Sex, Age, and Bodyweight as Determinants of Extracellular DNA in the Plasma of Mice: A Cross-Sectional Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Procedures
4.2. DNA Isolation and Quantification
4.3. DNase Activity
4.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DNase | Deoxyribonuclease |
| mtDNA | Mitochondrial DNA |
| ncDNA | Nuclear DNA |
| ecDNA | Extracellular DNA |
References
- Mandel, P.; Metais, P. Comptes rendus des seances de la Societe de biologie et de ses filiales. Journal de la Société de Biologie 1948, 142, 241–243. [Google Scholar]
- Poli, C.; Augusto, J.F.; Dauve, J.; Adam, C.; Preisser, L.; Larochette, V.; Pignon, P.; Savina, A.; Blanchard, S.; Subra, J.F.; et al. IL-26 Confers Proinflammatory Properties to Extracellular DNA. J. Immunol. 2017, 198, 3650–3661. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.; Hu, T.; Zhang, Y.; Zhang, C.; Li, T.; Wang, C.; Dong, Z.; Novakovic, V.A.; Hu, T.; et al. Neutrophil extracellular traps enhance procoagulant activity in patients with oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta; Int. J. Clin. Chem. 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Lo, Y.M.; Wainscoat, J.S.; Fleming, K.A. Non-invasive approach to prenatal diagnosis from maternal peripheral blood. Prenat. Diagn. 1992, 12, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar] [PubMed]
- Burnham, P.; Kim, M.S.; Agbor-Enoh, S.; Luikart, H.; Valantine, H.A.; Khush, K.K.; De Vlaminck, I. Single-stranded DNA library preparation uncovers the origin and diversity of ultrashort cell-free DNA in plasma. Sci. Rep. 2016, 6, 27859. [Google Scholar] [CrossRef]
- Lehmann-Werman, R.; Magenheim, J.; Moss, J.; Neiman, D.; Abraham, O.; Piyanzin, S.; Zemmour, H.; Fox, I.; Dor, T.; Grompe, M.; et al. Monitoring liver damage using hepatocyte-specific methylation markers in cell-free circulating DNA. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Hamaguchi, S.; Akeda, Y.; Yamamoto, N.; Seki, M.; Yamamoto, K.; Oishi, K.; Tomono, K. Origin of Circulating Free DNA in Sepsis: Analysis of the CLP Mouse Model. Mediat. Inflamm. 2015, 2015, 614518. [Google Scholar] [CrossRef]
