Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice
Abstract
1. Introduction
2. Results
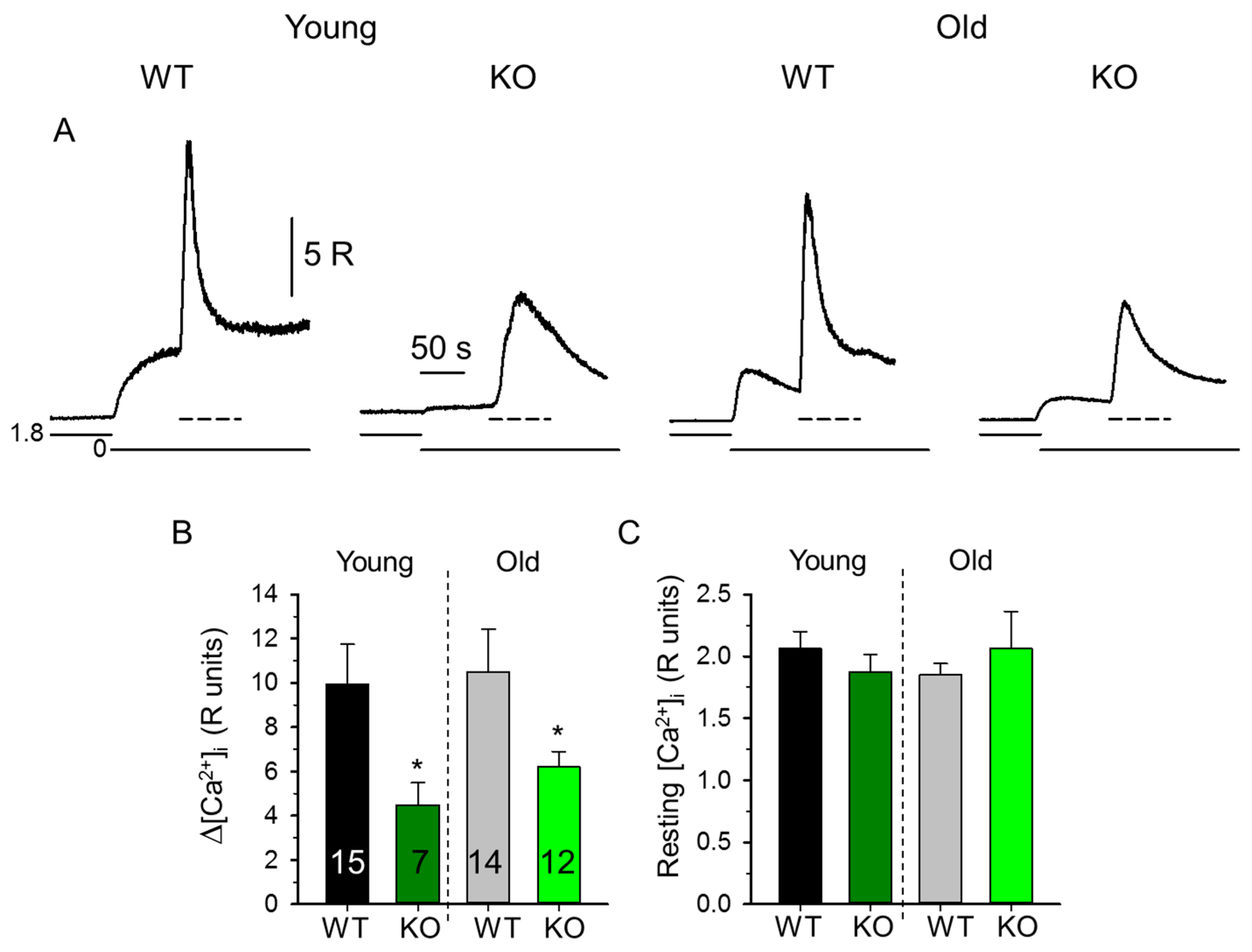
2.1. Decreased SR Ca2+ Content in Skeletal Muscle Fibers from sAnk1 KO Mice
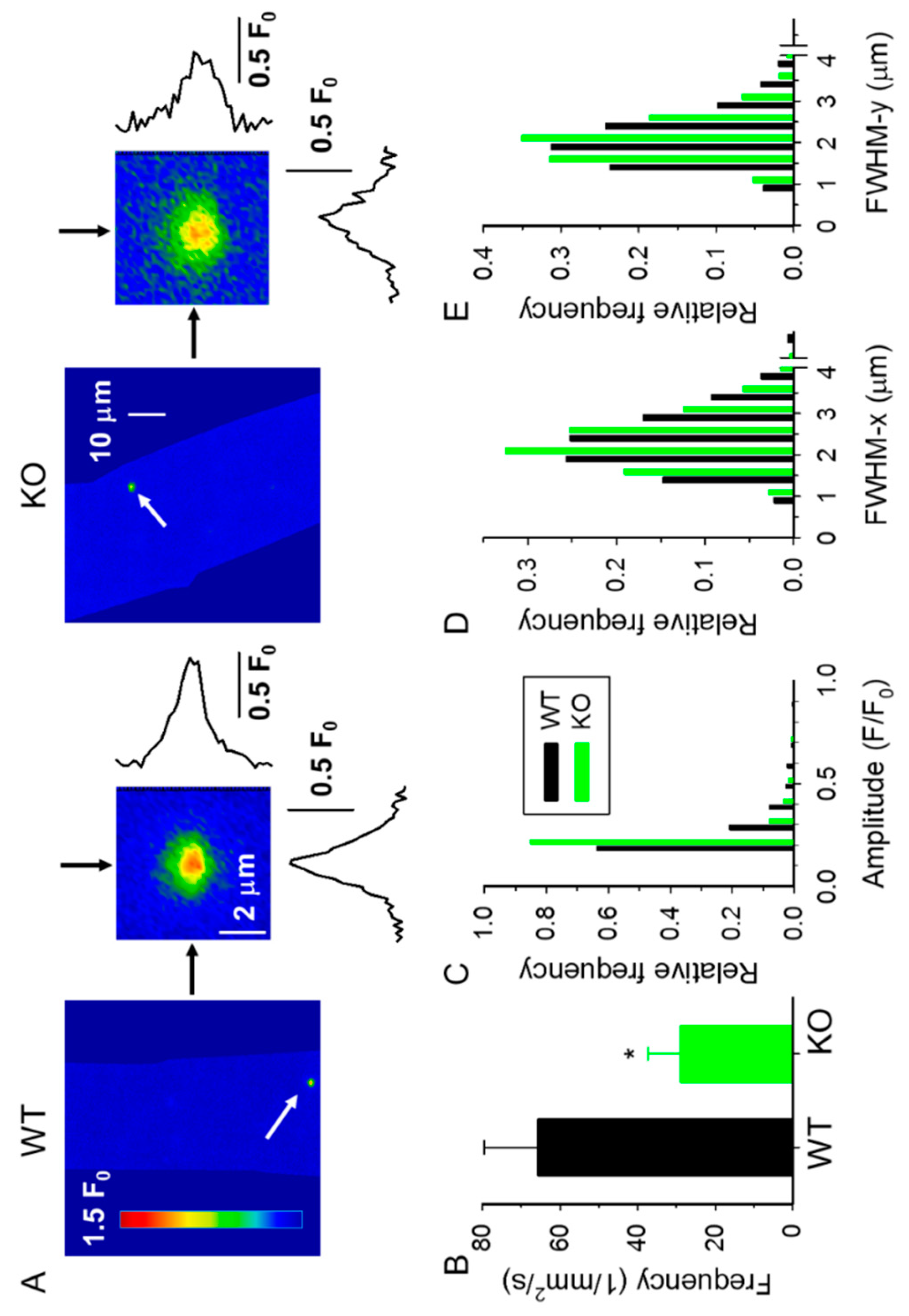
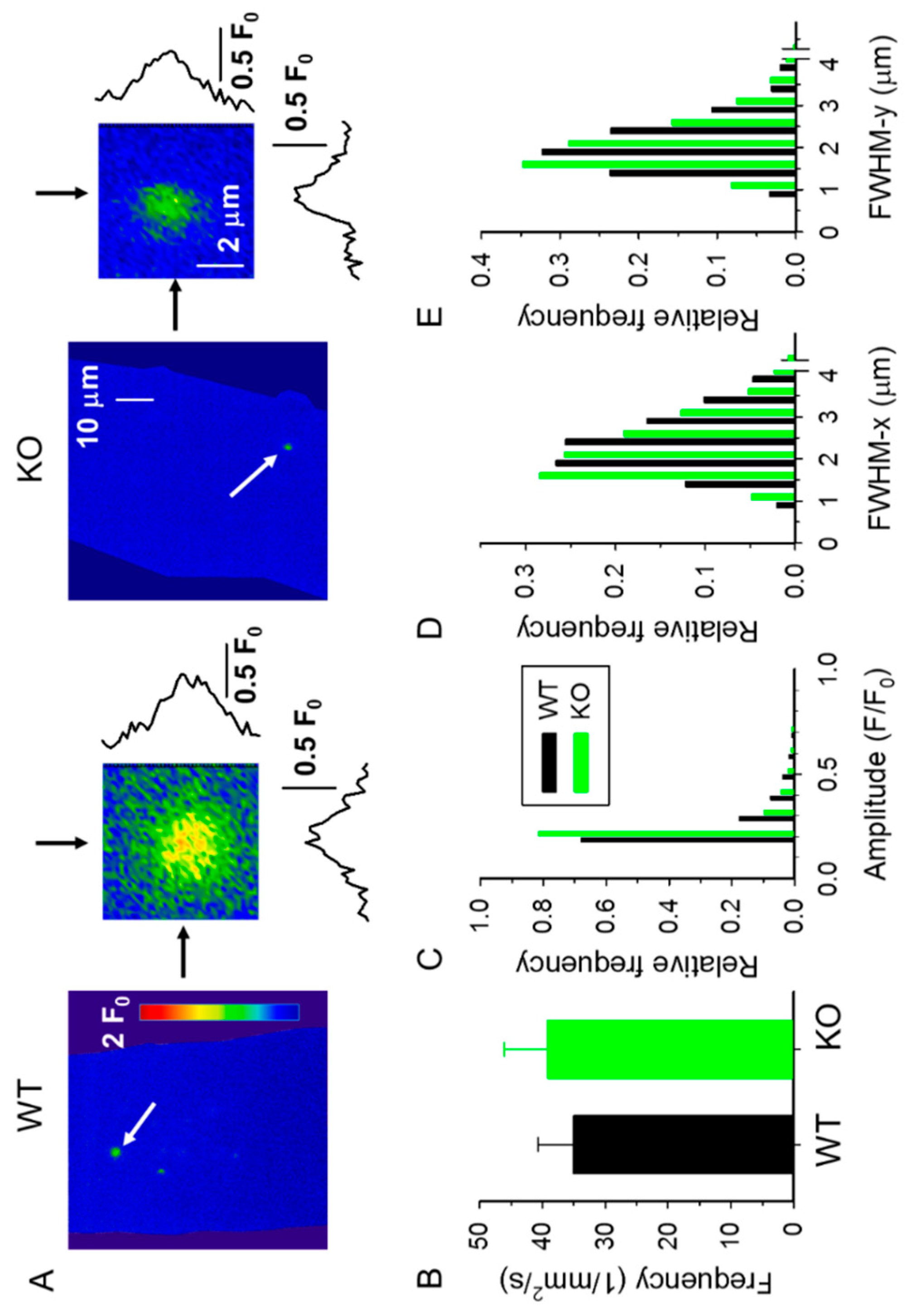
2.2. Spontaneous Ca2+ Release Events (CREs) Are Reduced in sAnk1 KO Mice
3. Discussion
4. Materials and Methods
4.1. Animal Care
4.2. Isolation of Single Skeletal Muscle Fibers
4.3. Whole Cell Intracellular Ca2+ Concentration Measurements
4.4. Measurement of Ca2+ Release Events (CREs) in Permeabilized Muscle Fibers
4.5. Voltage Clamp and Ca2+ Transient Analysis
4.6. Chemicals and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franzini-Armstrong, C. The relationship between form and function throughout the history of excitation–contraction coupling. J. Gen. Physiol. 2018, 150, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Lawlor, M.W.; Dirksen, R.T. Triadopathies: An emerging class of skeletal muscle diseases. Neurotherapeutics. 2014, 11, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E.; Figueroa, L.; Manno, C.; Kraeva, N.; Riazi, S. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J. Gen. Physiol. 2015, 145, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Vezzani, B.; Galli, L.; Paolini, C.; Toniolo, L.; Pierantozzi, E.; Spinozzi, S.; Barone, V.; Pegoraro, E.; Bello, L.; et al. A Mutation in the CASQ1 Gene Causes a Vacuolar Myopathy with Accumulation of Sarcoplasmic Reticulum Protein Aggregates. Hum Mutat. 