Phase I Clinical Trial Using Autologous Ex Vivo Expanded NK Cells and Cytotoxic T Lymphocytes for Cancer Treatment in Vietnam
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Immune Cell Expansion Ability
2.3. The Relative Relationship of Immune Cell Expansion Ability to the Patient’s Age and Gender
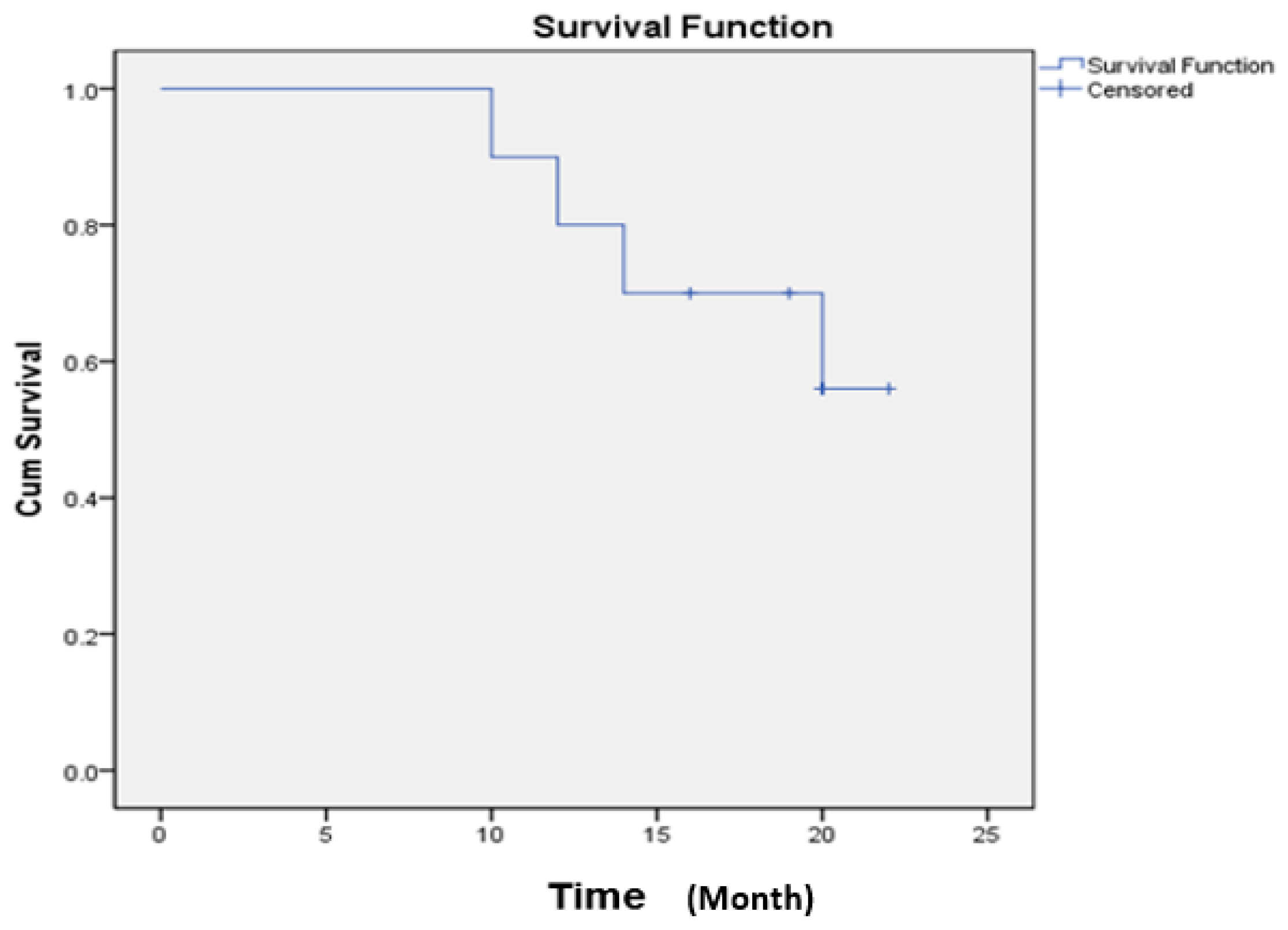
2.4. Safety of Ex Vivo Expanded Immune Cell Transfusion
3. Discussion
4. Methods
4.1. Patients
4.1.1. Inclusion Criteria
- Patients with confirmed diagnosis of colon, liver, or lung cancer by physician’s judgement.
- Eastern Cooperative Oncology Group performance status (ECOG/PS) ≥3.
