Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression
Abstract
1. Introduction
2. Results
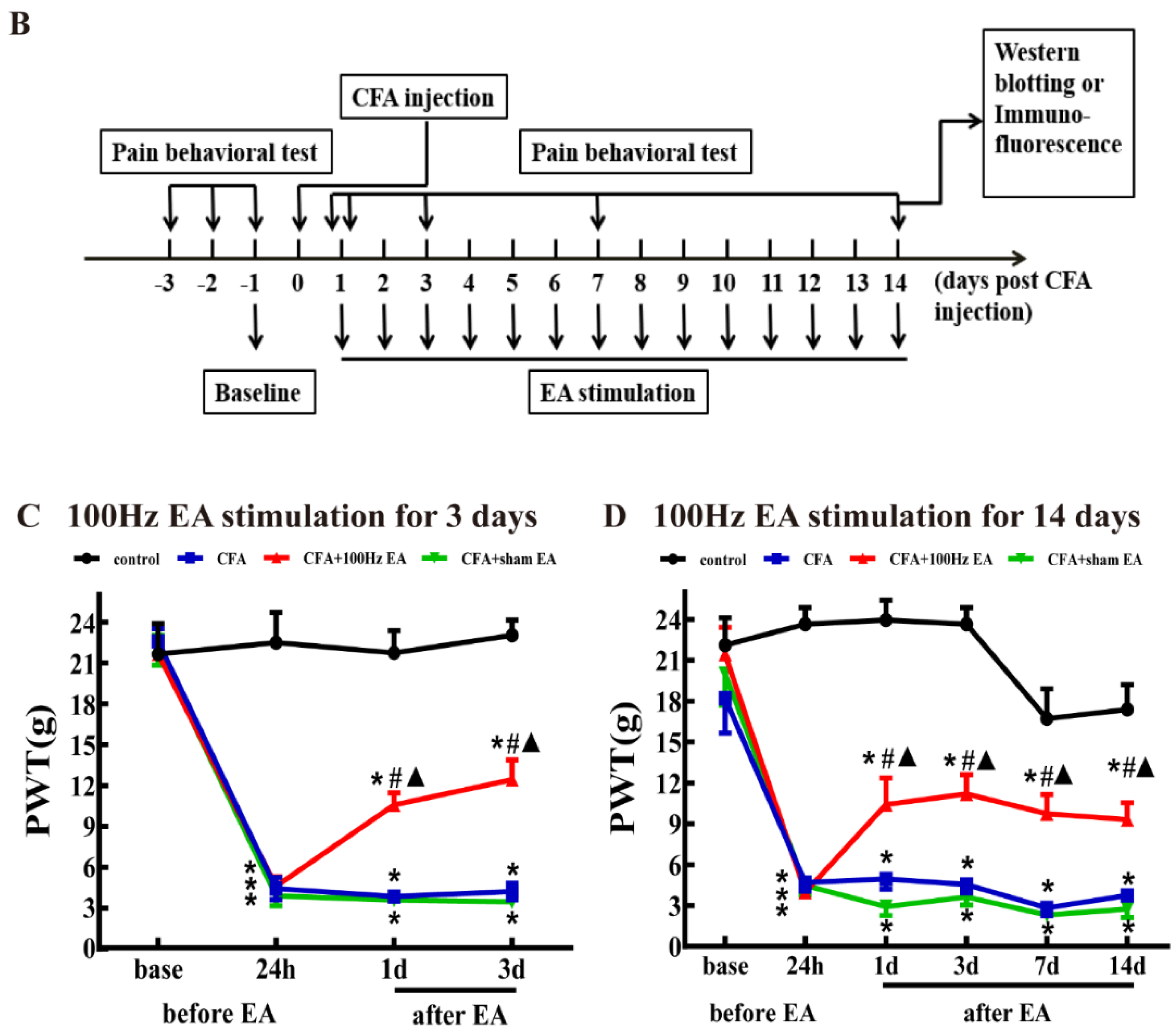
2.1. Both Short-Term and Long-Term EA Stimulation