Kang, S.H.; Gong, W.; Liu, M.; Aldape, K.D.; Sawaya, R.; Huang, S. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene 2007, 26, 6212–6219. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Patel, A.; Sabbineni, H.; Clarke, A.; Somanath, P.R. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016, 157, 52–61. [Google Scholar] [CrossRef]
- Ferragut, F.; Cagnoni, A.J.; Colombo, L.L.; Sanchez Terrero, C.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vanzulli, S.I.; Rabinovich, G.A.; Marino, K.V.; Elola, M.T. Dual knockdown of Galectin-8 and its glycosylated ligand, the activated leukocyte cell adhesion molecule (ALCAM/CD166), synergistically delays in vivo breast cancer growth. Biochim. Biophys. Acta Mol. Cell Res. 2019. [Google Scholar] [CrossRef]
- Piao, D.; Jiang, T.; Liu, G.; Wang, B.; Xu, J.; Zhu, A. Clinical implications of activated leukocyte cell adhesion molecule expression in breast cancer. Mol. Biol. Rep. 2012, 39, 661–668. [Google Scholar] [CrossRef]
- Hansen, A.G.; Freeman, T.J.; Arnold, S.A.; Starchenko, A.; Jones-Paris, C.R.; Gilger, M.A.; Washington, M.K.; Fan, K.H.; Shyr, Y.; Beauchamp, R.D.; et al. Elevated ALCAM shedding in colorectal cancer correlates with poor patient outcome. Cancer Res. 2013, 73, 2955–2964. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, C.; Jing, L.; Ren, J.; Guan, Y. Meta-analysis indicating that high ALCAM expression predicts poor prognosis in colorectal cancer. Oncotarget 2017, 8, 48272–48281. [Google Scholar] [CrossRef]
- Willrodt, A.H.; Beffinger, M.; Vranova, M.; Protsyuk, D.; Schuler, K.; Jadhav, M.; Heikenwalder, M.; van den Broek, M.; Borsig, L.; Halin, C. Stromal Expression of Activated Leukocyte Cell Adhesion Molecule Promotes Lung Tumor Growth and Metastasis. Am. J. Pathol. 2017, 187, 2558–2569. [Google Scholar] [CrossRef]
- Donizy, P.; Zietek, M.; Halon, A.; Leskiewicz, M.; Kozyra, C.; Matkowski, R. Prognostic significance of ALCAM (CD166/MEMD) expression in cutaneous melanoma patients. Diagn. Pathol. 2015, 10, 86. [Google Scholar] [CrossRef]
- Jannie, K.M.; Stipp, C.S.; Weiner, J.A. ALCAM regulates motility, invasiveness, and adherens junction formation in uveal melanoma cells. PLoS ONE 2012, 7, e39330. [Google Scholar] [CrossRef]
- Kijima, N.; Hosen, N.; Kagawa, N.; Hashimoto, N.; Nakano, A.; Fujimoto, Y.; Kinoshita, M.; Sugiyama, H.; Yoshimine, T. CD166/activated leukocyte cell adhesion molecule is expressed on glioblastoma progenitor cells and involved in the regulation of tumor cell invasion. Neuro Oncol. 2012, 14, 1254–1264. [Google Scholar] [CrossRef]
- Jannatipour, M.; Dion, P.; Khan, S.; Jindal, H.; Fan, X.; Laganiere, J.; Chishti, A.H.; Rouleau, G.A. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J. Biol. Chem. 2001, 276, 33093–33100. [Google Scholar] [CrossRef]