- Patients signed the written informed consent form, which was approved by the Ethics Committee of Vinmec International Hospital in 18th November 2015, project identification code Vingroup JSC ĐT-00.
4.1.2. Exclusion Criteria
- Severe health conditions, such as serious infection, autoimmune diseases, or the use of anti-rejection drugs, or T-cell lymphomas.
4.2. Study Design
4.3. Research Setting and Duration
- The study was carried out at the Oncology Department, Vinmec Times City International Hospital, from March 2016 to June 2017.
- This study was conducted with the permission of the Vietnam Ministry of Health (Hanoi, Vietnam) (document no. 2517/BYT-KCB).
4.4. Cohort Size
- During the study period, 10 patients met the inclusion criteria.
- A total of 10 peripheral blood samples were marked as PT1 to PT10, corresponding to 10 patients (PT), 7 lung cancer patients were labeled as PT1 to PT7, 2 liver cancer patients were labeled as PT8 and PT9, and 1 colon cancer patient labeled as PT10.
4.5. Isolation and Large-Scale Expansion of NK Cells and CTLs From Peripheral Blood
4.6. Dosage and Duration
4.7. Clinical Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenocarcinoma |
| AIET | Autologous immune enhancement therapy |
| BIJ | Biotherapy Institute of Japan |
| BINKIT | Immune cell expansion kit of Biotherapy Institute of Japan |
| CD | Cluster of differentiation |
| CIK cells | Cytokine-induced killer cells |
| CTL | Cytotoxic T Lymphocyte |
| D | Day of culture |
| DC | Dendritic cell |
| ECOG/PS scale | Eastern Cooperative Oncology Group/Performance Status scale |
| EORTC QLQ-C30 | Organization for Research and Treatment of Cancer Quality of Life Questionnaire Version 3.0 |
| HCC | Hepatocellular carcinoma |
| IL | Interleukin |
| MHC | Major histocompatibility complex |
| NK cell | Natural killer cell |
| OS | Overall survival |
| PBMNC | Peripheral blood mononuclear cell |
| PT | Patient |
| QoL | Quality of life |
| SqC | Squamous cell carcinoma |
| TCR | T-cell receptors |
| VAFS | Visual analog fatigue scale |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Löwer, M.; Van De Roemer, N.; De Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012, 72, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A.; Britten, C.M.; Huber, C.; O’donnell-Tormey, J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat. Biotechnol. 2011, 29, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335. [Google Scholar] [CrossRef]
- Terunuma, H.; Deng, X.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef]
- Srivastava, S.; Lundqvist, A.; Childs, R.W. Natural killer cell immunotherapy for cancer: A new hope. Cytotherapy 2008, 10, 775–783. [Google Scholar] [CrossRef]
- Villegas, F.R.; Coca, S.; Villarrubia, V.G.; Jiménez, R.; Chillón, M.J.; Jareño, J.; Zuil, M.; Callol, L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002, 35, 23–28. [Google Scholar] [CrossRef]
- Anfossi, N.; Andre, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Ferlazzo, G.; Munz, C. NK cell compartments and their activation by dendritic cells. J. Immunol. 2004, 172, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-killer cells and dendritic cells: L’union fait la force. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, H.G.; Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef]
- Nguyen-Pham, T.N.; Yang, D.H.; Nguyen, T.A.; Lim, M.S.; Hong, C.Y.; Kim, M.H.; Lee, H.J.; Lee, Y.K.; Cho, D.; Bae, S.Y.; et al. Optimal culture conditions for the generation of natural killer cell-induced dendritic cells for cancer immunotherapy. Cell. Mol. Immunol. 2012, 9, 45–53. [Google Scholar] [CrossRef]
- Piccioli, D.; Sbrana, S.; Melandri, E.; Valiante, N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 2002, 195, 335–341. [Google Scholar] [CrossRef]
- Gerosa, F.; Baldani-Guerra, B.; Nisii, C.; Marchesini, V.; Carra, G.; Trinchieri, G. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 2002, 195, 327–333. [Google Scholar] [CrossRef]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef]
- Mocikat, R.; Braumuller, H.; Gumy, A.; Egeter, O.; Ziegler, H.; Reusch, U.; Bubeck, A.; Louis, J.; Mailhammer, R.; Riethmüller, G.; et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 2003, 19, 561–569. [Google Scholar] [CrossRef]