Attenuated CFA Induced Mechanical Allodynia
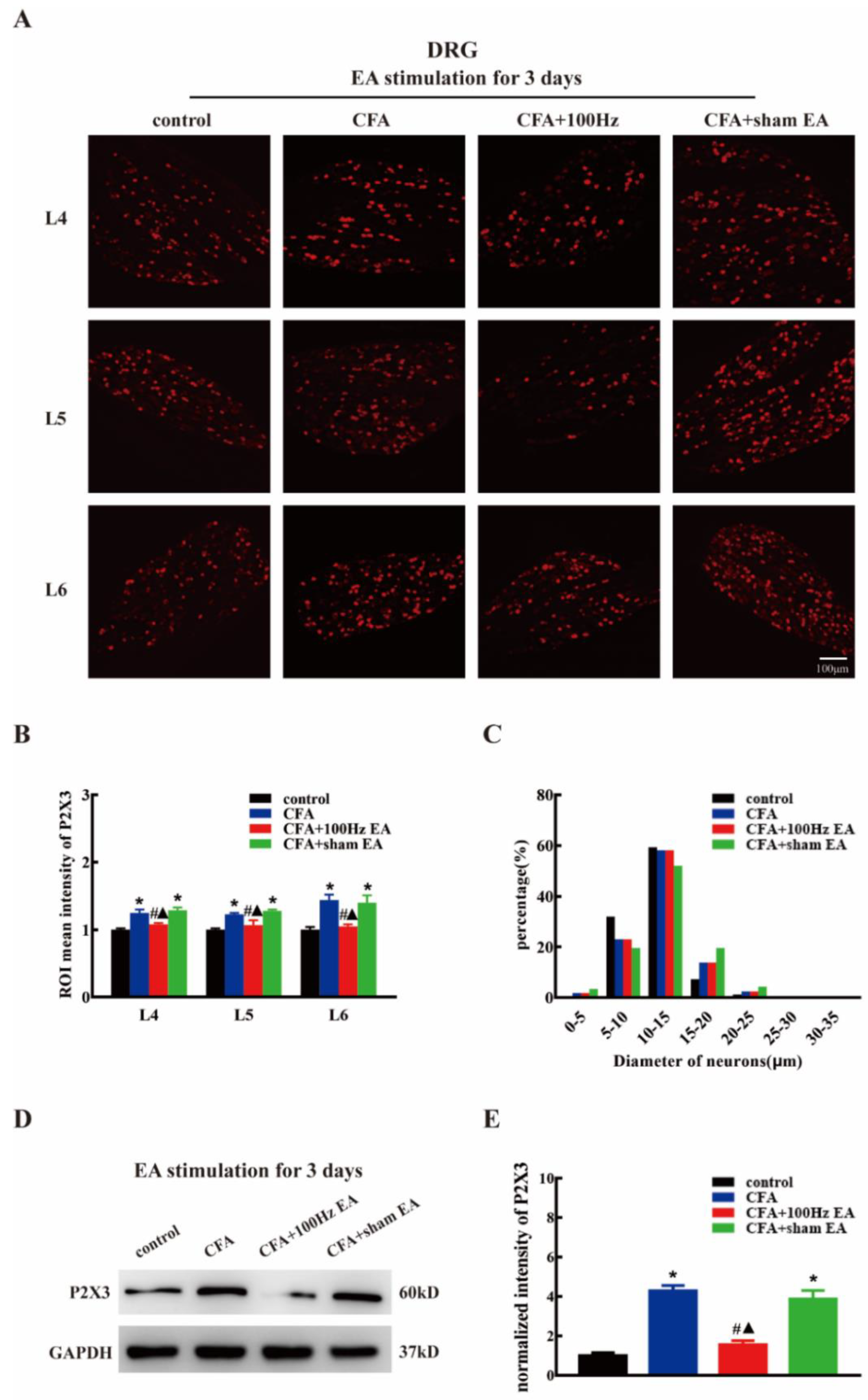
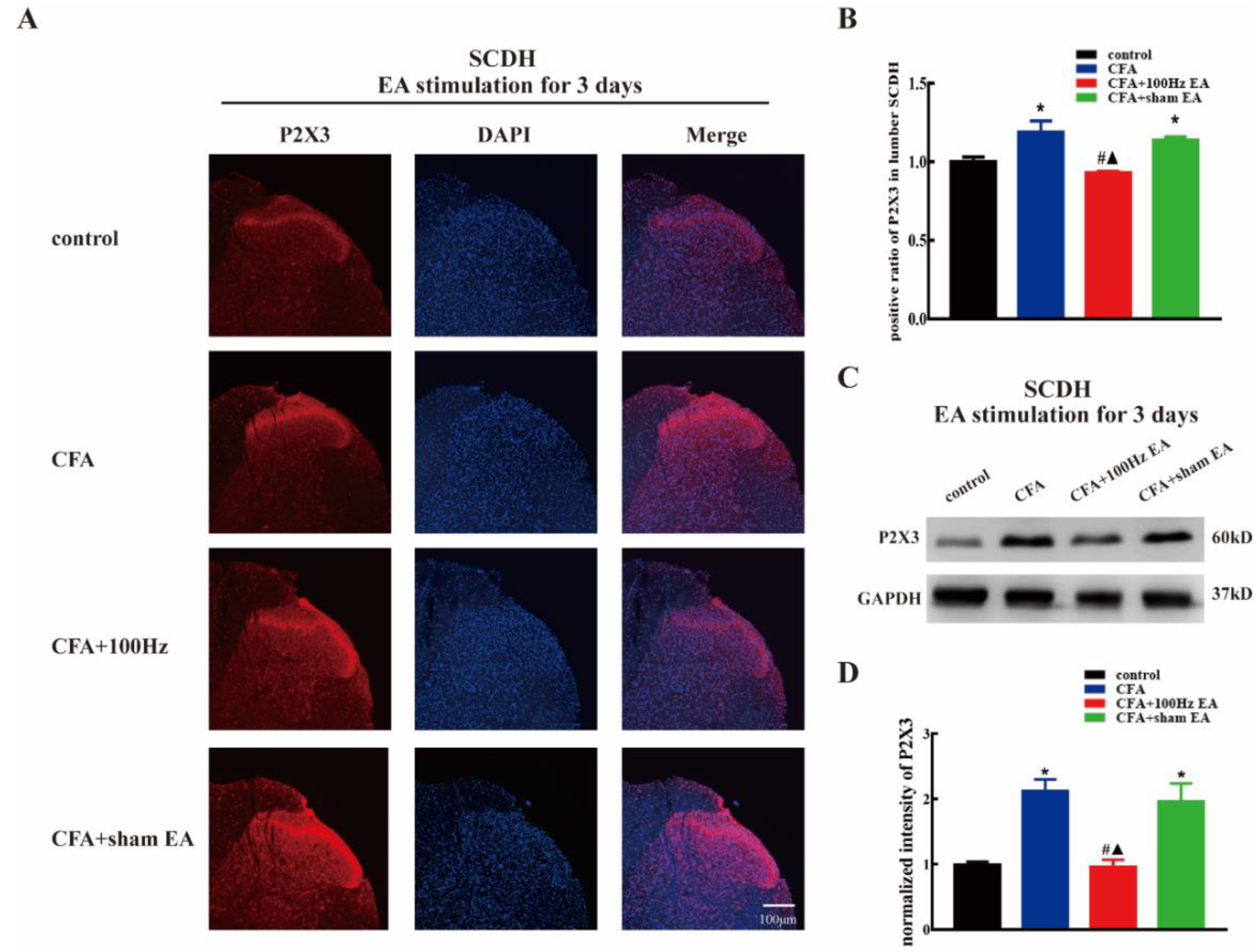
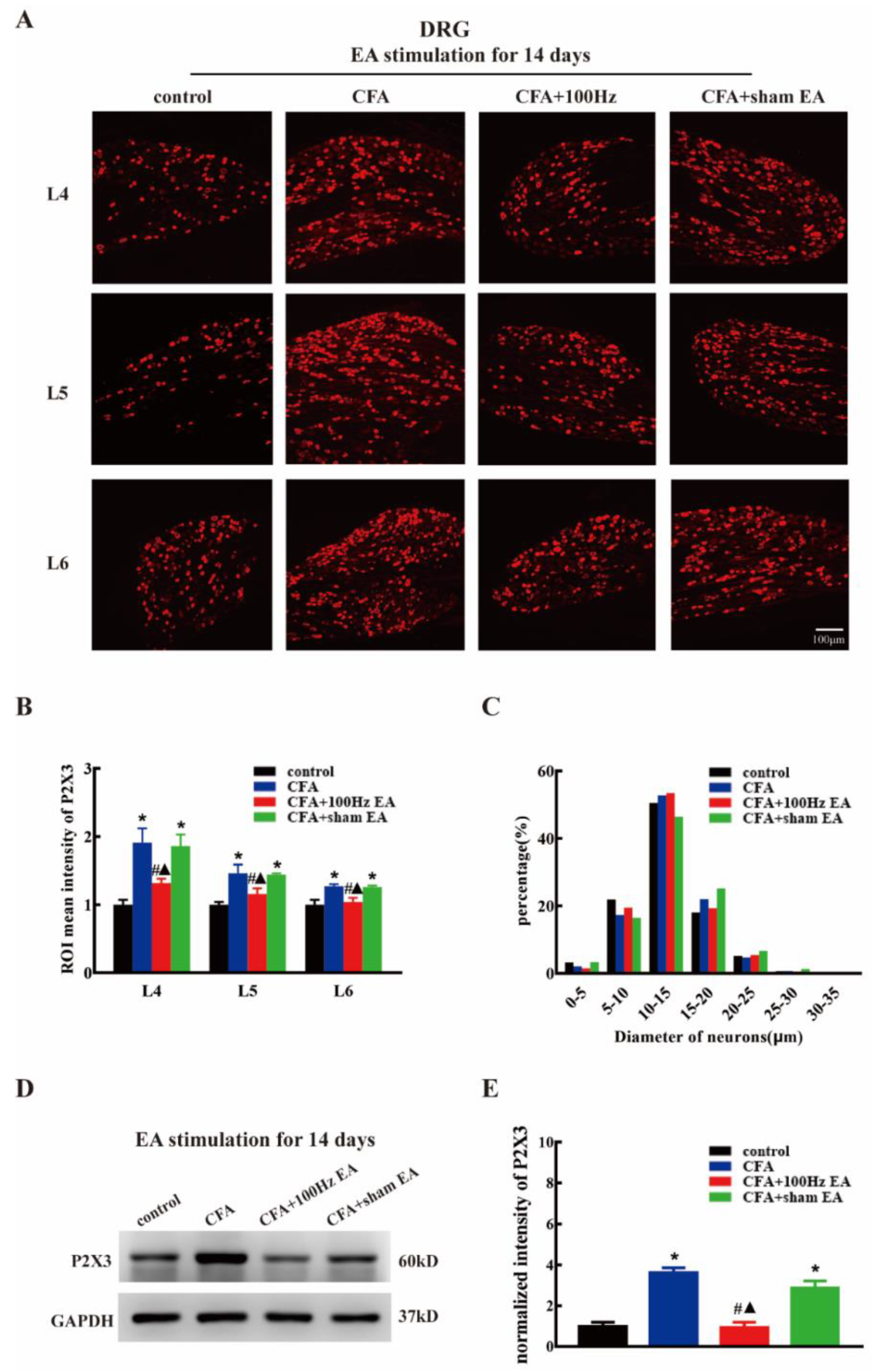