2014, 35, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Del Re, V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Treves, S.; Zorzato, F.; Sarkozy, A.; Ochala, J.; Sewry, C.; Phadke, R.; Gautel, M.; Muntoni, F. Congenital myopathies: Disorders of excitation-contraction coupling and muscle contraction. Nat Rev Neurol. 2018, 14, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Smolenicka, Z.; Guerini, D.; Carafoli, E.; Kress, W.; Liechti-Gallati, S. Detection of a new polymorphism in the plasma-membrane Ca2+ ATPase isoform-3 gene and its exclusion as a candidate for X-linked myotubular myopathy (MTM1). Hum. Genet. 1996, 98, 681–684. [Google Scholar] [CrossRef]
- Kutchukian, C.; Szentesi, P.; Allard, B.; Trochet, D.; Beuvin, M.; Berthier, C.; Tourneur, Y.; Guicheney, P.; Csernoch, L.; Bitoun, M.; et al. Impaired excitation-contraction coupling in muscle fibres from the dynamin2R465W mouse model of centronuclear myopathy. J Physiol. 2017, 595, 7369–7382. [Google Scholar] [CrossRef]
- Sorrentino, V. Sarcoplasmic reticulum: Structural determinants and protein dynamics. Int. J. Biochem. Cell Biol. 2011, 43, 1075–1078. [Google Scholar] [CrossRef]
- Bennett, V.; Chen, L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr. Opin. Cell Biol. 2001, 13, 61–67. [Google Scholar] [CrossRef]
- Bennett, V.; Healy, J. Membrane Domains Based on Ankyrin and Spectrin Associated with Cell–Cell Interaction. Cold Spring Harb. Perspect. Biol. 2009, 1, a003012. [Google Scholar] [CrossRef] [PubMed]