- Vokalova, L.; Laukova, L.; Conka, J.; Meliskova, V.; Borbelyova, V.; Babickova, J.; Tothova, L.; Hodosy, J.; Vlkova, B.; Celec, P. Deoxyribonuclease partially ameliorates thioacetamide-induced hepatorenal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G457–G463. [Google Scholar] [CrossRef]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.R.; Sato, F.; Bando, M.; Yagi, S.; et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016, 2, e1501332. [Google Scholar] [CrossRef] [PubMed]
- Revelo, X.S.; Ghazarian, M.; Chng, M.H.; Luck, H.; Kim, J.H.; Zeng, K.; Shi, S.Y.; Tsai, S.; Lei, H.; Kenkel, J.; et al. Nucleic Acid-Targeting Pathways Promote Inflammation in Obesity-Related Insulin Resistance. Cell Rep. 2016, 16, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, R.; Dache, Z.A.A.; Thezenas, S.; Otandault, A.; Tanos, R.; Pastor, B.; Sanchez, C.; Azzi, J.; Tousch, G.; Azan, S.; et al. Quantifying circulating cell-free DNA in humans. Sci. Rep. 2019, 9, 5220. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.C.; Chantler, P.D.; Barr, T.L. High Interspecimen Variability in Nucleic Acid Extraction Efficiency Necessitates the Use of Spike-In Control for Accurate qPCR-based Measurement of Plasma Cell-Free DNA Levels. Lab. Med. 2017, 48, 332–338. [Google Scholar] [CrossRef]
- Vlkova, B.; Turna, J.; Celec, P. Fetal DNA in maternal plasma in preeclamptic pregnancies. Hypertens. Pregnancy 2015, 34, 36–49. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Burk, M.R.; Troeger, C.; Kang, A.; Holzgreve, W.; Hahn, S. Fluctuation of maternal and fetal free extracellular circulatory DNA in maternal plasma. Obstet. Gynecol. 2000, 96, 991–996. [Google Scholar]
- Cheng, T.H.T.; Lui, K.O.; Peng, X.L.; Cheng, S.H.; Jiang, P.; Chan, K.C.A.; Chiu, R.W.K.; Lo, Y.M.D. DNase1 Does Not Appear to Play a Major Role in the Fragmentation of Plasma DNA in a Knockout Mouse Model. Clin. Chem. 2018, 64, 406–408. [Google Scholar] [CrossRef]
- Serpas, L.; Chan, R.W.Y.; Jiang, P.; Ni, M.; Sun, K.; Rashidfarrokhi, A.; Soni, C.; Sisirak, V.; Lee, W.S.; Cheng, S.H.; et al. Dnase1l3 deletion causes aberrations in length and end-motif frequencies in plasma DNA. Proc. Natl. Acad. Sci. USA 2019, 116, 641–649. [Google Scholar] [CrossRef]
- Jimenez-Alcazar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renne, C.; Renne, T.; et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017, 358, 1202–1206. [Google Scholar] [CrossRef]