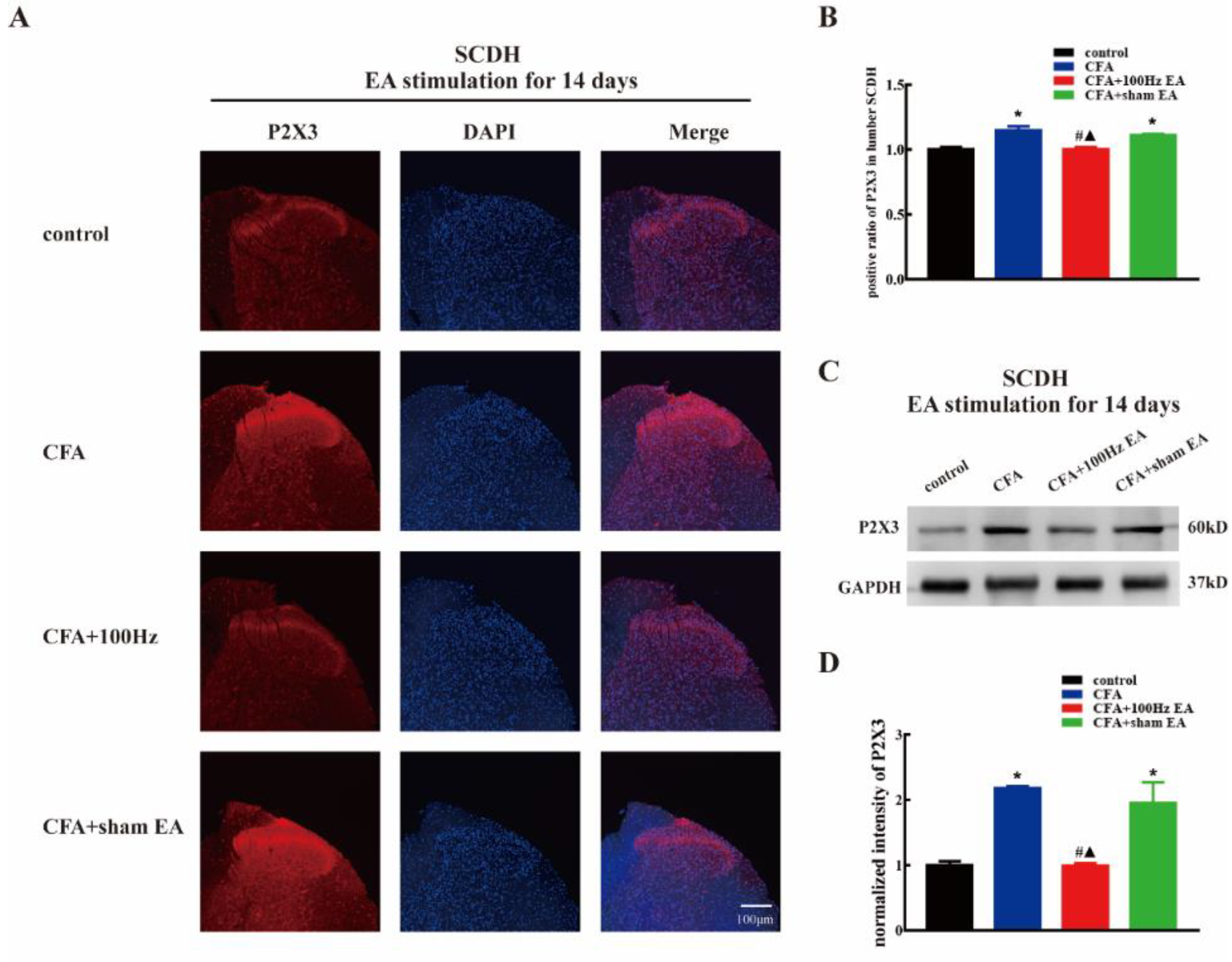
2.2. Both Short-Term and Long-Term 100 Hz EA Stimulation Reversed P2X3 Elevation in L4-6 DRG and SCDH after CFA Injection
2.3. Short-Term and Long-Term EA