- Tee, J.M.; Peppelenbosch, M.P. Anchoring skeletal muscle development and disease: The role of ankyrin repeat domain containing proteins in muscle physiology. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.R.; Mohler, P.J. Ankyrin-based cellular pathways for cardiac ion channel and transporter targeting and regulation. Semin. Cell Dev. Biol. 2011, 22, 166–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Randazzo, D.; Giacomello, E.; Lorenzini, S.; Rossi, D.; Pierantozzi, E.; Blaauw, B.; Reggiani, C.; Lange, S.; Peter, A.K.; Chen, J.; et al. Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity. J Cell Biol. 2013, 200, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Birkenmeier, C.S.; Williams, M.W.; Sharp, J.J.; Barker, J.E.; Bloch, R.J. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J. Cell Biol. 1997, 136, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.G.; Forget, B.G. An alternate promoter directs expression of a truncated, muscle-specific isoform of the human ankyrin 1 gene. J. Biol. Chem. 1998, 273, 1339–1348. [Google Scholar] [CrossRef]
- Kordeli, E.; Ludosky, M.A.; Deprette, C.; Frappier, T.; Cartaud, J. AnkyrinG is associated with the postsynaptic membrane and the sarcoplasmic reticulum in the skeletal muscle fiber. J. Cell Sci. 1998, 1, 2197–2207. [Google Scholar]
- Tuvia, S.; Buhusi, M.L.; Davis, M.; Reedy, V. Bennett, Ankyrin-B Is Required for Intracellular Sorting of Structurally Diverse Ca2+ Homeostasis Proteins. J. Cell Biol. 1999, 147, 995–1008. [Google Scholar]
- Armani, A.; Galli, S.; Giacomello, E.; Bagnato, P.; Barone, V.; Rossi, D.; Sorrentino, V. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp. Cell Res. 2006, 312, 3546–3558. [Google Scholar] [CrossRef]
- Bagnato, P.; Barone, V.; Giacomello, E.; Rossi, D.; Sorrentino, V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 2003, 160, 245–253. [Google Scholar] [CrossRef]