- Suck, D. DNA recognition by DNase I. J. Mol. Recognit. 1994, 7, 65–70. [Google Scholar] [CrossRef]
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488. [Google Scholar] [CrossRef]
- Giles, B.M.; Boackle, S.A. Linking complement and anti-dsDNA antibodies in the pathogenesis of systemic lupus erythematosus. Immunol. Res. 2013, 55, 10–21. [Google Scholar] [CrossRef]
- Koizumi, T. Tissue distribution of deoxyribonuclease I (DNase I) activity level in mice and its sexual dimorphism. Exp. Anim. 1995, 44, 181–185. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef]
- Botezatu, I.; Serdyuk, O.; Potapova, G.; Shelepov, V.; Alechina, R.; Molyaka, Y.; Ananev, V.; Bazin, I.; Garin, A.; Narimanov, M.; et al. Genetic analysis of DNA excreted in urine: A new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin. Chem. 2000, 46, 1078–1084. [Google Scholar]
- Hisazumi, J.; Kobayashi, N.; Nishikawa, M.; Takakura, Y. Significant role of liver sinusoidal endothelial cells in hepatic uptake and degradation of naked plasmid DNA after intravenous injection. Pharm. Res. 2004, 21, 1223–1228. [Google Scholar] [CrossRef]
- Galeazzi, M.; Morozzi, G.; Piccini, M.; Chen, J.; Bellisai, F.; Fineschi, S.; Marcolongo, R. Dosage and characterization of circulating DNA: Present usage and possible applications in systemic autoimmune disorders. Autoimmun Rev. 2003, 2, 50–55. [Google Scholar] [CrossRef]
- Lou, X.; Hou, Y.; Liang, D.; Peng, L.; Chen, H.; Ma, S.; Zhang, L. A novel Alu-based real-time PCR method for the quantitative detection of plasma circulating cell-free DNA: Sensitivity and specificity for the diagnosis of myocardial infarction. Int. J. Mol. Med. 2015, 35, 72–80. [Google Scholar] [CrossRef]
- Ale, A.; Zhang, Y.; Han, C.; Cai, D. Obesity-associated extracellular mtDNA activates central TGFbeta pathway to cause blood pressure increase. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E161–E174. [Google Scholar] [CrossRef]
- Ermakov, A.V.; Konkova, M.S.; Kostyuk, S.V.; Izevskaya, V.L.; Baranova, A.; Veiko, N.N. Oxidized extracellular DNA as a stress signal in human cells. Oxid. Med. Cell Longev. 2013, 2013, 649747. [Google Scholar] [CrossRef]