Stimulation Exerted Comparable Effects on Pain Hypersensitivity and P2X3 Expression
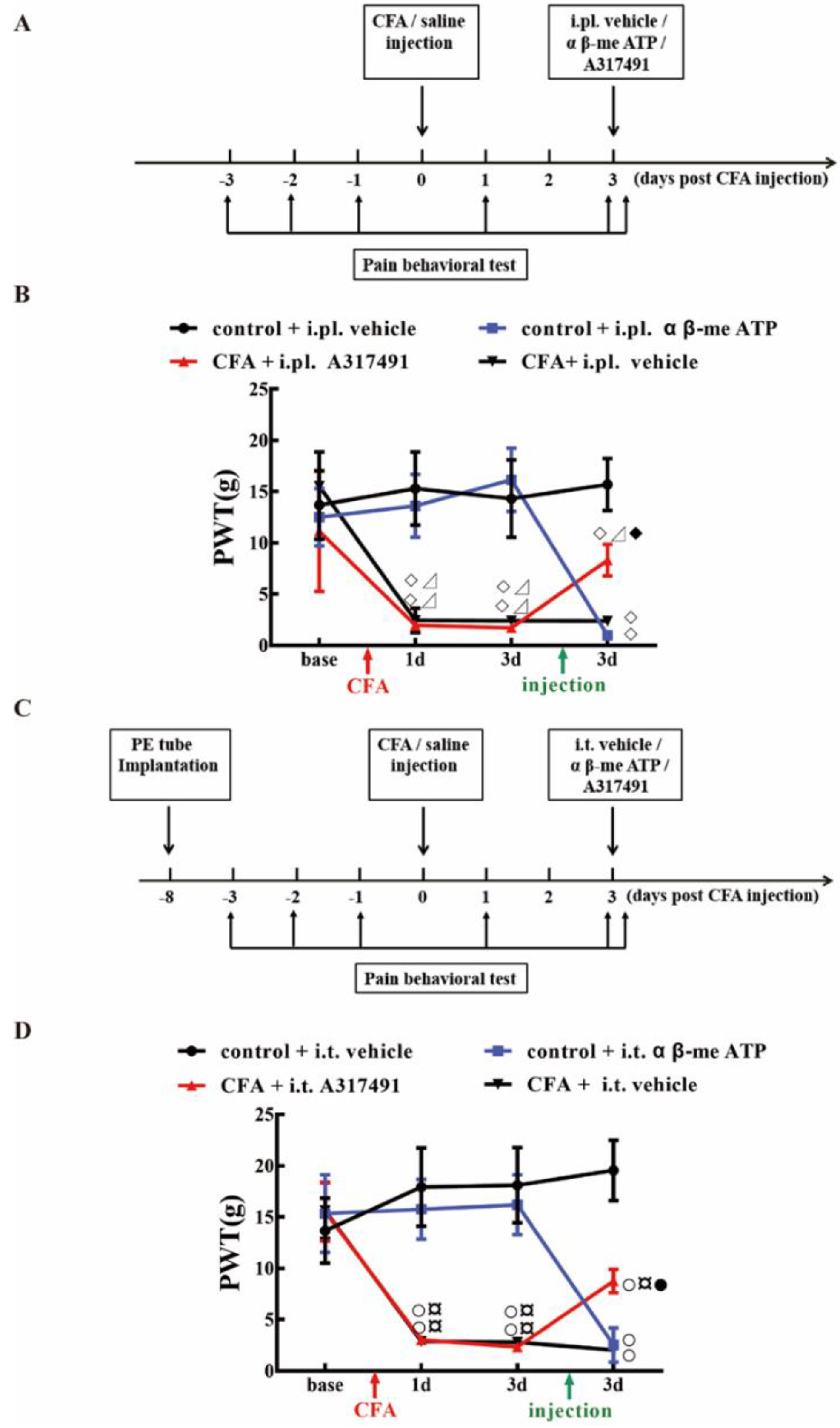
2.4. P2X3 Levels in L4-6 DRG and SCDH Are Involved in Chronic Inflammatory Pain