- Kontrogianni-Konstantopoulos, A.; Jones, E.M.; Van Rossum, D.B.; Bloch, R.J. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol. Biol. Cell 2003, 14, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Kontrogianni-Konstantopoulos, A.; Catino, D.H.; Strong, J.C.; Sutter, S.; Borisov, A.B.; Pumplin, D.W.; Russell, M.W.; Bloch, R.J. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006, 20, 2102–2121. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, D.; Blaauw, B.; Paolini, C.; Pierantozzi, E.; Spinozzi, S.; Lange, S.; Chen, J.; Protasi, F.; Reggiani, C.; Sorrentino, V. Exercise-induced alterations and loss of sarcomeric M-line organization in the diaphragm muscle of obscurin knockout mice. Am. J. Physiol.-Cell Physiol. 2017, 312, C16–C28. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, D.; Pierantozzi, E.; Rossi, D.; Sorrentino, V. The potential of obscurin as a therapeutic target in muscle disorders. Expert Opin. Ther. Targets. 2017, 21, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Palmio, J.; Evilä, A.; Galli, L.; Barone, V.; Caldwell, T.A.; Policke, R.A.; Aldkheil, E.; Berndsen, C.E.; Wright, N.T.; et al. A novel FLNC frameshift and an OBSCN variant in a family with distal muscular dystrophy. PLoS ONE 2017, 12, e0186642. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Randazzo, D.; Del Re, V.; Sorrentino, V.D. Rossi, Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 2015, 36, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.A.; Ziman, A.P.; Strong, J.; Zhang, Y.; Hartford, A.K.; Ward, C.W.; Randall, W.R.; Kontrogianni-Konstantopoulos, A.; Bloch, R.J. Integrity