Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity
Abstract
1. Introduction
2. Results
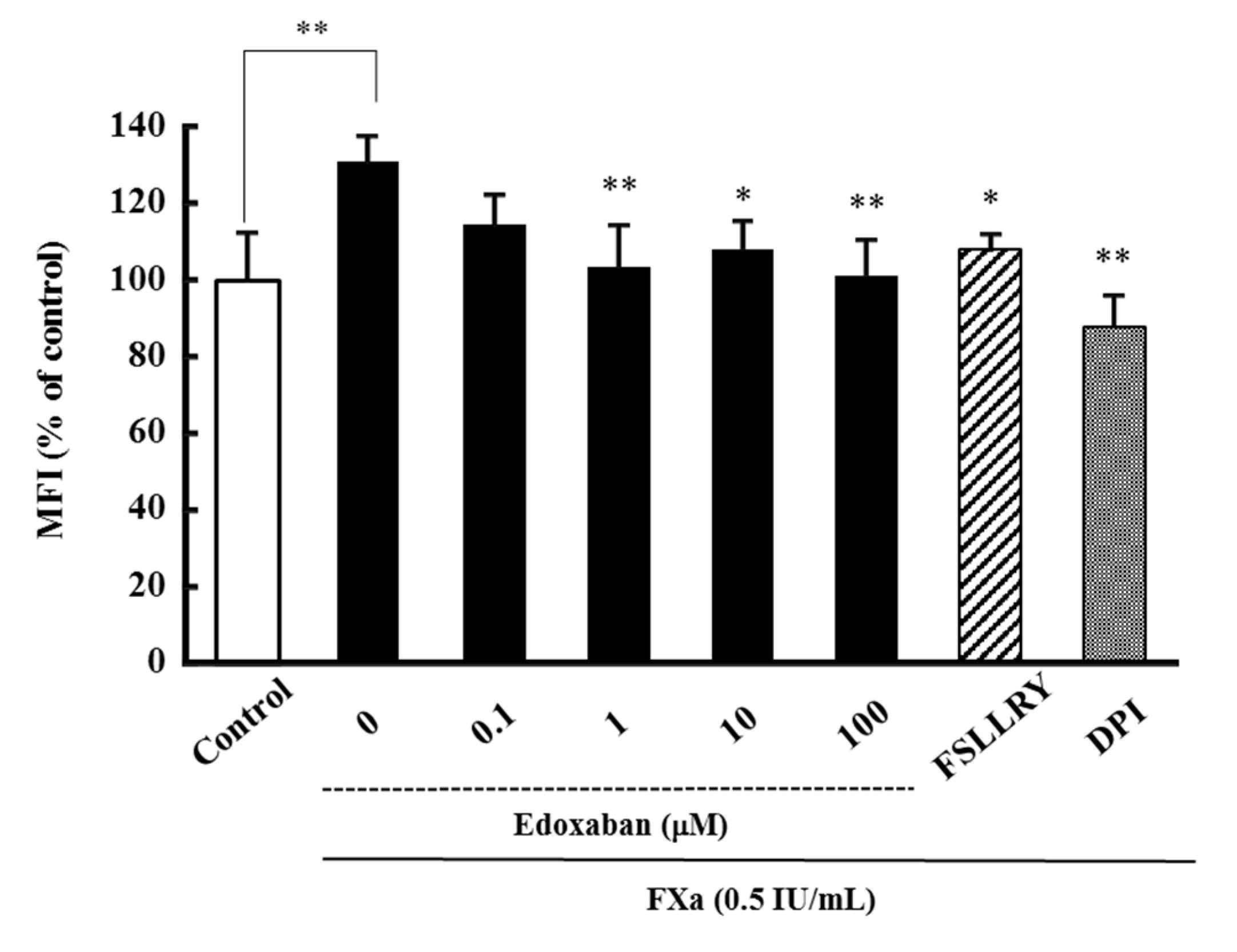
2.1. Effect of Edoxaban on Intracellular Reactive Oxygen Species Production Induced by FXa in HK-2 Cells
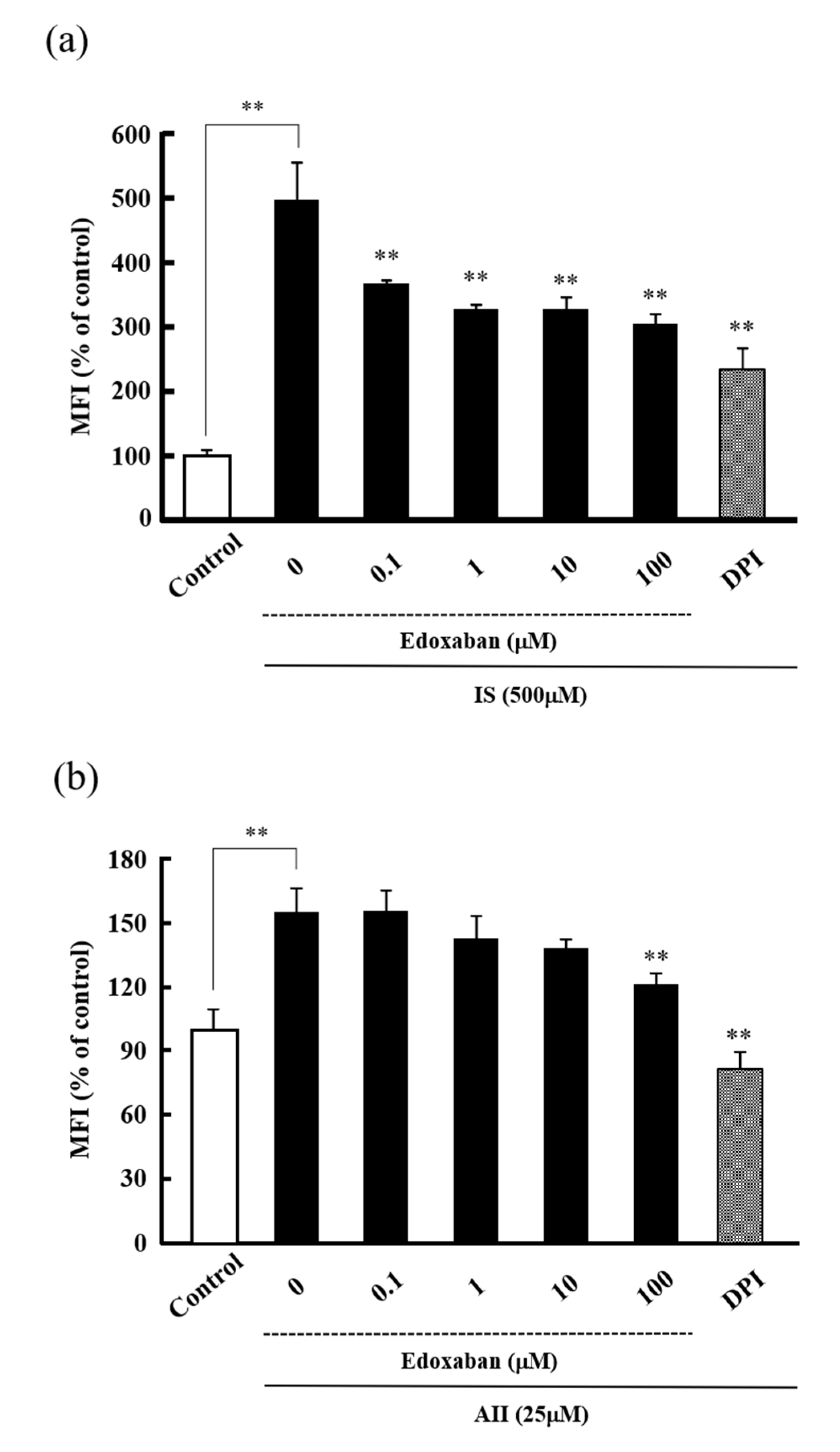
2.2. Effect of Edoxaban on Intracellular Reactive Oxygen Species Production Induced by Indoxyl Sulfate and Angiotensin II in HK-2 Cells
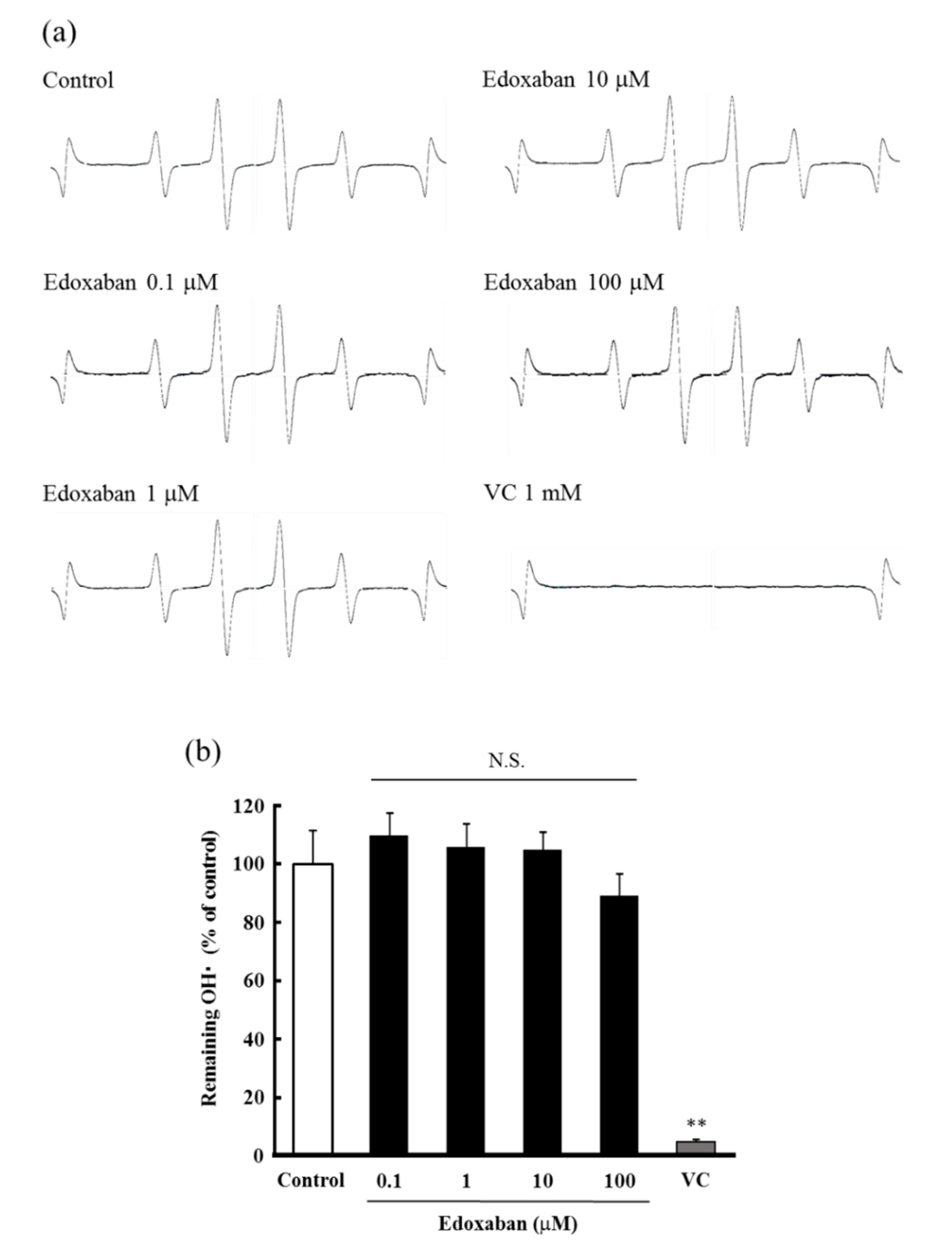
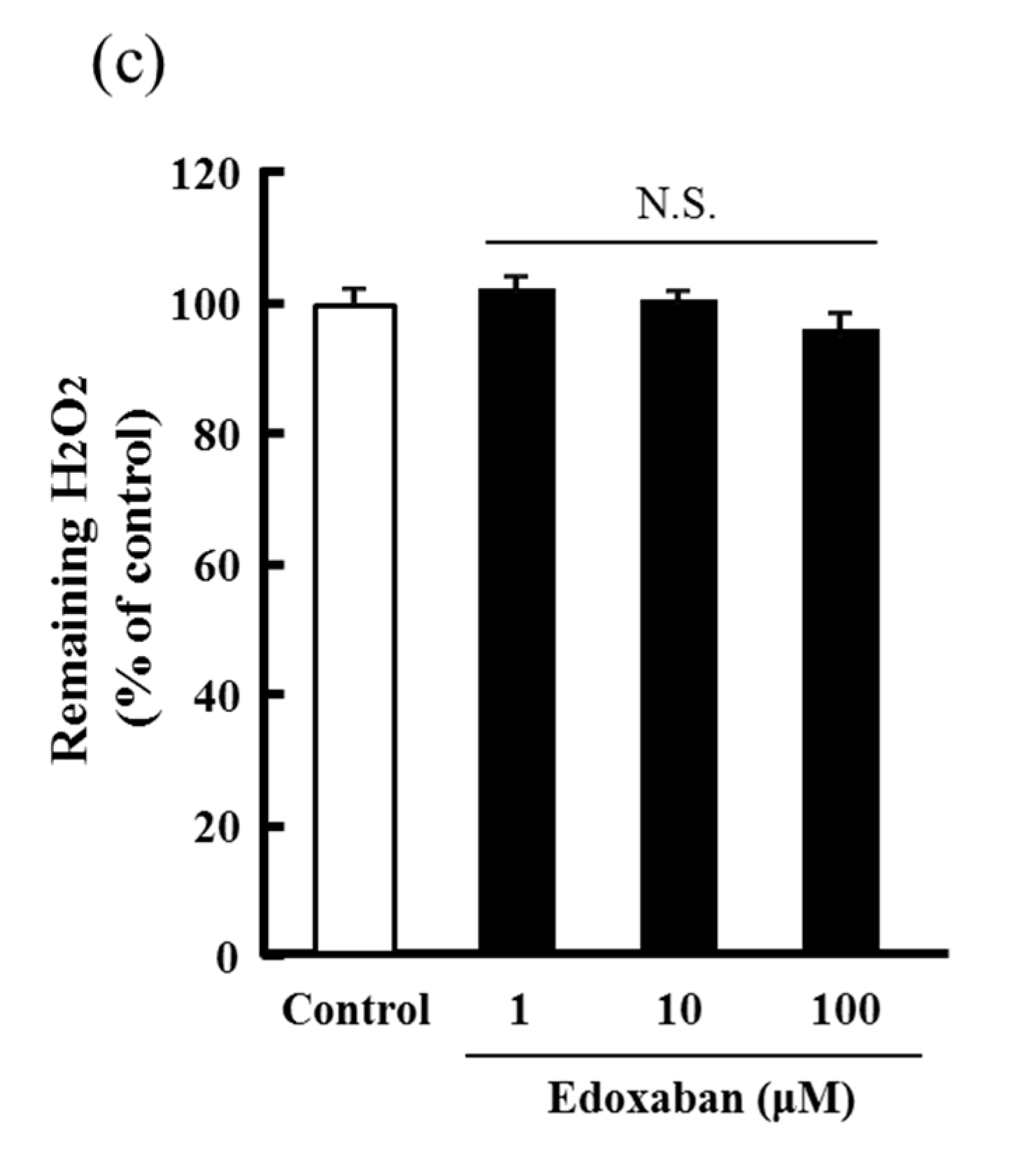
2.3. Radical Scavenging Activity of Edoxaban Against Hydroxyl Radical and Hydrogen Peroxide Decomposition Ability
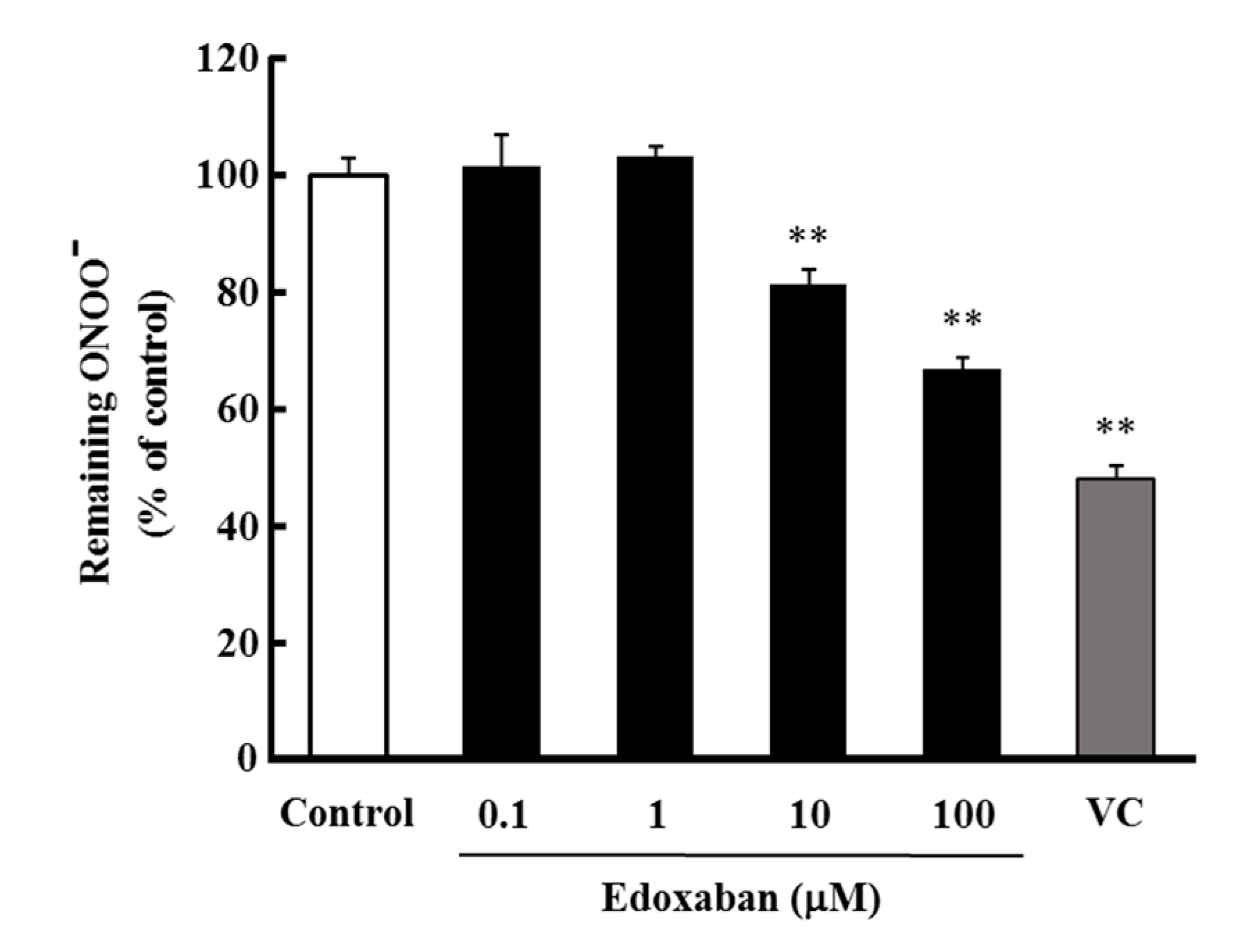
2.4. Radical Scavenging Activity of Edoxaban against Peroxynitrite
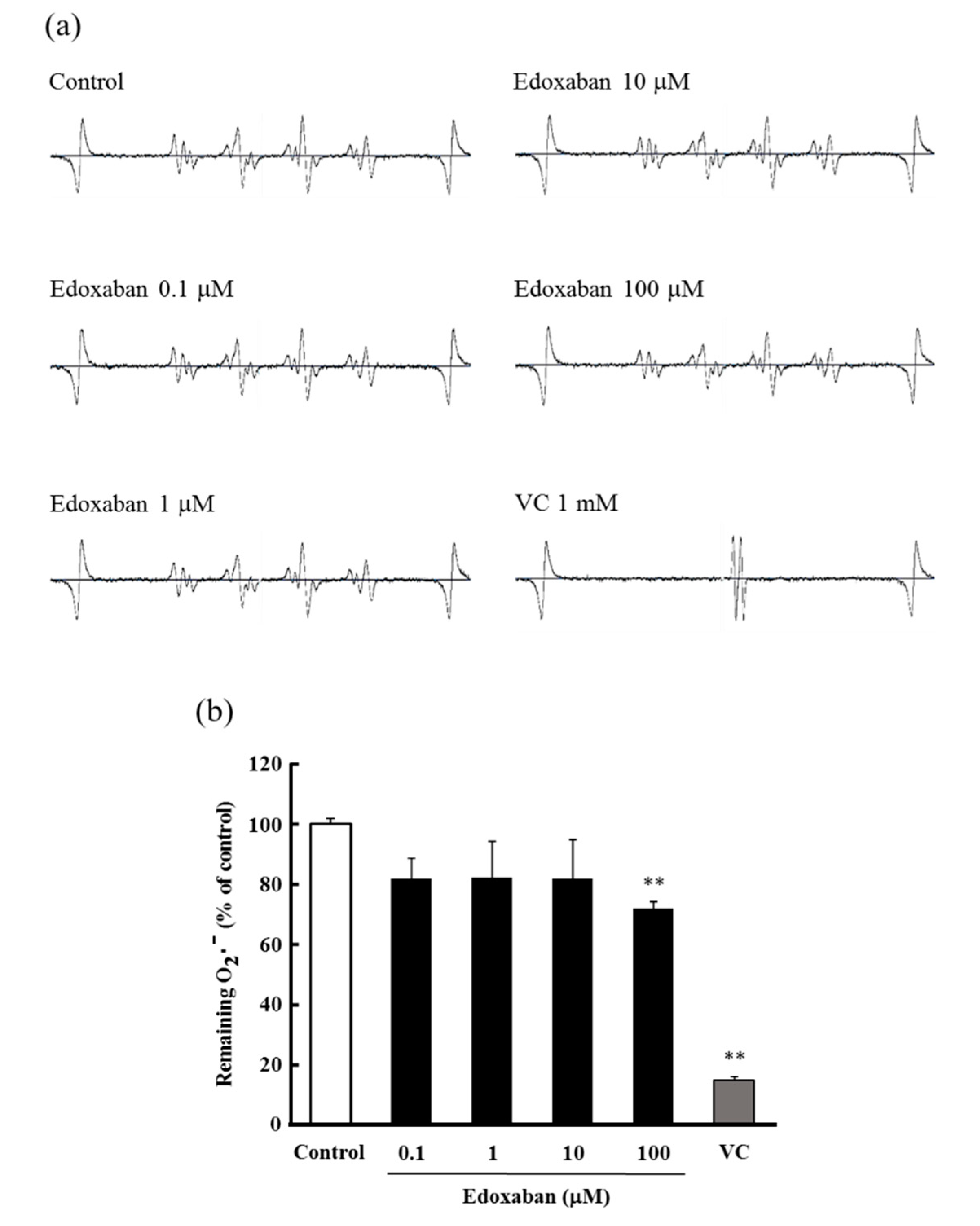
2.5. Radical Scavenging Activity of Edoxaban against Superoxide Radical
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of Reactive Oxygen Species Production
4.4. Electron Spin Resonance Spectroscopy
4.5. Measurement of Hydrogen Peroxide Decomposition