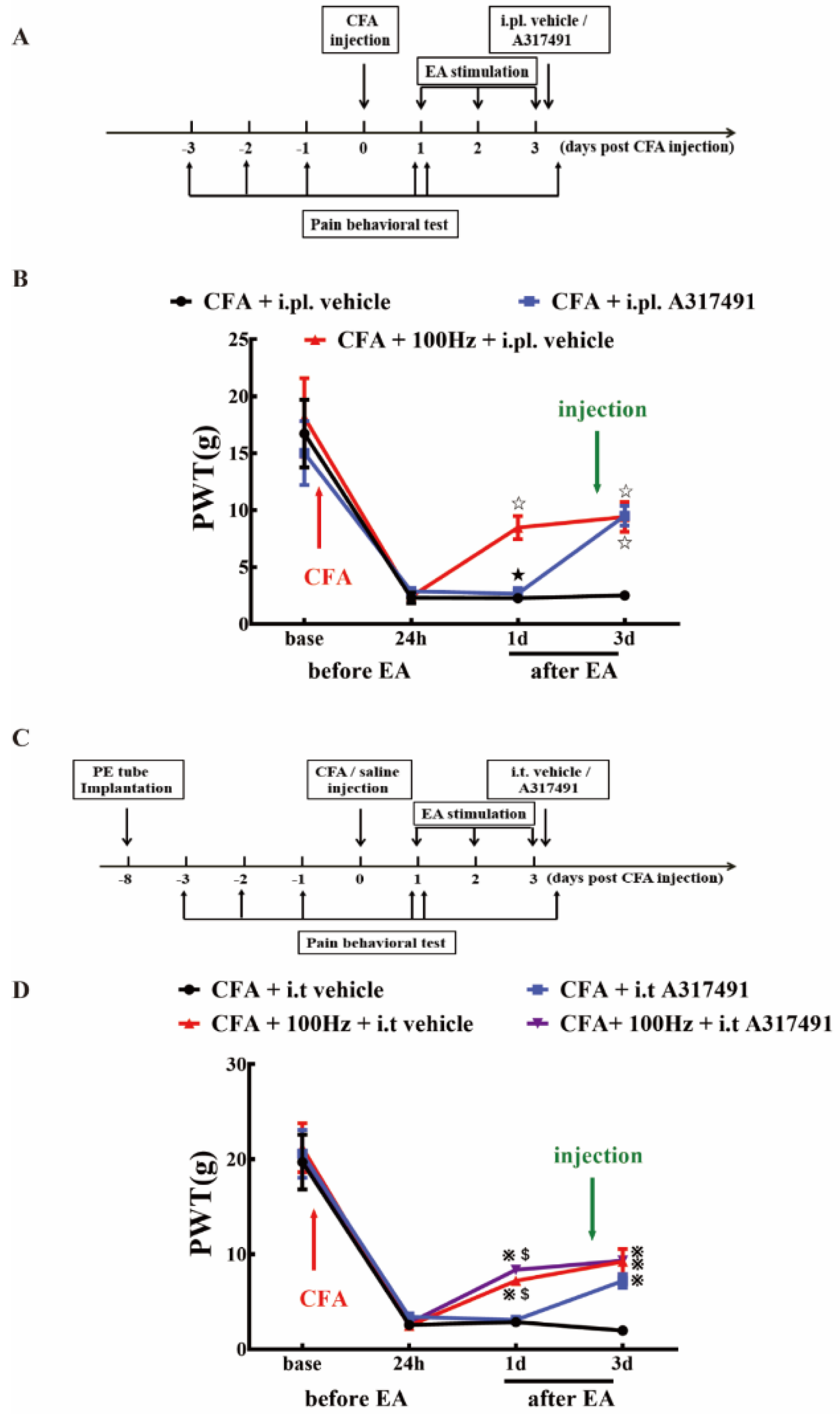
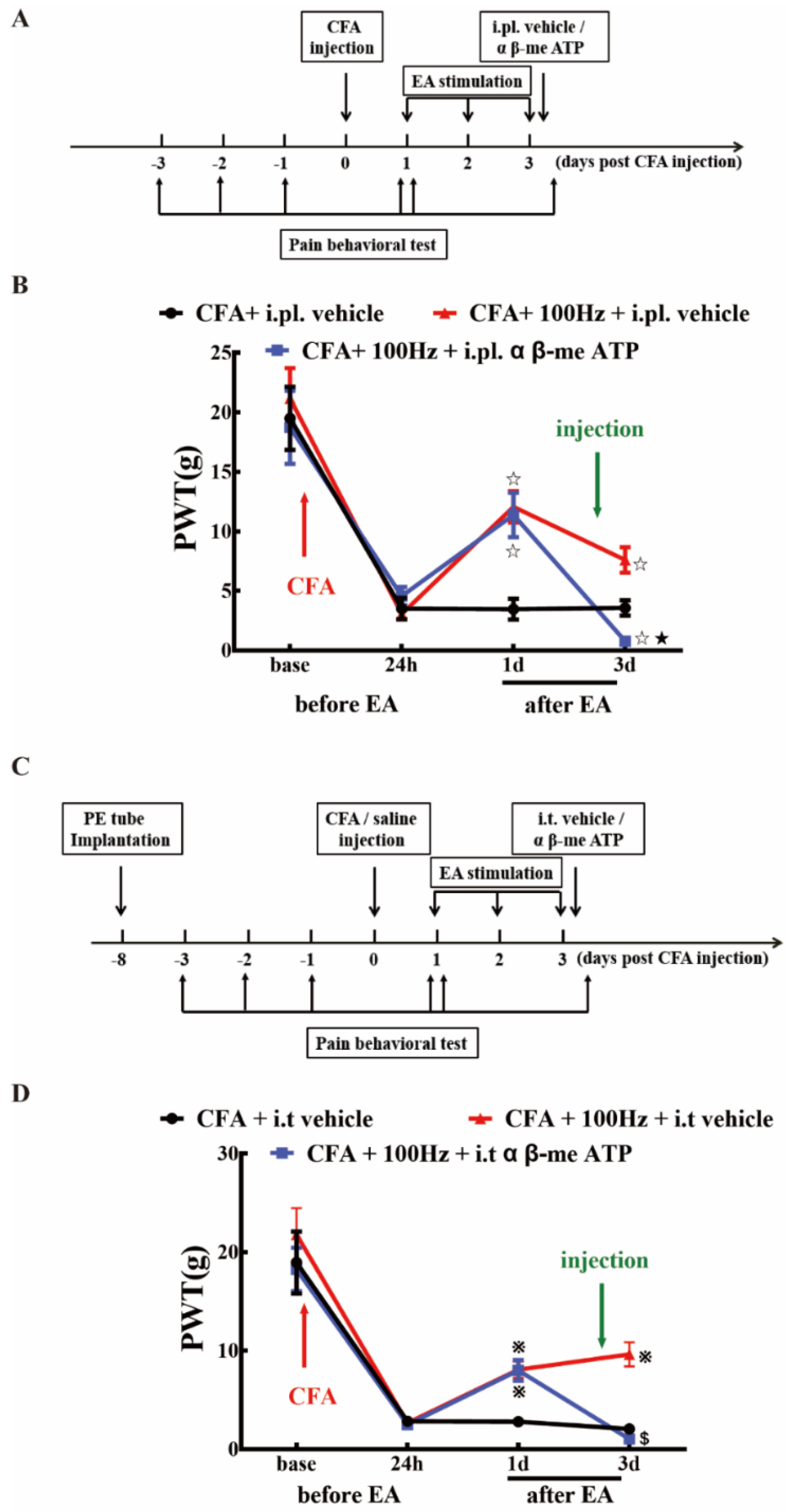
2.5. P2X3 Inhibition Contributed to the Analgesic Effects of 100 Hz EA on CFA-Induced Inflammatory Pain