of the network sarcoplasmic reticulum in skeletal muscle requires small ankyrin 1. J. Cell Sci. 2011, 124, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Quarta, M.; Paolini, C.; Squecco, R.; Fusco, P.; Toniolo, L.; Blaauw, B.; Formoso, L.; Rossi, D.; Birkenmeier, C.; et al. Deletion of small ankyrin 1 (sAnk1) isoforms results in structural and functional alterations in aging skeletal muscle fibers. Am. J. Physiol.-Cell Physiol. 2015, 308, C123–C138. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Ouyang, K.; Meyer, G.; Cui, L.; Cheng, H.; Lieber, R.L.; Chen, J. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J. Cell Sci. 2009, 122, 2640–2650. [Google Scholar] [CrossRef]
- Kirsch, W.G.; Uttenweiler, D.; Fink, R.H. Spark- and ember-like elementary Ca2+ release events in skinned fibres of adult mammalian skeletal muscle. J. Physiol. 2001, 537, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Lukács, B.; Sztretye, M.; Almássy, J.; Sárközi, S.; Dienes, B.; Mabrouk, K.; Simut, C.; Szabó, L.; Szentesi, P.; De Waard, M.; et al. Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor. Biophys. J. 2008, 95, 3497–3509. [Google Scholar] [CrossRef]
- Reggiani, C. Calcium handling in muscle fibers of mice and men: Evolutionary adaptation in different species to optimize performance and save energy. J. Physiol. 2014, 592, 1173–1174. [Google Scholar] [CrossRef]
- Rossi, A.E.; Dirksen, R.T. Sarcoplasmic reticulum: The dynamic calcium governor of muscle. Muscle Nerve 2006, 33, 715–731. [Google Scholar] [CrossRef]