4.6. Peroxynitrite Scavenging Assay Using Dihydrorhodamine123
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AII | Angiotensin II |
| CKD | Chronic kidney disease |
| CM-H2DCFDA | Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate |
| Ccr | Creatinine clearance |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMPO | Dimethyl-1-pyrroline-N-oxide |
| DHR123 | Dihydrorhodamine123 |
| D-PBS | Dulbecco’s phosphate buffered saline |
| DPI | Diphenyleneiodonium chloride |
| ESR | Electron spin resonance |
| FXa | Factor Xa |
| H2O2 | Hydrogen peroxide |
| HK-2 cells | Human proximal tubular cells |
| IS | Indoxyl sulfate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PAR | Protease-activated receptor |
| ROS | Reactive oxygen species |
| SIN-1 | Sydnonimine hydrochloride |
References
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Ozgoren, A.A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Modlinger, P.S.; Wilcox, C.S.; Aslam, S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin. Nephrol. 2004, 24, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.R.; Seatter, M.J.; Kanke, T.; Hunter, G.D.; Plevin, R. Proteinase-activated receptors. Pharmacol. Rev. 2001, 53, 245–282. [Google Scholar] [PubMed]
- Borensztajn, K.; Peppelenbosch, M.P.; Spek, C.A. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol. Med. 2008, 14, 429–440. [Google Scholar] [CrossRef]
- Kawabata, A.; Kuroda, R. Protease-activated receptor (PAR), a novel family of G protein-coupled seven trans-membrane domain receptors: activation mechanisms and physiological roles. Jpn. J. Pharmacol. 2000, 82, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Vergnolle, N.; Wallace, J.L.; Bunnett, N.W.; Hollenberg, M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001, 22, 146–152. [Google Scholar] [CrossRef]
- Oe, Y.; Hayashi, S.; Fushima, T.; Sato, E.; Kisu, K.; Sato, H.; Ito, S.; Takahashi, N. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1525–1533. [Google Scholar] [CrossRef]
