Complications for a Hoyeraal–Hreidarsson Syndrome Patient with a Germline DKC1 A353V Variant Undergoing Unrelated Peripheral Blood Stem Cell Transplantation
Abstract
1. Introduction
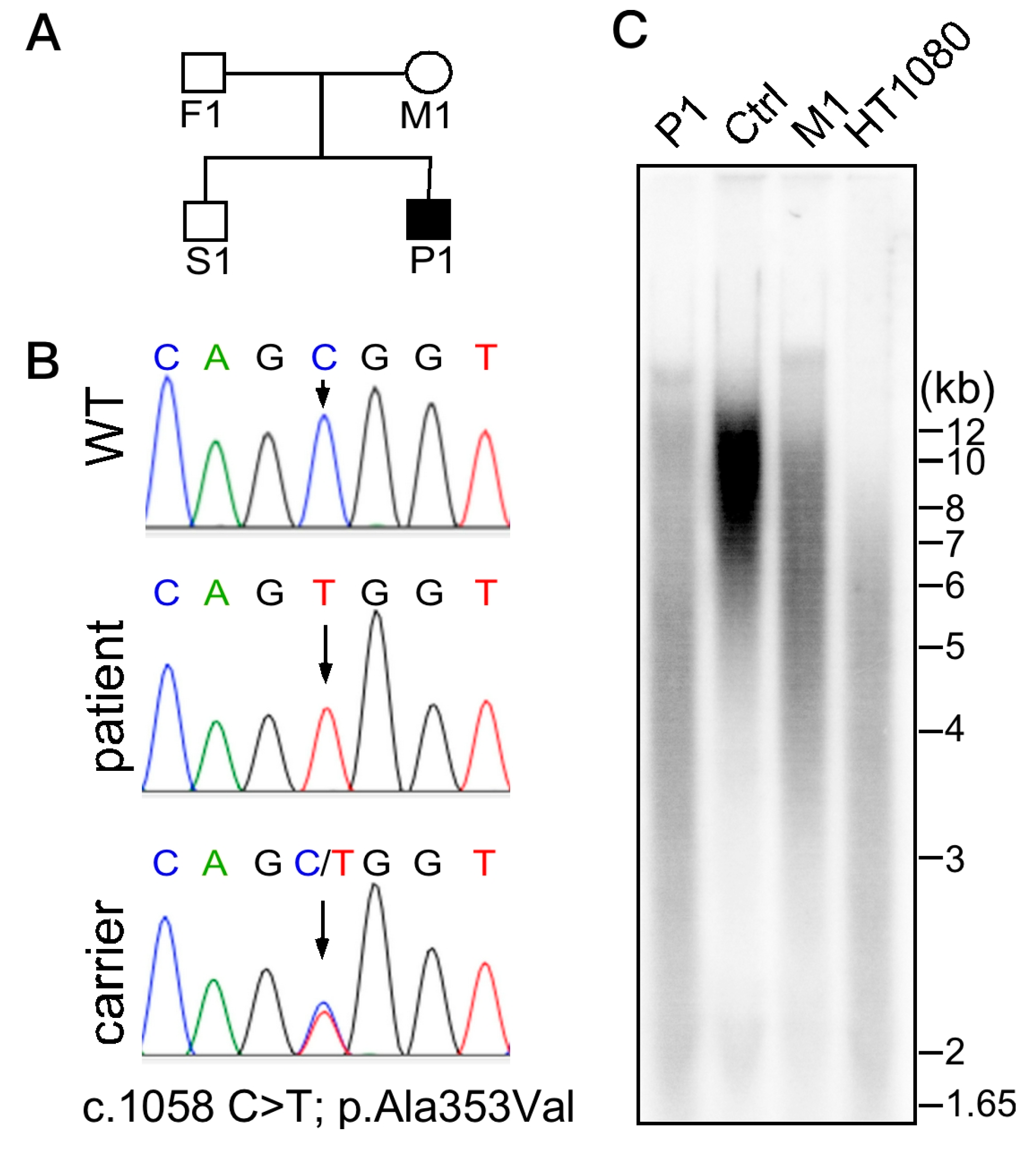
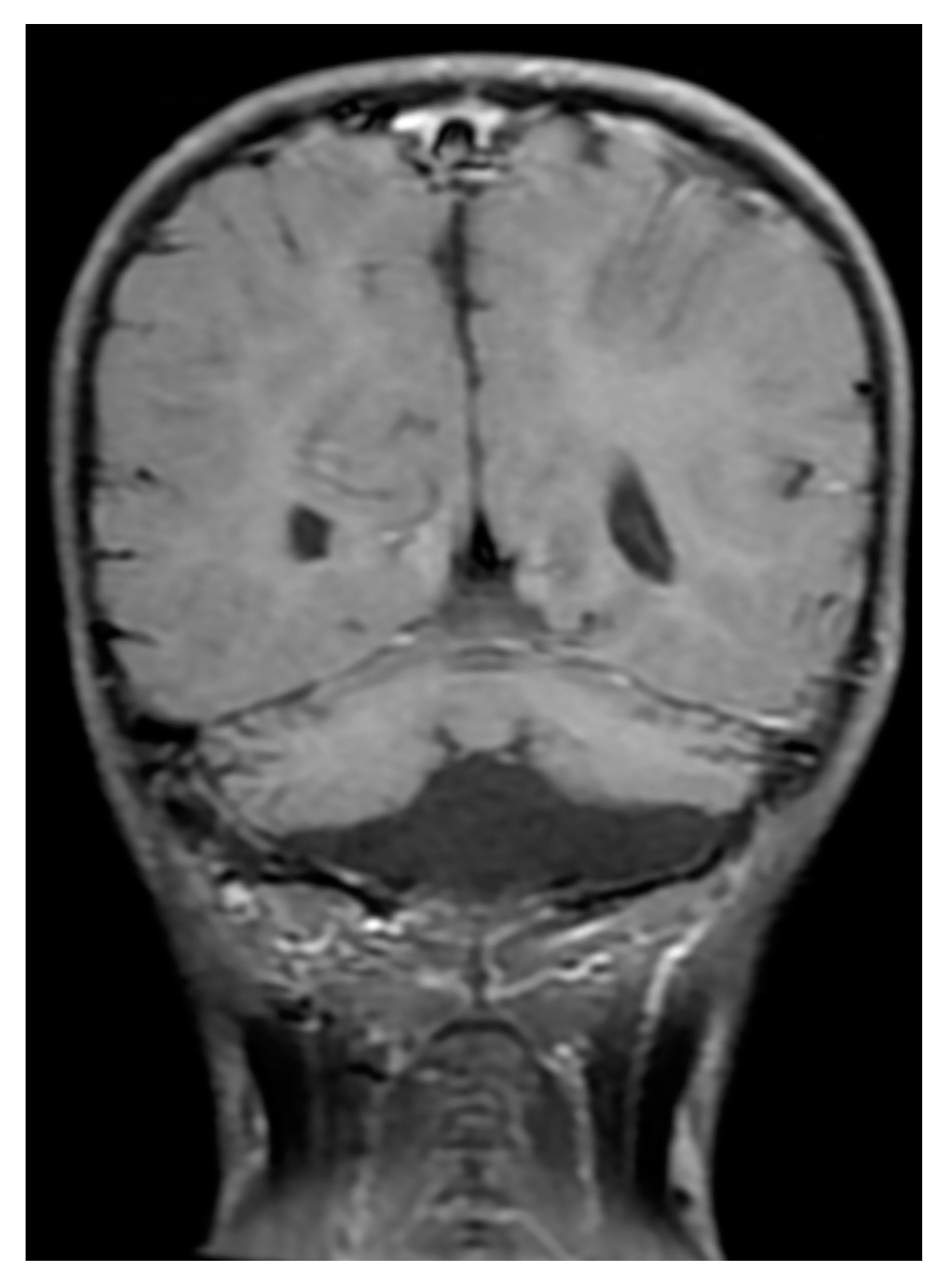
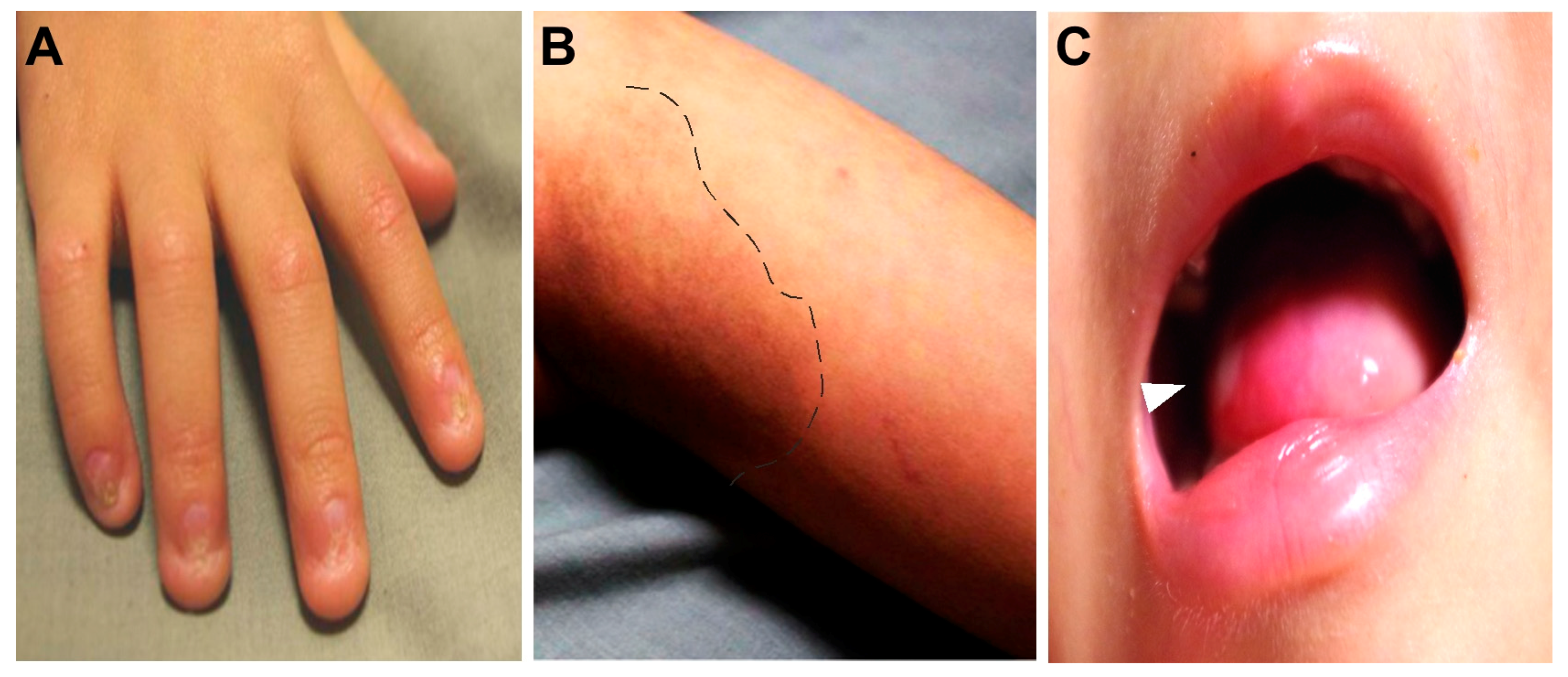
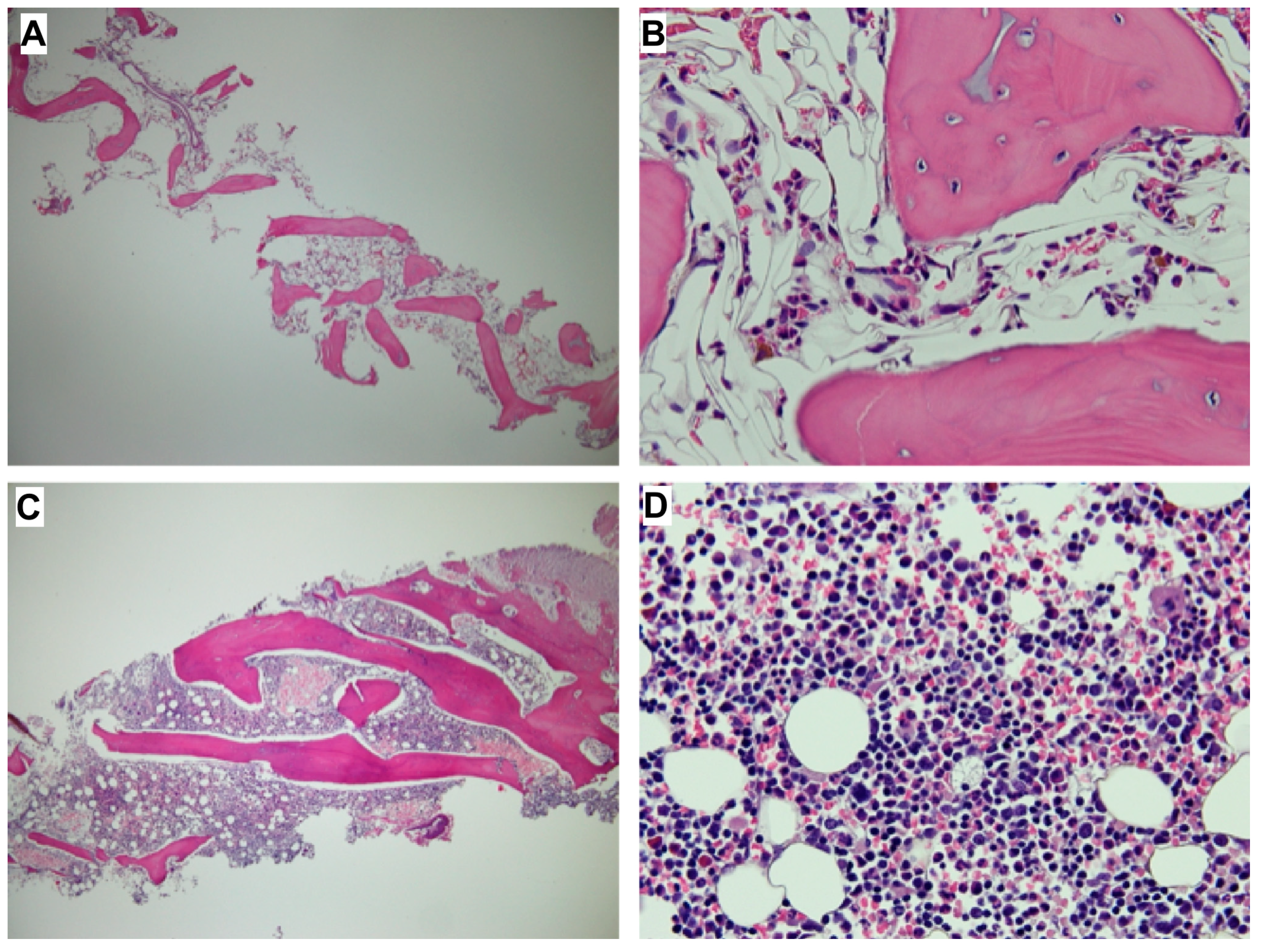
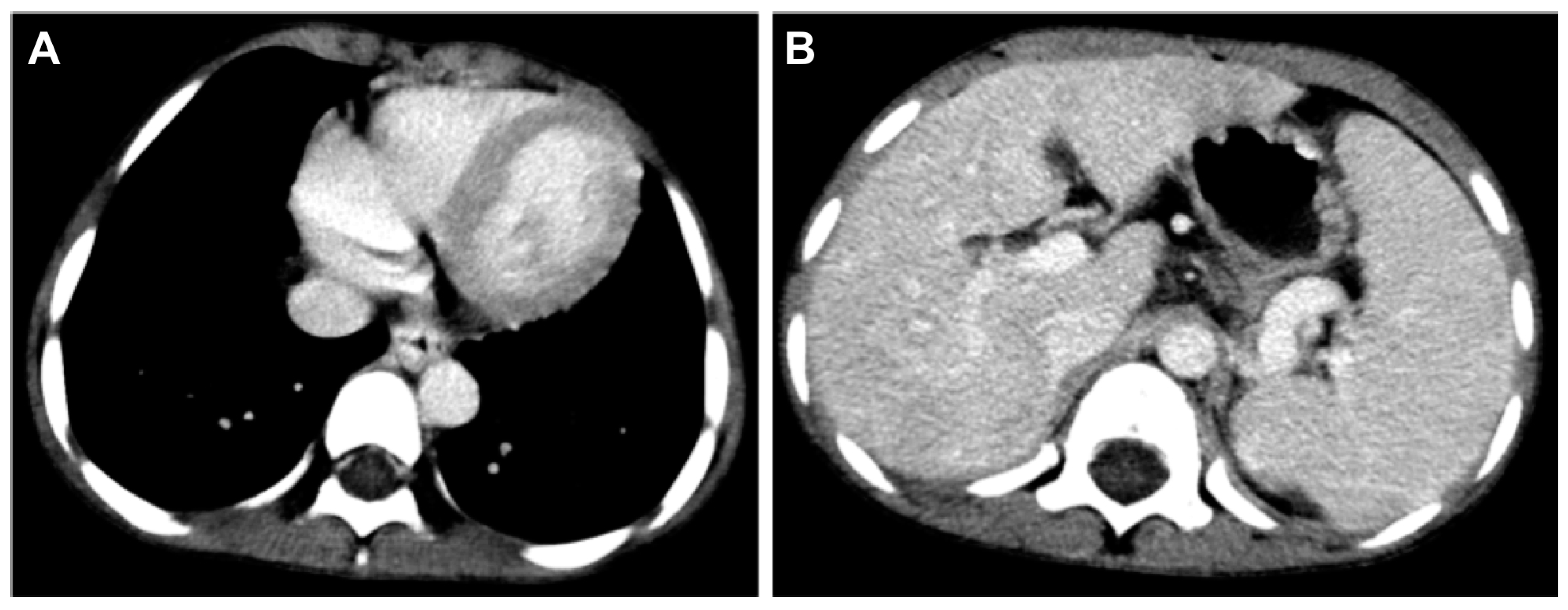
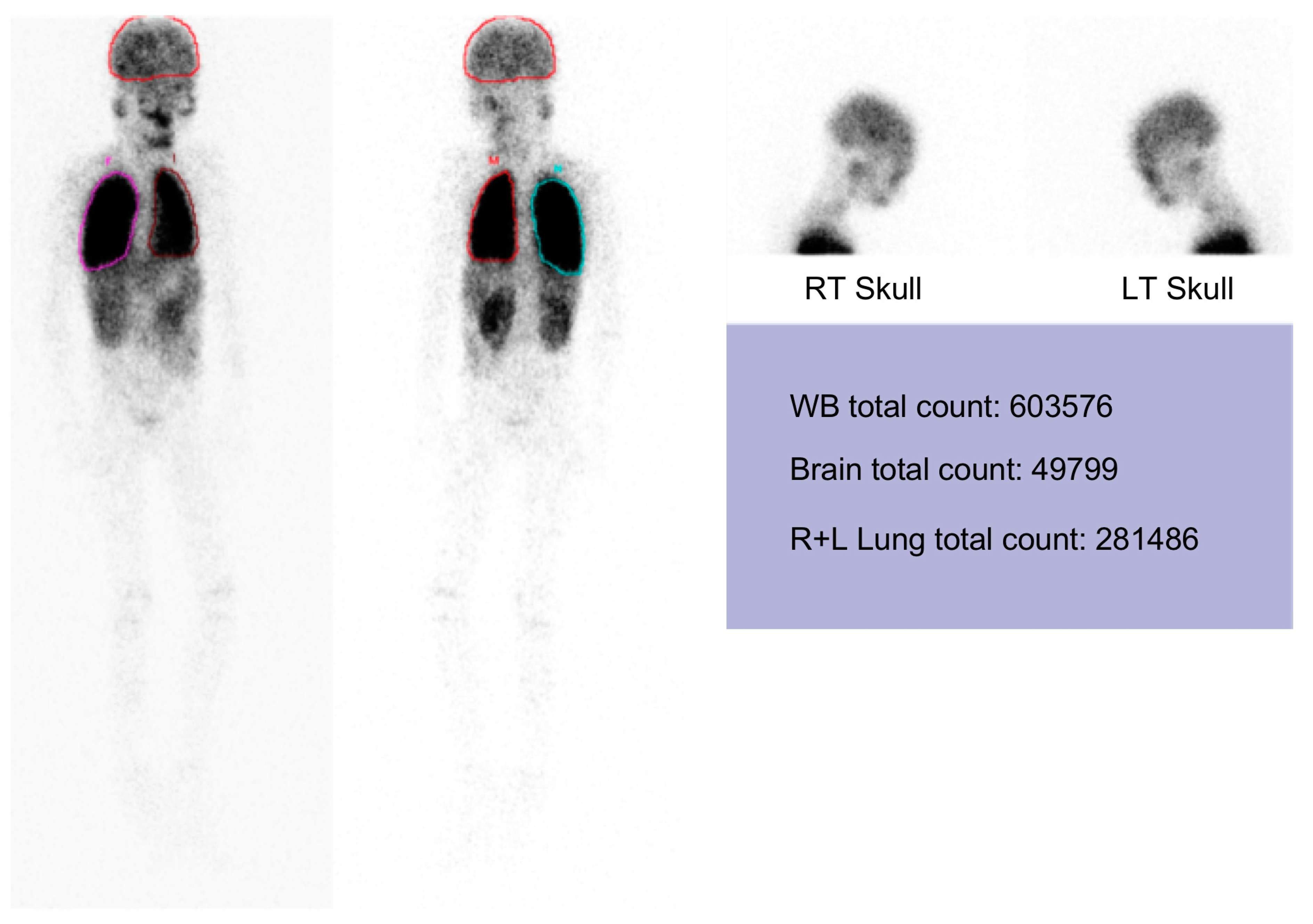
2. Case Reports
3. Discussion
4. Materials and Methods
4.1. Whole Exome Sequencing (WES)
4.2. Telomere Length Analysis
4.3. Tecnetium-99m MAA Lung and Brain Scanning
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hreidarsson syndrome, a complex telomere biology disorder. Br. J. Haematol. 2015, 170, 457–471. [CrossRef] [PubMed]
- Vulliamy, T.J.; Marrone, A.; Knight, S.W.; Walne, A.; Mason, P.J.; Dokal, I. Mutations in dyskeratosis congenita: Their impact on telomere length and the diversity of clinical presentation. Blood 2006, 107, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.W.; Heiss, N.S.; Vulliamy, T.J.; Aalfs, C.M.; McMahon, C.; Richmond, P.; Jones, A.; Hennekam, R.C.; Poustka, A.; Mason, P.J.; et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br. J. Haematol. 1999, 107, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Coman, D.; Herbert, A.; McGill, J.; Lockwood, L.; Hallahan, A. Unrelated cord blood transplantation in a girl with Hoyeraal-Hreidarsson syndrome. Bone Marrow Transpl. 2008, 42, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Gramatges, M.M.; Qi, X.; Sasa, G.S.; Chen, J.J.; Bertuch, A.A. A homozygous telomerase T-motif variant resulting in markedly reduced repeat addition processivity in siblings with Hoyeraal-Hreidarsson syndrome. Blood 2013, 121, 3586–3593. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Tan, A.M.; Chan, M.Y.; Jamuar, S.S.; Foo, R.; Iyer, P. TCR-αβ and CD19-depleted haploidentical stem cell transplant with reduced intensity conditioning for Hoyeraal–Hreidarsson syndrome with RTEL1 mutation. Bone Marrow Transpl. 2016, 51, 753–754. [Google Scholar] [CrossRef]
- Moriya, K.; Niizuma, H.; Rikiishi, T.; Yamaguchi, H.; Sasahara, Y.; Kure, S. Novel compound heterozygous RTEL1 gene mutations in a patient with Hoyeraal-Hreidarsson syndrome. Pediatr. Blood Cancer 2016, 63, 1683–1684. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Williams, D.A.; London, W.B.; Agarwal, S. Full donor myeloid engraftment with minimal toxicity in dyskeratosis congenita patients undergoing allogeneic bone marrow transplantation without radiation or alkylating agents. Blood 2014, 124, 2941. [Google Scholar]
- Savage, S.A.; Dokal, I.; Armanios, M.; Aubert, G.; Cowen, E.W.; Domingo, D.L.; Giri, N.; Greene, M.H.; Orchard, P.J.; Tolar, J.; et al. Dyskeratosis congenita: The first NIH clinical research workshop. Pediatr. Blood Cancer 2009, 53, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Ayas, M.; Nassar, A.; Hamidieh, A.A.; Kharfan-Dabaja, M.; Othman, T.B.; Elhaddad, A.; Seraihy, A.; Hussain, F.; Alimoghaddam, K.; Ladeb, S.; et al. Reduced intensity conditioning is effective for hematopoietic SCT in dyskeratosis congenita-related BM failure. Bone Marrow Transpl. 