- Barton, G.M.; Kagan, J.C.; Medzhitov, R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006, 7, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, N.; Tall, A.R.; Tabas, I. Mitochondrial Oxidative Stress Promotes Atherosclerosis and Neutrophil Extracellular Traps in Aged Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e99–e107. [Google Scholar] [CrossRef]
- Kishi, K.; Yasuda, T.; Takeshita, H. DNase I: Structure, function, and use in medicine and forensic science. Leg. Med. 2001, 3, 69–83. [Google Scholar] [CrossRef]
- Velders, M.; Treff, G.; Machus, K.; Bosnyak, E.; Steinacker, J.; Schumann, U. Exercise is a potent stimulus for enhancing circulating DNase activity. Clin. Biochem. 2014, 47, 471–474. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Kang, T.H.; Reardon, J.T.; Lee, J.H.; Ozturk, N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010, 584, 2618–2625. [Google Scholar] [CrossRef]
- Jaini, R.; Altuntas, C.Z.; Loya, M.G.; Tuohy, V.K. Disruption of estrous cycle homeostasis in mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2015, 279, 71–74. [Google Scholar] [CrossRef]
- Chim, S.S.; Tong, Y.K.; Chiu, R.W.; Lau, T.K.; Leung, T.N.; Chan, L.Y.; Oudejans, C.B.; Ding, C.; Lo, Y.M. Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc. Natl. Acad. Sci. USA 2005, 102, 14753–14758. [Google Scholar] [CrossRef]
- Zinkova, A.; Brynychova, I.; Svacina, A.; Jirkovska, M.; Korabecna, M. Cell-free DNA from human plasma and serum differs in content of telomeric sequences and its ability to promote immune response. Sci. Rep. 2017, 7, 2591. [Google Scholar] [CrossRef]
- Kolarevic, A.; Yancheva, D.; Kocic, G.; Smelcerovic, A. Deoxyribonuclease inhibitors. Eur. J. Med. Chem. 2014, 88, 101–111. [Google Scholar] [CrossRef]
- Rooney, J.P.; Ryde, I.T.; Sanders, L.H.; Howlett, E.H.; Colton, M.D.; Germ, K.E.; Mayer, G.D.; Greenamyre, J.T.; Meyer, J.N. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol. Biol. 2015, 1241, 23–38. [Google Scholar] [CrossRef]
- Wai, T.; Ao, A.; Zhang, X.; Cyr, D.; Dufort, D.; Shoubridge, E.A. The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 2010, 83, 52–62. [Google Scholar] [CrossRef]
- Nadano, D.; Yasuda, T.; Kishi, K. Measurement of deoxyribonuclease I activity in human tissues and body fluids by a single radial enzyme-diffusion method. Clin. Chem. 1993, 39, 448–452. [Google Scholar]
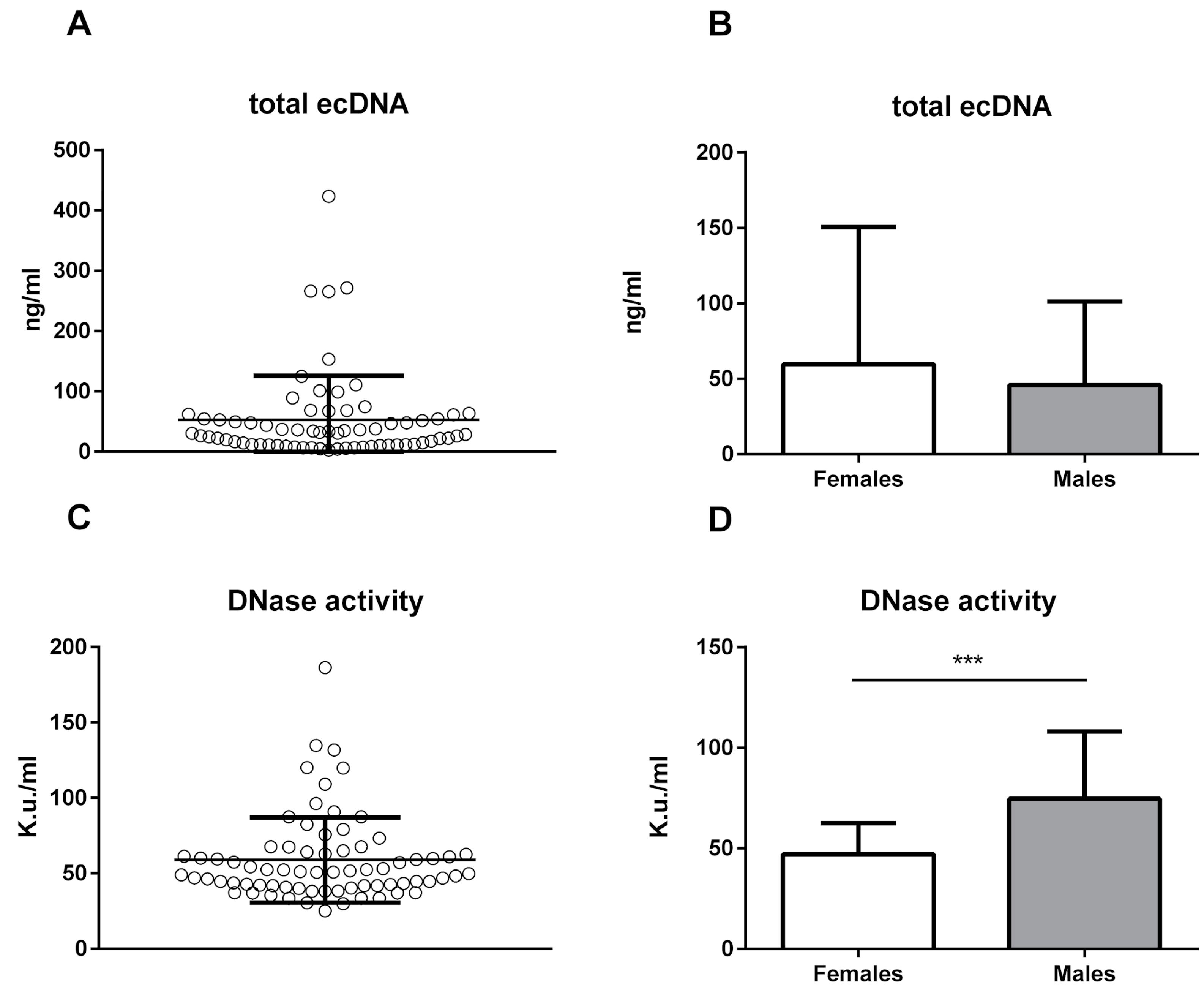