- Horinouchi, Y.; Ikeda, Y.; Fukushima, K.; Imanishi, M.; Hamano, H.; Izawa-Ishizawa, Y.; Zamami, Y.; Takechi, K.; Miyamoto, L.; Fujino, H.; et al. Renoprotective effects of a factor Xa inhibitor: fusion of basic research and a database analysis. Sci. Rep. 2018, 8, 10858. [Google Scholar] [CrossRef]
- Moñux, G.; Zamorano-León, J.J.; Marqués, P.; Sopeña, B.; García-García, J.M.; Laich de Koller, G.; Calvo-Rico, B.; García-Fernandez, M.A.; Serrano, J.; López-Farré, A. FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br. J. Clin. Pharmacol. 2017, 83, 2661–2670. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Ueda, S.; Fukami, K.; Yamagishi, S. Advanced glycation end products potentiate citrated plasma-evoked oxidative and inflammatory reactions in endothelial cells by up-regulating protease-activated receptor-1 expression. Cardiovasc. Diabetol. 2014, 13, 60. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Fukami, K.; Ueda, S.; Okuda, S.; Yamagishi, S. Rivaroxaban inhibits oxidative and inflammatory reactions in advanced glycation end product-exposed tubular cells by blocking thrombin/protease-activated receptor-2 system. Thromb. Res. 2015, 135, 770–773. [Google Scholar] [CrossRef]