- Kegelman, T.P.; Wu, B.; Das, S.K.; Talukdar, S.; Beckta, J.M.; Hu, B.; Emdad, L.; Valerie, K.; Sarkar, D.; Furnari, F.B.; et al. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc. Natl. Acad. Sci. USA 2017, 114, 370–375. [Google Scholar] [CrossRef]
- Yu, Y.; Li, S.; Wang, K.; Wan, X. A PDZ Protein MDA-9/Syntenin: As a Target for Cancer Therapy. Comput. Struct. Biotechnol. J. 2019, 17, 136–141. [Google Scholar] [CrossRef]
- Chivet, M.; Javalet, C.; Hemming, F.; Pernet-Gallay, K.; Laulagnier, K.; Fraboulet, S.; Sadoul, R. Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 2013, 41, 241–244. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Kramer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Higa, G.S.; de Sousa, E.; Walter, L.T.; Kinjo, E.R.; Resende, R.R.; Kihara, A.H. MicroRNAs in neuronal communication. Mol. Neurobiol. 2014, 49, 1309–1326. [Google Scholar] [CrossRef]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef]
- Fares, J.; Kashyap, R.; Zimmermann, P. Syntenin: Key player in cancer exosome biogenesis and uptake? Cell Adhes. Migr. 2017, 11, 124–126. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [CrossRef]
- Escola, J.-M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Odorizzi, G. The multiple personalities of Alix. J. Cell Sci. 2006, 119, 3025–3032. [Google Scholar] [CrossRef][Green Version]
- Fujii, K.; Hurley, J.H.; Freed, E.O. Beyond Tsg101: The role of Alix in ‘ESCRTing’ HIV-1. Nat. Rev. Microbiol. 2007, 5, 912–916. [Google Scholar] [CrossRef]
- Grassel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Spoden, G.A.; Tenzer, S.; Boller, K.; Bago, R.; Rajesh, S.; Overduin, M.; et al. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 32337. [Google Scholar] [CrossRef]
- Imjeti, N.S.; Menck, K.; Egea-Jimenez, A.L.; Lecointre, C.; Lembo, F.; Bouguenina, H.; Badache, A.; Ghossoub, R.; David, G.; Roche, S.; et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2017, 114, 12495–12500. [Google Scholar] [CrossRef]
- Hikita, T.; Kuwahara, A.; Watanabe, R.; Miyata, M.; Oneyama, C. Src in endosomal membranes promotes exosome secretion and tumor progression. Sci. Rep. 2019, 9, 3265. [Google Scholar] [CrossRef]
- Tomlinson, P.R.; Zheng, Y.; Fischer, R.; Heidasch, R.; Gardiner, C.; Evetts, S.; Hu, M.; Wade-Martins, R.; Turner, M.R.; Morris, J.; et al. Identification of distinct circulating exosomes in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 353–361. [Google Scholar] [CrossRef]
- Papinski, D.; Kraft, C. Regulation of Autophagy By Signaling Through the Atg1/ULK1 Complex. J. Mol. Biol. 2016, 428, 1725–1741. [Google Scholar] [CrossRef]