3. Discussion
4. Materials and Methods
4.1. Animals
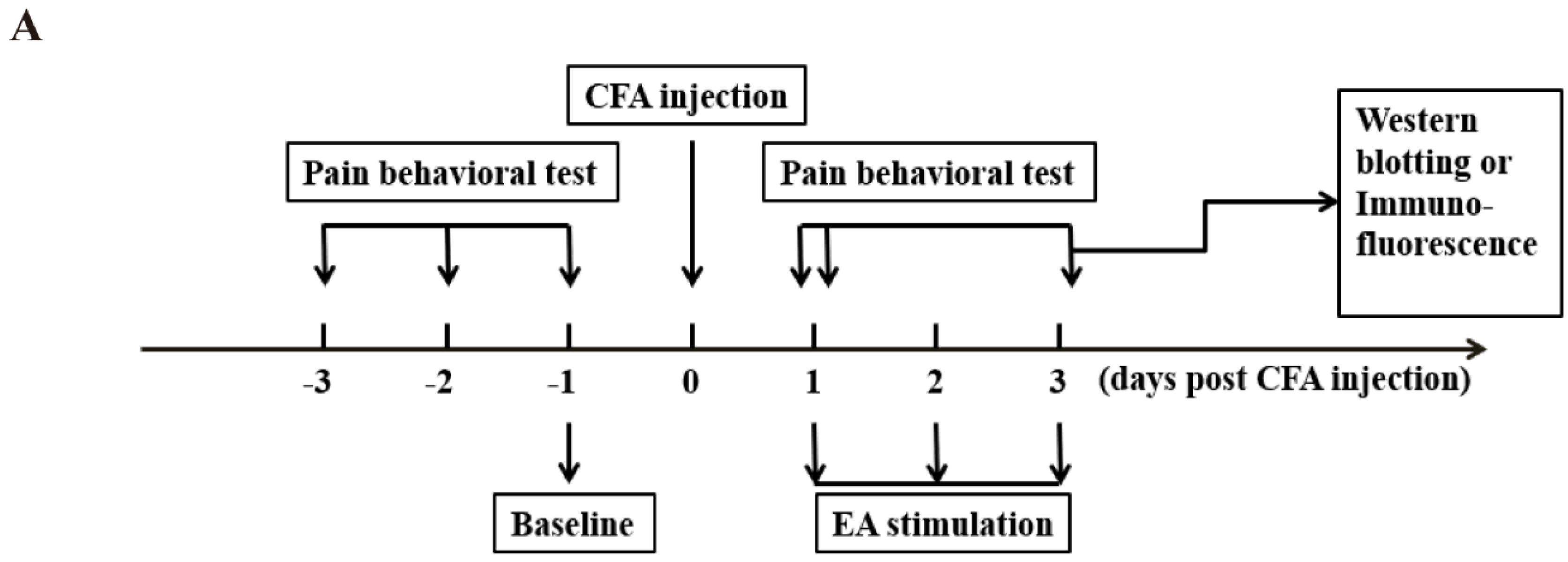
4.2. Experimental Design
This study was divided into three parts.