- Yokozawa, T.; Zheng, P.D.; Oura, H.; Koizumi, F. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef]
- Hara, T.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis 2015, 242, 639–646. [Google Scholar] [CrossRef]
- Zuo, P.; Zhou, Q.; Zuo, Z.; Wang, X.; Chen, L.; Ma, G. Effects of the factor Xa inhibitor, fondaparinux, on the stability of atherosclerotic lesions in apolipoprotein E-deficient mice. Circ. J. 2015, 79, 2499–2508. [Google Scholar] [CrossRef]
- Shinagawa, K.; Martin, J.A.; Ploplis, V.A.; Castellino, F.J. Coagulation factor Xa modulates airway remodeling in a murine model of asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 136–143. [Google Scholar] [CrossRef]
- Grandaliano, G.; Pontrelli, P.; Cerullo, G.; Monno, R.; Ranieri, E.; Ursi, M.; Loverre, A.; Gesualdo, L.; Schena, F.P. Protease-activated receptor-2 expression in IgA nephropathy: apotential role in the pathogenesis of interstitial fibrosis. J. Am. Soc. Nephrol. 2003, 14, 2072–2083. [Google Scholar] [CrossRef]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int. 2003, 63, 1671–1680. [Google Scholar] [CrossRef]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br. J. Pharmacol. 2002, 135, 555–563. [Google Scholar] [CrossRef]
- Shimoishi, K.; Anraku, M.; Kitamura, K.; Tasaki, Y.; Taguchi, K.; Hashimoto, M.; Fukunaga, E.; Maruyama, T.; Otagiri, M. An oral adsorbent, AST-120 protects against the progression of oxidative stress by reducing the accumulation of indoxyl sulfate in the systemic circulation in renal failure. Pharm. Res. 2007, 24, 1283–1289. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Lassègue, B.; Griendling, K.K. Reactive oxygen species in hypertension; An update. Am. J. Hypertens. 2004, 17, 852–860. [Google Scholar] [CrossRef]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7A–11A. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef]
- Park, Y.; Yang, J.; Zhang, H.; Chen, X.; Zhang, C. Effect of PAR2 in regulating TNF-α and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic. Res. Cardiol. 2011, 106, 111–123. [Google Scholar] [CrossRef]