- Jiménez-Moreno, R.; Wang, Z.M.; Gerring, R.C.; Delbono, O. Sarcoplasmic Reticulum Ca2+ Release Declines in Muscle Fibers from Aging Mice. Biophys. J. 2008, 94, 3178–3188. [Google Scholar] [CrossRef]
- Cheng, H.; Lederer, W.J. Calcium Sparks. Physiol. Rev. 2008, 88, 1491–1545. [Google Scholar] [CrossRef]
- Ríos, E.; Stern, M.D.; González, A.; Pizarro, G.; Shirokova, N. Calcium Release Flux Underlying Ca2+ Sparks of Frog Skeletal Muscle. J. Gen. Physiol. 1999, 114, 31–48. [Google Scholar] [CrossRef]
- Burdakov, D.; Petersen, O.H.; Verkhratsky, A. Intraluminal calcium as a primary regulatorof endoplasmic reticulum function. Cell Calcium 2005, 38, 303–310. [Google Scholar] [CrossRef]
- Desmond, P.F.; Muriel, J.; Markwardt, M.L.; Rizzo, M.A.; Bloch, R.J. Identification of Small Ankyrin 1 as a Novel Sarco(endo)plasmic Reticulum Ca2+-ATPase 1 (SERCA1) Regulatory Protein in Skeletal Muscle. J. Biol. Chem. 2015, 290, 27854–27867. [Google Scholar] [CrossRef]
- Desmond, P.F.; Labuza, A.; Muriel, J. Interactions between small ankyrin 1 and sarcolipin coordinately regulate activity of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA1). J. Biol. Chem. 2017, 292, 10961–10972. [Google Scholar] [CrossRef]
- Fodor, J.; Gönczi, M.; Sztretye, M.; Dienes, B.; Oláh, T.; Szabó, L.; Csoma, E.; Szentesi, P.; Szigeti, G.P.; Marty, I.; et al. Altered expression of triadin 95 causes parallel changes in localized Ca2+ release events and global Ca2+ signals in skeletal muscle cells in culture. J. Physiol. 2008, 586, 5803–5818. [Google Scholar] [CrossRef]
- Csernoch, L.; Pouvreau, S.; Ronjat, M.; Jacquemond, V. Voltage-activated elementary calcium release events in isolated mouse skeletal muscle fibers. J. Membr. Biol. 2008, 226, 43–55. [Google Scholar] [CrossRef]