- McLauchlan, H.; Newell, J.; Morrice, N.; Osborne, A.; West, M.; Smythe, E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr. Biol. 1998, 8, 34–45. [Google Scholar] [CrossRef]
- Villaseñor, R.; Kalaidzidis, Y.; Zerial, M. Signal processing by the endosomal system. Curr. Opin. Cell Biol. 2016, 39, 53–60. [Google Scholar] [CrossRef]
- Chen, H.J.; Rojas-Soto, M.; Oguni, A.; Kennedy, M.B. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 1998, 20, 895–904. [Google Scholar] [CrossRef]
- Kim, J.H.; Liao, D.; Lau, L.F.; Huganir, R.L. SynGAP: A synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron 1998, 20, 683–691. [Google Scholar] [CrossRef]
- Diehn, M.; Majeti, R. Metastatic cancer stem cells: An opportunity for improving cancer treatment? Cell Stem Cell 2010, 6, 502–503. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells-what challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef]
- Talukdar, S.; Pradhan, A.K.; Bhoopathi, P.; Shen, X.N.; August, L.A.; Windle, J.J.; Sarkar, D.; Furnari, F.B.; Cavenee, W.K.; Das, S.K.; et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, 5768–5773. [Google Scholar] [CrossRef]
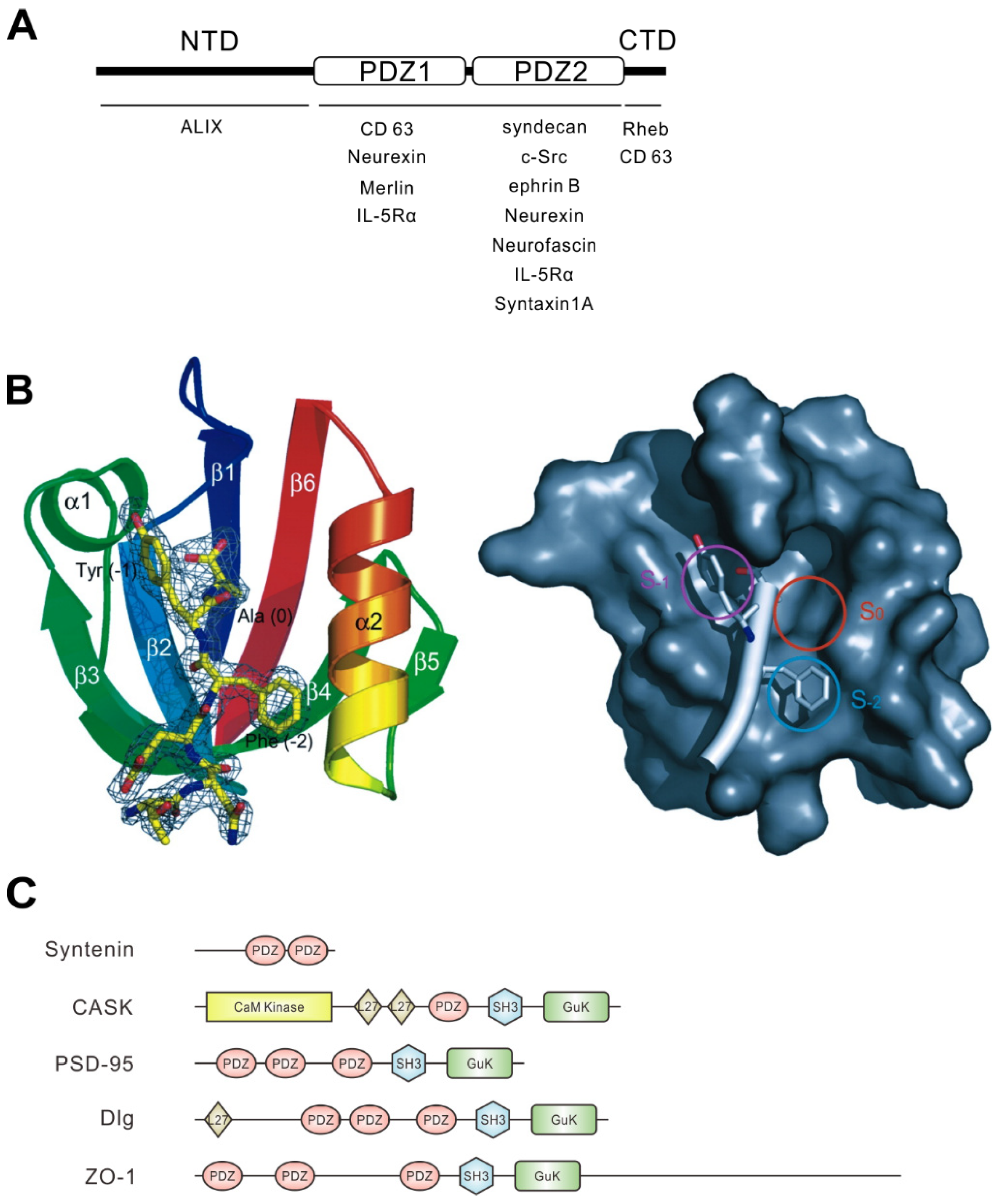

| Binding Partner | Where to Interact with Syntenin | Reference |
|---|---|---|
| ALG-2 interacting protein X (ALIX) | NTD | [12] |
| Cluster of differentiation (CD)63 | PDZ1 and CTD | [24] |
| Neurexin | PDZ1 and PDZ2 | [16,26] |
| Merlin | PDZ1 | [22] |
| IL-5Rα | PDZ1 and PDZ2 | [22] |
| Syndecan | PDZ2 | [16,22] |
| cellular Src kinase (c-Src) | PDZ2 | [27] |
| Ephrin B | PDZ2 | [25] |
| Neurofascin | PDZ2 | [16] |
| Syntaxin 1A | PDZ2 | [7] |
| Ras homolog enriched in brain (Rheb) | CTD | [10] |
| Activated leukocyte cell adhesion molecule (ALCAM) | Not examined. ALCAM has PDZ binding motif at its C-terminal (-TEA, class I) | [15] |
| Uncoordinated (Unc)51.1 | PDZ1 and PDZ2 ? | [11] |
| Ras-associated binding (Rab)5 | PDZ1 and PDZ2 ? | [11] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171. https://doi.org/10.3390/ijms20174171
Shimada T, Yasuda S, Sugiura H, Yamagata K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. International Journal of Molecular Sciences. 2019; 20(17):4171. https://doi.org/10.3390/ijms20174171
Chicago/Turabian StyleShimada, Tadayuki, Shin Yasuda, Hiroko Sugiura, and Kanato Yamagata. 2019. "Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions" International Journal of Molecular Sciences 20, no. 17: 4171. https://doi.org/10.3390/ijms20174171
APA StyleShimada, T., Yasuda, S., Sugiura, H., & Yamagata, K. (2019). Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. International Journal of Molecular Sciences, 20(17), 4171. https://doi.org/10.3390/ijms20174171