- Jain, N.; Reilly, R.F. Clinical Pharmacology of Oral Anticoagulants in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018. [Google Scholar] [CrossRef]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef]
- Wang, L.Z.; Sun, W.C.; Zhu, X.Z. Ethyl pyruvate protects PC12 cells from dopamine-induced apoptosis. Eur. J. Pharmacol. 2005, 508, 57–68. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free. Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Chung, H.Y.; Choi, H.R.; Park, H.J.; Choi, J.S.; Choi, W.C. Peroxynitrite scavenging and cytoprotective activity of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether from the marine alga Symphyocladia latiuscula. J. Agric. Food Chem. 2001, 49, 3614–3621. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narita, Y.; Hamamura, K.; Kashiyama, M.; Utsumi, S.; Kakizoe, Y.; Kondo, Y.; Ishitsuka, Y.; Jono, H.; Irie, T.; Mukoyama, M.; et al. Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity. Int. J. Mol. Sci. 2019, 20, 4140. https://doi.org/10.3390/ijms20174140
Narita Y, Hamamura K, Kashiyama M, Utsumi S, Kakizoe Y, Kondo Y, Ishitsuka Y, Jono H, Irie T, Mukoyama M, et al. Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity. International Journal of Molecular Sciences. 2019; 20(17):4140. https://doi.org/10.3390/ijms20174140
Chicago/Turabian StyleNarita, Yuki, Kana Hamamura, Mami Kashiyama, Sara Utsumi, Yutaka Kakizoe, Yuki Kondo, Yoichi Ishitsuka, Hirofumi Jono, Tetsumi Irie, Masashi Mukoyama, and et al. 2019. "Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity" International Journal of Molecular Sciences 20, no. 17: 4140. https://doi.org/10.3390/ijms20174140
APA StyleNarita, Y., Hamamura, K., Kashiyama, M., Utsumi, S., Kakizoe, Y., Kondo, Y., Ishitsuka, Y., Jono, H., Irie, T., Mukoyama, M., Saito, H., Kadowaki, D., Hirata, S., & Kitamura, K. (2019). Edoxaban Exerts Antioxidant Effects Through FXa Inhibition and Direct Radical-Scavenging Activity. International Journal of Molecular Sciences, 20(17), 4140. https://doi.org/10.3390/ijms20174140