4.3. Persistent Inflammatory Pain Model
4.4. Paw Withdraw Threshold (PWT)
4.5. EA Treatment
4.6. Drug Treatment
4.7. Immunofluorescence
4.8. Western Blotting Analysis
4.9. Statistical analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luo, C.; Kuner, T.; Kuner, R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014, 37, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Marret, E.; Kurdi, O.; Zufferey, P.; Bonnet, F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: Meta-analysis of randomized controlled trials. Anesthesiology 2005, 102, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xiang, H.C.; Li, H.P.; Jia, M.; Pan, X.L.; Pan, H.L.; Li, M. Electroacupuncture Inhibits NLRP3 Inflammasome Activation through CB2 Receptors in Inflammatory Pain. Brain Behav. Immun. 2017, 67. [Google Scholar] [CrossRef] [PubMed]
- Ooi Thye, C.; Critchley, H.O.D.; Horne, A.W.; Robert, E.; Erna, H.; Marie, F. The BMEA study: The impact of meridian balanced method electroacupuncture on women with chronic pelvic pain-a three-arm randomised controlled pilot study using a mixed-methods approach. BMJ Open 2015, 5, e008621. [Google Scholar]
- Ulett, G.A.; Han, S.; Han, J.S. Electroacupuncture: Mechanisms and clinical application. Biol. Psychiatry 1998, 44, 129–138. [Google Scholar] [CrossRef]
- Han, J.S. Acupuncture and endorphins. Neurosci. Lett. 2004, 361, 258–261. [Google Scholar] [CrossRef]
- Fang, J.Q.; Du, J.Y.; Fang, J.F.; Xiao, T.; Le, X.Q.; Pan, N.F.; Yu, J.; Liu, B.Y. Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats. Life Sci. 2018, 200, 69–80. [Google Scholar] [CrossRef]
- Shiozaki, Y.; Sato, M.; Kimura, M.; Sato, T.; Tazaki, M.; Shibukawa, Y. Ionotropic P2X ATP Receptor Channels Mediate Purinergic Signaling in Mouse Odontoblasts. Front. Physiol. 2017, 8, 3. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnstock, G.; Mcmahon, S.B. The Expression of P2X 3 Purinoreceptors in Sensory Neurons: Effects of Axotomy and Glial-Derived Neurotrophic Factor. Mol. Cell. Neurosci. 1998, 12, 256–268. [Google Scholar] [CrossRef]
- Liu, S.; Lv, Y.; Wan, X.X.; Song, Z.J.; Liu, Y.P.; Miao, S.; Wang, G.L.; Liu, G.J. Hedgehog signaling contributes to bone cancer pain by regulating sensory neuron excitability in rats. Mol. Pain 2018, 14, 1744806918767560. [Google Scholar] [CrossRef]
- Wang, W.S.; Tu, W.Z.; Cheng, R.D.; He, R.; Ruan, L.H.; Zhang, L.; Gong, Y.S.; Fan, X.F.; Hu, J.; Cheng, B. Electroacupuncture and A-317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J. Neurosci. Res. 2015, 92, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Meisner, J.G.; Reid, A.R.; Sawynok, J. Adrenergic regulation of P2X3 and TRPV1 receptors: Differential effects of spared nerve injury. Neurosci. Lett. 2008, 444, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, W.X.; Sun, J.R.; Zhu, T.T.; Fan, J.; Yu, L.H.; Burnstock, G.; Yang, H.; Ma, B. Inhibitory effect of estrogen receptor beta on P2X3 receptors during inflammation in rats. Purinergic Signal. 2016, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brederson, J.D.; Jarvis, M.F. Homomeric and heteromeric P2X3 receptors in peripheral sensory neurons. Curr. Opin. Investig. Drugs 2008, 9, 716–725. [Google Scholar] [PubMed]
- Vulchanova, L.; Riedl, M.S.; Shuster, S.J.; Stone, L.S.; Hargreaves, K.M.; Buell, G.; Surprenant, A.; North, R.A.; Elde, R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur. J. Neurosci. 1998, 10, 3470–3478. [Google Scholar] [CrossRef]
- Kaan, T.K.Y.; Yip, P.K.; John, G.; Cefalu, J.S.; Nunn, P.A.; Ford, A.P.D.W.; Yu, Z.; Mcmahon, S.B. Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 4503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.B.; Zhang, Y.L.; Li, Q.; Liu, Y.G.; Wang, X.D.; Yang, B.L.; Zhu, G.C.; Zhou, C.F.; Gao, Y.; Liu, Z.X. Effects of 1,8-cineole on neuropathic pain mediated by P2X2 receptor in the spinal cord dorsal horn. Sci. Rep. 2019, 9, 7909. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wang, L.; Wang, X.; Ren, K.; Berman, B.M.; Lao, L. Electroacupuncture combined with MK-801 prolongs anti-hyperalgesia in rats with peripheral inflammation. Pharmacol. Biochem. Behav. 2005, 81, 146–151. [Google Scholar] [CrossRef]
- Jianbo, Y.; Cong, Z.; Xiaoqin, L. The effects of electroacupuncture on the extracellular signal-regulated kinase 1/2/P2X3 signal pathway in the spinal cord of rats with chronic constriction injury. Anesth. Analg. 2013, 116, 239–246. [Google Scholar]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Lin, J.G.; Lo, M.W.; Wen, Y.R.; Hsieh, C.L.; Tsai, S.K.; Sun, W.Z. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 2002, 99, 509–514. [Google Scholar] [CrossRef]
- He, X.; Wei, J.; Shou, S.; Fang, J.; Jiang, Y. Effects of electroacupuncture at 2 and 100 Hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J. Zhejiang Univ. Sci. B 2017, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Silva, J.R.T.; Prado, W.A. 100-Hz Electroacupuncture but not 2-Hz Electroacupuncture is Preemptive Against Postincision Pain in Rats. J. Acupunct. Meridian Stud. 2016, 9, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Chen, S.; Duanmu, C.; Zhang, J.; Feng, X.; Yan, Y.; Liu, J.; Litscher, G. The Effect of Repeated Electroacupuncture Analgesia on Neurotrophic and Cytokine Factors in Neuropathic Pain Rats. Evid. -Based Complementray Altern. Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Wang, J.Y.; Qiao, L.N.; Chen, S.P.; Tan, L.H.; Xu, Q.L.; Liu, J.L. NK cells mediate the cumulative analgesic effect of electroacupuncture in a rat model of neuropathic pain. BMC Complementary Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Y.; Qin, Z.; Zhou, K.; Xu, H.; He, L.; Li, N.; Su, T.; Sun, J.; Yue, Z.; et al. Electroacupuncture Versus Pelvic Floor Muscle Training Plus Solifenacin for Women With Mixed Urinary Incontinence: A Randomized Noninferiority Trial. Mayo Clin. Proc. 2019, 94, 54–65. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Xu, H.; He, L.; Chen, Y.; Fu, L.; Li, N.; Lu, Y.; Su, T.; Sun, J.; et al. Effect of Electroacupuncture on Urinary Leakage Among Women With Stress Urinary Incontinence: A Randomized Clinical Trial. JAMA 2017, 317, 2493–2501. [Google Scholar] [CrossRef]