2013, 48, 1168–1172. [Google Scholar] [CrossRef]
- Gadalla, S.M.; Sales-Bonfim, C.; Carreras, J.; Alter, B.P.; Antin, J.H.; Ayas, M.; Bodhi, P.; Davis, J.; Davies, S.M.; Deconinck, E.; et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biol. Blood Marrow Transpl. 2013, 19, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Fioredda, F.; Iacobelli, S.; Korthof, E.T.; Knol, C.; van Biezen, A.; Bresters, D.; Veys, P.; Yoshimi, A.; Fagioli, F.; Mats, B.; et al. Outcome of haematopoietic stem cell transplantation in dyskeratosis congenita. Br. J. Haematol. 2018, 183, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.C.; Orchard, P.J.; Baker, K.S.; Giller, R.H.; Savage, S.A.; Alter, B.P.; Tolar, J. Disease-specific hematopoietic cell transplantation: Nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transpl. 2011, 46, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jonassaint, N.L.; Guo, N.; Califano, J.A.; Montgomery, E.A.; Armanios, M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell 2013, 12, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Brudno, J.; Mehta, P.; Kovacs, J.J.; Wu, C.; Zago, M.A.; Chanock, S.J.; Boyer, T.D.; Young, N.S. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology 2011, 53, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Srivastava, U.; Thaler, M.; Kleinhans, K.N.; N’kontchou, G.; Scheffold, A.; Bauer, K.; Kratzer, R.F.; Kloos, N.; Katz, S.F.; et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology 2011, 53, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.Y.; Chen, J.J.; Cogan, J.D.; Alder, J.K.; Ingersoll, R.G.; Markin, C.; Lawson, W.E.; Xie, M.; Vulto, I.; Phillips, J.A., III; et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007, 356, 1317–1326. [Google Scholar] [CrossRef]
- Rodríguez-Roisin, R.; Krowka, M.J. Hepatopulmonary syndrome—A liver-induced lung vascular disorder. N. Engl. J. Med. 2008, 358, 2378–2387. [Google Scholar] [CrossRef]