- Szabó, L.Z.; Vincze, J.; Csernoch, L.; Szentesi, P. Improved spark and ember detection using stationary wavelet transforms. J. Theor. Biol. 2010, 264, 1279–1292. [Google Scholar] [CrossRef]
- Hollingworth, S.; Peet, J.; Chandler, W.K.; Baylor, S.M. Calcium sparks in intact skeletal muscle fibers of the frog. J. Gen. Physiol. 2001, 118, 653–678. [Google Scholar] [CrossRef]
- Royer, L.; Pouvreau, S.; Ríos, E. Evolution and modulation of intracellular calcium release during long-lasting, depleting depolarization in mouse muscle. J. Physiol. 2008, 586, 4609–4629. [Google Scholar] [CrossRef]
- Melzer, W.; Rios, E.; Schneider, M.F. Time course of calcium release and removal in skeletal muscle fibers. Biophys. J. 1984, 45, 637–641. [Google Scholar] [CrossRef]
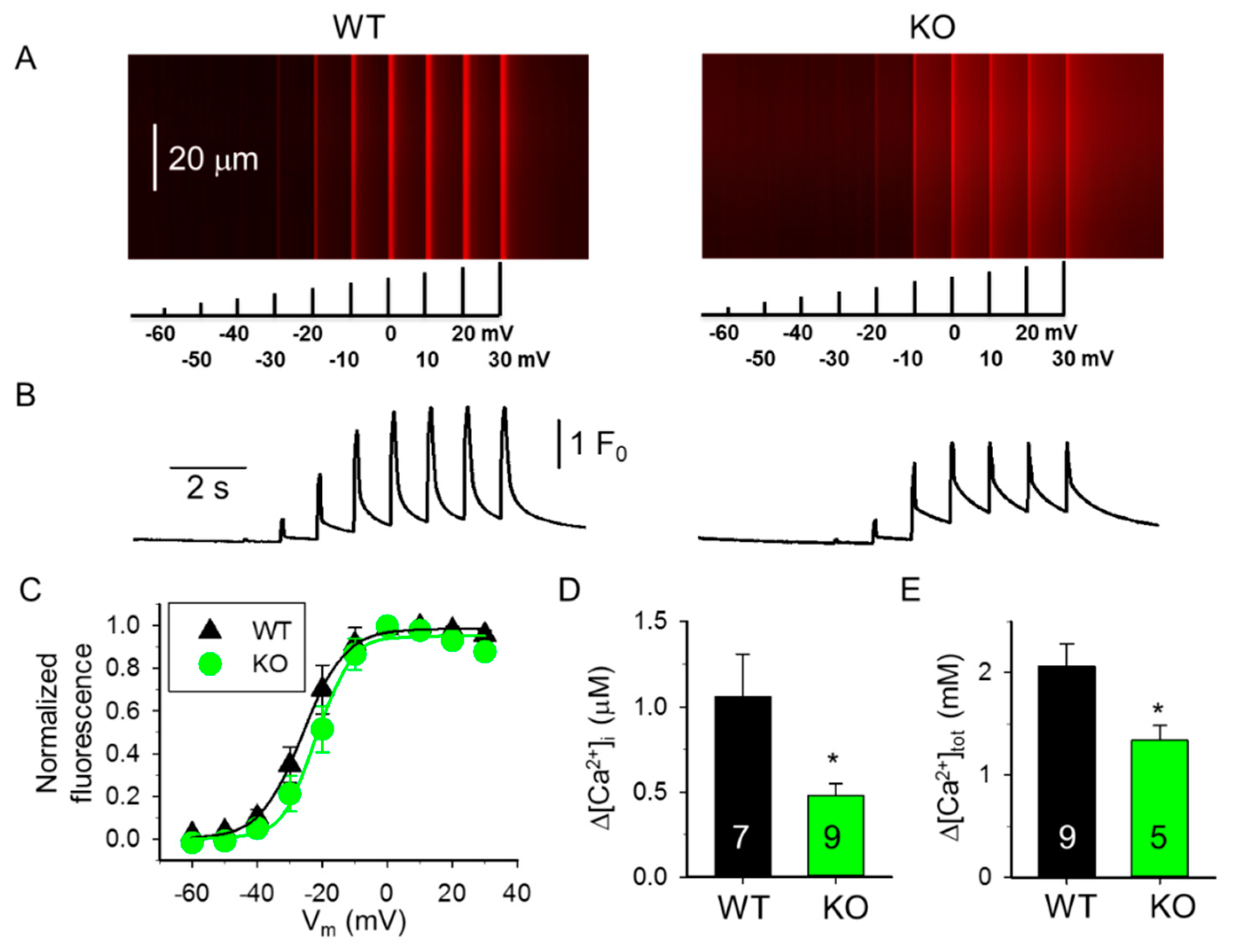
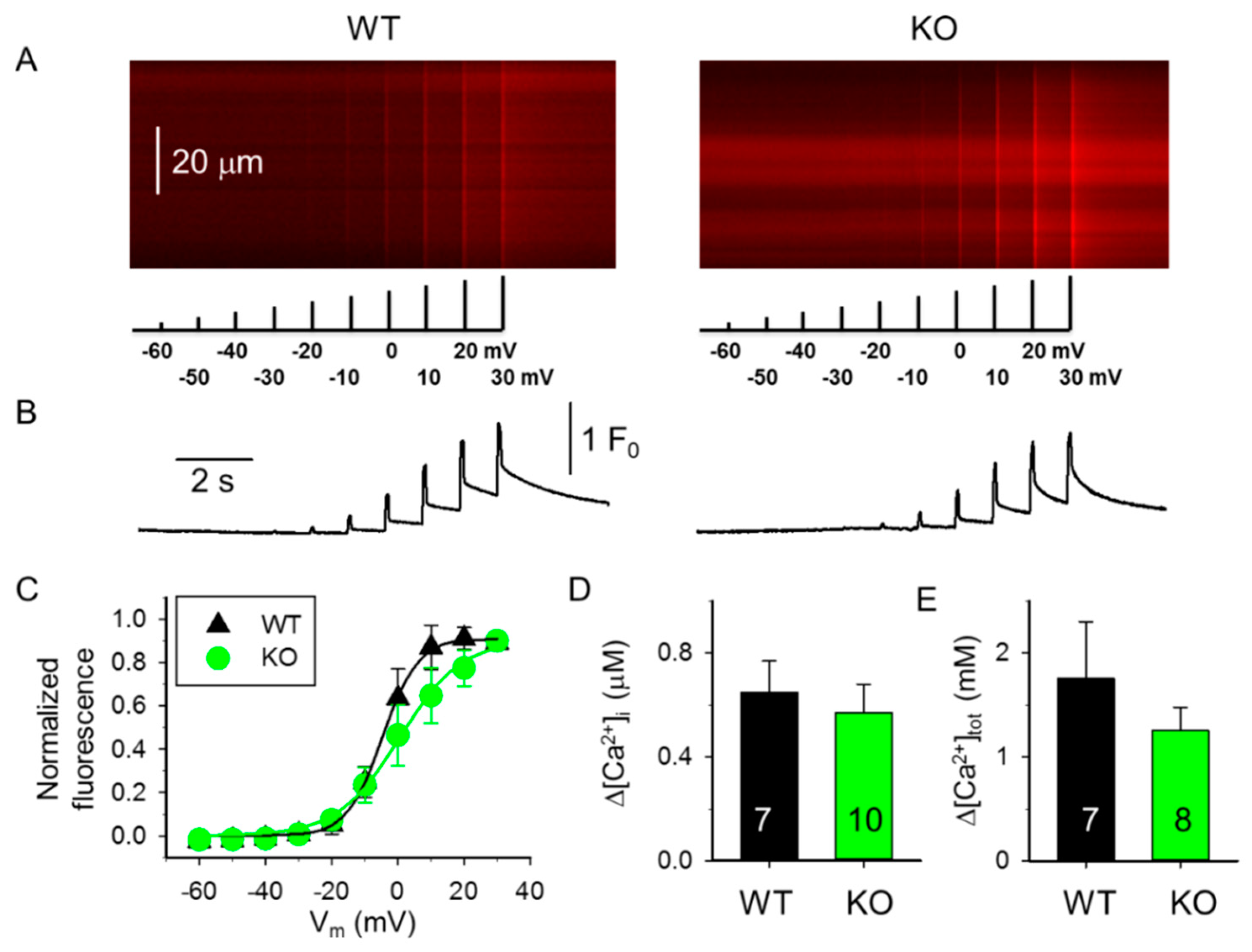

| Boltzmann Parameters † | WT 4-Month-Old (4 Mice) (9 Fibers) | KO 4-Month-Old (5 Mice) (9 Fibers) | WT 10-Month-Old (4 Mice) (9 Fibers) | KO 10-Month-Old (4 Mice) (9 Fibers) |
|---|---|---|---|---|
| max | 1.91 ± 0.27 | 1.11 ± 0.19 * | 1.61 ± 0.34 | 1.39 ± 0.32 |
| k (mV) | 7.25 ± 1.09 | 5.57 ± 0.85 | 5.88 ± 0.92 | 8.67 ± 1.16 # |
| V50 (mV) | −21.84 ± 4.96 | −20.68 ± 2.97 | −4.76 ± 4.44 # | −3.62 ± 6.17 # |
| WT 4-Month-Old (3 Mice) (43 Fibers) | KO 4-Month-Old (9 Mice) (87 Fibers) | WT 10-Month-Old (3 Mice) (76 Fibers) | KO 10-Month-Old (4 Mice) (105 Fibers) | |
|---|---|---|---|---|
| Number of sparks | 1084 | 1231 | 1063 | 1646 |
| Amplitude (F/F0) | 0.216 ± 0.005 | 0.158 ± 0.003 *** | 0.205 ± 0.006 | 0.171 ± 0.003 ***,## |