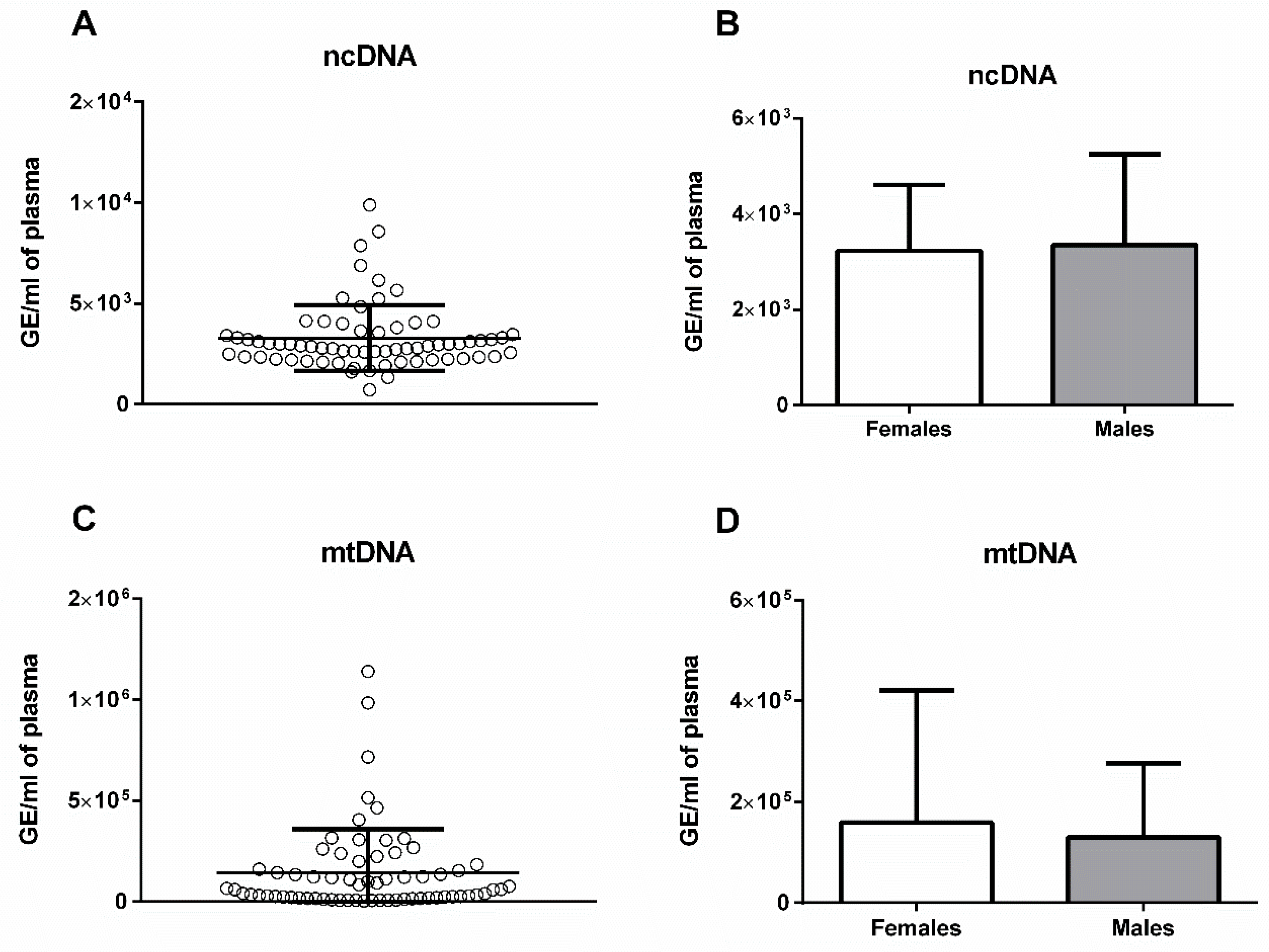
| Bodyweight (g) | Age (Days) | Total ecDNA (ng/mL) | ncDNA (GE/mL) | mtDNA (GE/mL) | DNase Activity (K.u./mL) | |
|---|---|---|---|---|---|---|
| Mean | 38.06 | 226 | 52.7 | 3.3 × 103 | 1.4 × 105 | 59.0 |
| Standard Error | 0.72 | 14 | 8.8 | 2.0 × 102 | 2.6 × 104 | 3.4 |
| Median | 38.53 | 195 | 31.1 | 2.9 × 103 | 6.0 ×104 | 51.0 |
| Standard Deviation | 6.16 | 122 | 73.0 | 1.6 × 103 | 2.1 × 105 | 28.4 |
| Minimum | 24.62 | 99 | 2.9 | 7.2 × 102 | 3.1 × 103 | 25.1 |
| Maximum | 52.11 | 468 | 423.2 | 9.9 × 103 | 1.1 × 106 | 186.4 |
| Coefficient of Variance | 16% | 54% | 139% | 50% | 149% | 48% |
| Bodyweight | Age | Total ecDNA | ncDNA | mtDNA | DNase Activity | |
|---|---|---|---|---|---|---|
| Bodyweight | 0.60 *** | 0.01 | 0.07 | −0.01 | 0.19 | |
| Age | 0.60 *** | 0.03 | 0.04 | 0.03 | −0.11 | |
| Total ecDNA | 0.01 | 0.03 | 0.66 *** | 0.90 *** | −0.11 | |
| ncDNA | 0.07 | 0.04 | 0.66 *** | 0.63 *** | −0.02 | |
| mtDNA | −0.01 | 0.03 | 0.90 *** | 0.63 *** | −0.02 | |
| DNase activity | 0.19 | −0.11 | −0.11 | −0.02 | −0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovičová, Ľ.; Konečná, B.; Vokálová, L.; Lauková, L.; Vlková, B.; Celec, P. Sex, Age, and Bodyweight as Determinants of Extracellular DNA in the Plasma of Mice: A Cross-Sectional Study. Int. J. Mol. Sci. 2019, 20, 4163. https://doi.org/10.3390/ijms20174163
Janovičová Ľ, Konečná B, Vokálová L, Lauková L, Vlková B, Celec P. Sex, Age, and Bodyweight as Determinants of Extracellular DNA in the Plasma of Mice: A Cross-Sectional Study. International Journal of Molecular Sciences. 2019; 20(17):4163. https://doi.org/10.3390/ijms20174163
Chicago/Turabian StyleJanovičová, Ľubica, Barbora Konečná, Lenka Vokálová, Lucia Lauková, Barbora Vlková, and Peter Celec. 2019. "Sex, Age, and Bodyweight as Determinants of Extracellular DNA in the Plasma of Mice: A Cross-Sectional Study" International Journal of Molecular Sciences 20, no. 17: 4163. https://doi.org/10.3390/ijms20174163
APA StyleJanovičová, Ľ., Konečná, B., Vokálová, L., Lauková, L., Vlková, B., & Celec, P. (2019). Sex, Age, and Bodyweight as Determinants of Extracellular DNA in the Plasma of Mice: A Cross-Sectional Study. International Journal of Molecular Sciences, 20(17), 4163. https://doi.org/10.3390/ijms20174163