- Kelly, J.M.; Darcy, P.K.; Markby, J.L.; Godfrey, D.I.; Takeda, K.; Yagita, H.; Smyth, M.J. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat. Immunol. 2002, 3, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N. Eng. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Sekine, T.; Makuuchi, M.; Yamasaki, S.; Kosuge, T.; Yamamoto, J.; Shimada, K.; Sakamoto, M.; Hirohashi, S.; Ohashi, Y.; et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: A randomised trial. Lancet 2000, 356, 802–807. [Google Scholar] [CrossRef]
- Iwai, K.; Soejima, K.; Kudoh, S.; Umezato, Y.; Kaneko, T.; Yoshimori, K.; Tokuda, H.; Yamaguchi, T.; Mizoo, A.; Setoguchi, Y.; et al. Extended survival observed in adoptive activated T lymphocyte immunotherapy for advanced lung cancer: Results of a multicenter historical cohort study. Cancer Immunol. Immunother. 2012, 61, 1781–1790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goto, S.; Kaneko, T.; Miyamoto, Y.; Eriguchi, M.; Kato, A.; Akeyama, T.; Fujimoto, K.; Tomonaga, M.; Egawa, K. Combined immunocell therapy using activated lymphocytes and monocyte-derived dendritic cells for malignant melanoma. Anticancer Res. 2005, 25, 3741–3746. [Google Scholar] [PubMed]
- Nhung, H.T.M.; Anh, B.V.; Huyen, T.L.; Hiep, D.T.; Thao, C.T.; Lam, P.N.; Liem, N.T. Ex vivo expansion of human peripheral blood natural killer cells and cytotoxic T lymphocytes from lung cancer patients. Oncol. Lett. 2018, 15, 5730–5738. [Google Scholar] [PubMed]
- Terunuma, H.; Deng, X.; Nishino, N.; Watanabe, K. NK cell-based autologous immune enhancement therapy (AIET) for cancer. J. Stem Cells Regen. Med. 2013, 9, 9–13. [Google Scholar]
- Terunuma, H. Autologous Immune Enhancement Therapy for Cancer—Our experience since 2004. J. Stem Cells Regen. Med. 2012, 8, 205–206. [Google Scholar]
- Ratnavelu, K.; Subramani, B.; Pullai, C.R.; Krishnan, K.; Sugadan, S.D.; Rao, M.S.; Veerakumarasivam, A.; Deng, X.; Hiroshi, T. Autologous immune enhancement therapy against an advanced epithelioid sarcoma: A case report. Oncol. Lett. 2013, 5, 1457–1460. [Google Scholar] [CrossRef]
- Premkumar, S.; Dedeepiya, V.D.; Terunuma, H.; Senthilkumar, R.; Srinivasan, T.; Reena, H.C.; Preethy, S.; Abraham, S.J. Cell Based Autologous Immune Enhancement Therapy (AIET) after Radiotherapy in a Locally Advanced Carcinoma of the Cervix. Case Rep. Oncol. Med. 2013, 903094. [Google Scholar] [CrossRef][Green Version]
- Baskar, S.; Kananathan, R.; Chithre, R.; Kohila, K.; Sheela, D.S.; Xuewen, D.; Terunuma, H. Autologous immune enhancement therapy: A case report of a stage IV colonic cancer. Oncol. Lett. 2013, 5, 1611–1614. [Google Scholar]
- Raj, R.; Deenadayalan, M.; Kumar, G.V.; Khandelwal, V.; Sri, K.; Senthilkumar, R.; Abraham, S.J.; Hiroshi, T. Autologous Immune Enhancement Therapy in Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Indian J. Hematol. Blood Transfus. 2012, 30, 202–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nguyen, K.S.H.; Stehr, H.; Zhou, L.; Nguyen, A.H.; Hiep, P.N.; Van Cau, N.; Duy, P.C.; Thorp, R.; Wakelee, H.A.; Diehn, M.; et al. Comparison of genomic driver oncogenes in Vietnamese patients with non-small-cell lung cancer in the United States and Vietnam. J. Glob. Oncol. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Dinh, S.H.; Do, A.; Pham, T.; Dao, D.Y.; Nguy, T.N.; Chen, M.S., Jr. High burden of hepatocellular carcinoma and viral hepatitis in Southern and Central Vietnam: Experience of a large tertiary referral center, 2010 to 2016. World J. Hepatol. 2018, 10, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Dedeepiya, V.; Terunuma, H.; Manjunath, S.; Senthilkumar, R.; Thamaraikannan, P.; Srinivasan, T.; HelenReena, C.; Preethy, S.; Abraham, S. Autologous Immune Enhancement Therapy for cancer using NK cells and CTLs without feeder layers; our six-year experience in India. J. Stem Cells Regen. Med. 2011, 7, 95. [Google Scholar] [PubMed]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023–1028. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Eng. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Tseng, B.Y.; Gajewski, B.J.; Kluding, P.M. Reliability, responsiveness, and validity of the visual analog fatigue scale to measure exertionfatigue in people with chronic stroke: A preliminary study. Stroke Res. Treat. 2010, 412964. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- EORTC Quality of Life website|EORTC Quality of Life Group website [Internet]. Available online: https://qol.eortc.org/ (accessed on 28 August 2015).