- Khincha, P.P.; Bertuch, A.A.; Agarwal, S.; Townsley, D.M.; Young, N.S.; Keel, S.; Shimamura, A.; Boulad, F.; Simoneau, T.; Justino, H.; et al. Pulmonary arteriovenous malformations: An uncharacterized phenotype of Dyskeratosis Congenita and related Telomere Biology Disorders. Eur. Respir. J. 2017, 49, 1601640. [Google Scholar] [CrossRef]
- Chen, L.Y.; Majerská, J.; Lingner, J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev. 2013, 27, 2099–2108. [Google Scholar] [CrossRef]
- Krowka, M.J.; Wiseman, G.A.; Burnett, O.L.; Spivey, J.R.; Therneau, T.; Porayko, M.K.; Wiesner, R.H. Hepatopulmonary syndrome: A prospective study of relationships between severity of liver disease, PaO2 response to 100% oxygen, and brain uptake after 99mTc MAA lung scanning. Chest 2000, 118, 615–624. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-L.; Lin, K.K.; Chen, L.-Y. Complications for a Hoyeraal–Hreidarsson Syndrome Patient with a Germline DKC1 A353V Variant Undergoing Unrelated Peripheral Blood Stem Cell Transplantation. Int. J. Mol. Sci. 2019, 20, 3261. https://doi.org/10.3390/ijms20133261
Chen R-L, Lin KK, Chen L-Y. Complications for a Hoyeraal–Hreidarsson Syndrome Patient with a Germline DKC1 A353V Variant Undergoing Unrelated Peripheral Blood Stem Cell Transplantation. International Journal of Molecular Sciences. 2019; 20(13):3261. https://doi.org/10.3390/ijms20133261
Chicago/Turabian StyleChen, Rong-Long, Kuanyin K Lin, and Liuh-Yow Chen. 2019. "Complications for a Hoyeraal–Hreidarsson Syndrome Patient with a Germline DKC1 A353V Variant Undergoing Unrelated Peripheral Blood Stem Cell Transplantation" International Journal of Molecular Sciences 20, no. 13: 3261. https://doi.org/10.3390/ijms20133261
APA StyleChen, R.-L., Lin, K. K., & Chen, L.-Y. (2019). Complications for a Hoyeraal–Hreidarsson Syndrome Patient with a Germline DKC1 A353V Variant Undergoing Unrelated Peripheral Blood Stem Cell Transplantation. International Journal of Molecular Sciences, 20(13), 3261. https://doi.org/10.3390/ijms20133261