| FWHM-x (µm) | 2.239 ± 0.023 | 2.048 ± 0.020 *** | 2.312 ± 0.025 # | 1.972 ± 0.020 ***,## |
| FWHM-y (µm) | 1.968 ± 0.020 | 1.784 ± 0.017 *** | 1.991 ± 0.022 | 1.761 ± 0.017 *** |
| Signal mass (SM) (µm3) | 3.195 ± 0.130 | 1.852 ± 0.090 *** | 2.909 ± 0.124 | 1.996 ± 0.088 *** |
| Frequency (mm−2 s−1) | 65.8 ± 13.8 | 28.8 ± 8.6 * | 35.0 ± 5.7 # | 39.1 ± 6.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierantozzi, E.; Szentesi, P.; Al-Gaadi, D.; Oláh, T.; Dienes, B.; Sztretye, M.; Rossi, D.; Sorrentino, V.; Csernoch, L. Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice. Int. J. Mol. Sci. 2019, 20, 3361. https://doi.org/10.3390/ijms20133361
Pierantozzi E, Szentesi P, Al-Gaadi D, Oláh T, Dienes B, Sztretye M, Rossi D, Sorrentino V, Csernoch L. Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice. International Journal of Molecular Sciences. 2019; 20(13):3361. https://doi.org/10.3390/ijms20133361
Chicago/Turabian StylePierantozzi, Enrico, Péter Szentesi, Dána Al-Gaadi, Tamás Oláh, Beatrix Dienes, Mónika Sztretye, Daniela Rossi, Vincenzo Sorrentino, and László Csernoch. 2019. "Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice" International Journal of Molecular Sciences 20, no. 13: 3361. https://doi.org/10.3390/ijms20133361
APA StylePierantozzi, E., Szentesi, P., Al-Gaadi, D., Oláh, T., Dienes, B., Sztretye, M., Rossi, D., Sorrentino, V., & Csernoch, L. (2019). Calcium Homeostasis Is Modified in Skeletal Muscle Fibers of Small Ankyrin1 Knockout Mice. International Journal of Molecular Sciences, 20(13), 3361. https://doi.org/10.3390/ijms20133361