| Patients | PT1 | PT2 | PT3 | PT4 | PT5 | PT6 | PT7 | PT8 | PT9 | PT10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 84 | 64 | 30 | 53 | 66 | 62 | 40 | 69 | 80 | 59 |
| Gender | F | F | F | F | M | M | F | M | M | F |
| Cancer type | Lung | Lung | Lung | Lung | Lung | Lung | Lung | Liver | Liver | Colon |
| Histological Type | AC | AC | AC | AC | SqC | AC | AC | HCC | HCC | AC |
| Stage | IV | IV | IV | IV | IV | IV | IV | IV | IV | IV |
| Disease Stage (Metastatic or recurrent) | M1m (bone, lung) | M0 | M1m (lung, bone) | M1m (lung, brain, bone) | M1m (liver) | M1m (brain, bone) | M1m (bone) | M0 | M1m (lung) | M1m (liver) |
| Prior-treatment | Chemo, targeted Taxol | Chemo Radio therapy | Targeted Taxol | Chemo, Targeted Taxol, Check point inhibitor | Chemo | Chemo Radio therapy chemo | Targeted Taxol | None | None | Chemo |
| ECOG/PS at blood collection | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 3 |
| ECOG/PS 6 months | 2 | 2 | 3 | 1 | 2 | 3 | 0 | 2 | 3 | 2 |
| ECOG/PS 12 months | 2 | 1 | 1 | 2 | 2 | 2 | 3 | 2 | 2 | 2 |
| Estimated survival prior to treatment (months) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 12 | 12 |
| Survival (time) at last evaluation | +21 | +19 | +20 | +19 | −22 | +18 | −17 | −16 | −20 | +16 |
| NK Cell Culture | CTL Culture | ||||||
|---|---|---|---|---|---|---|---|
| Patients | Sitting Number | Total Cell Infused (×106)/Sitting (Mean ± SD) | NK Cell Number (×106)/Sitting (Mean ± SD) | Fold Increase of NK Cell (Mean ± SD) | Total Cell Infused (×106)/Sitting (Mean ± SD) | CTL Number (×106)/Sitting (Mean ± SD) | Fold Increase of CTL (Mean ± SD) |
| PT1 | 2 | 5238.3 ± 6472.2 | 4804.3 ± 6022.7 | 263.6 ± 351.1 | 3431.5 ± 1120.8 | 2359.6 ± 618.4 | 169.09 ± 30.9 |
| PT2 | 2 | 2540.8 ± 1395.3 | 1311.5 ± 597.0 | 16.3 ± 57.5 | 21,106.2 ± 14,390.3 | 11,580.5 ± 8042.0 | 1014.9 ± 704.8 |
| PT3 | 2 | 3637.2 ± 490.7 | 2943.8 ± 208.9 | 251.9 ± 17.9 | 12,178.3 ± 7436.6 | 10,007.9 ± 6532.9 | 863.5 ± 563.7 |
| PT4 | 4 | 1787.6 ± 329.6 | 1553.8 ± 315.9 | 169.1 ± 35.7 | 9550.9 ± 1667.1 | 6389.2 ± 1502.8 | 492.7 ± 85.5 |
| PT5 | 4 | 5935.5 ± 3418.2 | 4244.9 ± 2487.7 | 892.3 ± 1003.9 | 4862.9 ± 3251.5 | 2392.2 ± 1991.2 | 602.6 ± 346.4 |
| PT6 | 2 | 3673.9 ± 461.0 | 3213.0 ± 506.4 | 1451.3 ± 228.7 | 4048.1 ± 723.3 | 2545.6 ± 394.9 | 454.3 ± 70.5 |
| PT7 | 1 | 3697.3 | 3077.9 | 524.3 | 7241.4 | 5288.6 | 595.97 |
| PT8 | 2 | 3405.8 ± 1367.8 | 2816.2 ± 1200.6 | 231.5 ± 143.9 | 6819.0 ± 5094.1 | 5581.5 ± 4283.0 | 284.19 ± 156.2 |
| PT9 | 9 | 1870.6 ± 621.9 | 1658.3 ± 566.7 | 623.4 ± 401.3 | 2377.5 ± 1913.8 | 1059.5 ± 900.8 | 360.27 ± 333.1 |