- Alvarado-Sanchez, B.G.; Salgado-Ceballos, H.; Torres-Castillo, S.; Rodriguez-Silverio, J.; Lopez-Hernandez, M.E.; Quiroz-Gonzalez, S.; Sanchez-Torres, S.; Mondragon-Lozano, R.; Fabela-Sanchez, O. Electroacupuncture and Curcumin Promote Oxidative Balance and Motor Function Recovery in Rats Following Traumatic Spinal Cord Injury. Neurochem. Res. 2019. [Google Scholar] [CrossRef]
- Cheng, R.S.; Pomeranz, B. Electroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systems. Life Sci. 1979, 25, 1957–1962. [Google Scholar] [CrossRef]
- Wang, Y.; Gehringer, R.; Mousa, S.A.; Hackel, D.; Brack, A.; Rittner, H.L. CXCL10 controls inflammatory pain via opioid peptide-containing macrophages in electroacupuncture. PLoS ONE 2014, 9, e94696. [Google Scholar] [CrossRef]
- Kim, H.W.; Uh, D.K.; Yoon, S.Y.; Roh, D.H. Low-frequency electroacupuncture suppresses carrageenan-induced paw inflammation in mice via sympathetic post-ganglionic neurons, while high-frequency EA suppression is mediated by the sympathoadrenal medullary axis. Brain Res. Bull. 2008, 75, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Berrocoso, E.M.; Chen, L.; Mcnaughton, P.A. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011, 333, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.B.; Stephens, G.J. Recent advances in targeting ion channels to treat chronic pain. Br. J. Pharmacol. 2018, 175, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Ying, X.M.; He, X.F.; Shou, S.Y.; Wei, J.J.; Tai, Z.X.; Shao, X.M.; Liang, Y.; Fang, F.; Fang, J.Q.; et al. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018, 14, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Badinez, P.; Sepulveda, H.; Diaz, E.; Greffrath, W.; Treede, R.D.; Stehberg, J.; Montecino, M.; van Zundert, B. Variable transcriptional responsiveness of the P2X3 receptor gene during CFA-induced inflammatory hyperalgesia. J. Cell Biochem. 2018, 119, 3922–3935. [Google Scholar] [CrossRef] [PubMed]
- Tariba, P.K.; Vukman, R.; Antonić, R.; Kovač, Z.; Uhač, I.; SimonićKocijan, S. The role of P2X3receptors in bilateral masseter muscle allodynia in rats. Croat. Med. J. 2016, 57, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, G.; Chen, Y.; Huang, L.Y. Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 2016, 157, 1541–1550. [Google Scholar] [CrossRef]
- Cheng, R.D.; Tu, W.Z.; Wang, W.S.; Zou, E.M.; Cao, F.; Cheng, B.; Wang, J.Z.; Jiang, Y.X.; Jiang, S.H. Effect of electroacupuncture on the pathomorphology of the sciatic nerve and the sensitization of P2X(3) receptors in the dorsal root ganglion in rats with chronic constrictive injury. Chin. J. Integr. Med. 2013, 19, 374–379. [Google Scholar] [CrossRef]
- Weng, Z.J.; Wu, L.Y.; Zhou, C.L.; Dou, C.Z.; Shi, Y.; Liu, H.R.; Wu, H.G. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015, 11, 321–329. [Google Scholar] [CrossRef]
- Li, M.H.; Suchland, K.L.; Ingram, S.L. Compensatory activation of cannabinoid cb2 receptor inhibition of gaba release in the rostral ventromedial medulla in inflammatory pain. J. Neurosci. 2017, 37, 626–636. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Cui, R.; Chu, H. Polysaccharopeptide from trametes versicolor blocks inflammatory osteoarthritis pain-morphine tolerance effects via activating cannabinoid type 2 receptor. Int. J. Biol. Macromol. 2019, 126, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Yamashita, A.; Matsuda, M.; Kawai, K.; Sawa, T.; Amaya, F. The nlrp2 inflammasome in dorsal root ganglion as a novel molecular platform that produces inflammatory pain hypersensitivity. Pain 2019. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.; Wang, S.; Shao, F.; Fang, J.; Xu, Y.; Wang, W.; Sun, H.; Liu, X.; Du, J.; Fang, J. Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression. Int. J. Mol. Sci. 2019, 20, 3248. https://doi.org/10.3390/ijms20133248
Xiang X, Wang S, Shao F, Fang J, Xu Y, Wang W, Sun H, Liu X, Du J, Fang J. Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression. International Journal of Molecular Sciences. 2019; 20(13):3248. https://doi.org/10.3390/ijms20133248
Chicago/Turabian StyleXiang, Xuaner, Sisi Wang, Fangbing Shao, Junfan Fang, Yingling Xu, Wen Wang, Haiju Sun, Xiaodong Liu, Junying Du, and Jianqiao Fang. 2019. "Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression" International Journal of Molecular Sciences 20, no. 13: 3248. https://doi.org/10.3390/ijms20133248
APA StyleXiang, X., Wang, S., Shao, F., Fang, J., Xu, Y., Wang, W., Sun, H., Liu, X., Du, J., & Fang, J. (2019). Electroacupuncture Stimulation Alleviates CFA-Induced Inflammatory Pain Via Suppressing P2X3 Expression. International Journal of Molecular Sciences, 20(13), 3248. https://doi.org/10.3390/ijms20133248