| PT10 | 1 | 4401.7 | 3555.5 | 962.49 | 8334.7 | 6015.4 | 730.57 |
| Mean value * | 3248.2 | 2616.9 | 548.9 | 6693.5 | 4251.9 | 505.8 | |
| Min value* | 661.8 | 545.6 | 15.3 | 686.7 | 286.9 | 70.9 | |
| Max value* | 10,105.5 | 9062.9 | 2374.9 | 31,281.7 | 17,267.04 | 1513.3 | |
| Items | Means ± Standard Deviation | ||
|---|---|---|---|
| Baseline | 6 months | 12 months | |
| Visual analog fatigue scale | 8.0 ± 0.8 | 4.7 ± 1.1 * | 3.6 ± 0.8 * |
| Eastern Cooperative Oncology Group | 3.2 ± 0.4 | 2.0 ± 0.7 * | 1.9 ± 0.9 * |
| Items | Means ± Standard Deviation | ||
|---|---|---|---|
| Baseline | 6months | 12 months | |
| Global Health Status | 29.2 ± 9.8 | 59.2 ± 8.3 * | 60 ± 6.6 * |
| Physical functioning | 30.6 ± 7.8 | 50.7 ± 15.1 * | 62.0 ± 16.9 * |
| Role functioning | 25.0 ± 11.8 | 50.0 ± 13.6 * | 58.3 ± 8.8 * |
| Emotional Functioning | 34.2 ± 9.2 | 61.7 ± 9.0 * | 76.7 ± 17.0 * |
| Cognitive functioning | 31.7 ± 14.6 | 55.0 ± 13.7 * | 61.7 ± 11.2 * |
| Social functioning | 26.7 ± 11.7 | 51.7 ± 12.3 * | 56.7 ± 14.1 * |
| Symptom scales | |||
| Fatigue | 56.7 ± 9.5 | 32.5 ± 8.3 * | 25.8 ± 7.3 * |
| Nausea and Vomiting | 26.3 ± 9.2 | 13.8 ± 10.9 * | 7.5 ± 6.5 * |
| Pains | 43.8 ± 15.9 | 30.0 ± 8.7 * | 27.5 ± 11.5 * |
| Dyspnea | 40.0 ± 21.1 | 22.5 ± 14.2 * | 20.0 ± 15.8 * |
| Insomnia | 45.0 ± 10.5 | 35.0 ± 12.9 | 37.5 ± 13.2 |
| Appetite loss | 57.5 ± 16.9 | 42.5 ± 20.6 | 27.5 ± 14.2 * |
| Constipation | 27.5 ± 14.2 | 10.0 ± 12.9 * | 5.0 ± 10.5 * |
| Diarrhea | 20.0 ± 10.5 | 2.5 ± 7.9 * | 0 |
| Financial difficulties | 62.5 ± 13.2 | 47.5 ± 7.9 * | 50.0 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liem, N.T.; Van Phong, N.; Kien, N.T.; Anh, B.V.; Huyen, T.L.; Thao, C.T.; Tu, N.D.; Hiep, D.T.; Hoai Thu, D.T.; Nhung, H.T.M. Phase I Clinical Trial Using Autologous Ex Vivo Expanded NK Cells and Cytotoxic T Lymphocytes for Cancer Treatment in Vietnam. Int. J. Mol. Sci. 2019, 20, 3166. https://doi.org/10.3390/ijms20133166
Liem NT, Van Phong N, Kien NT, Anh BV, Huyen TL, Thao CT, Tu ND, Hiep DT, Hoai Thu DT, Nhung HTM. Phase I Clinical Trial Using Autologous Ex Vivo Expanded NK Cells and Cytotoxic T Lymphocytes for Cancer Treatment in Vietnam. International Journal of Molecular Sciences. 2019; 20(13):3166. https://doi.org/10.3390/ijms20133166
Chicago/Turabian StyleLiem, Nguyen Thanh, Nguyen Van Phong, Nguyen Trung Kien, Bui Viet Anh, Truong Linh Huyen, Chu Thi Thao, Nguyen Dac Tu, Doan Trung Hiep, Do Thi Hoai Thu, and Hoang Thi My Nhung. 2019. "Phase I Clinical Trial Using Autologous Ex Vivo Expanded NK Cells and Cytotoxic T Lymphocytes for Cancer Treatment in Vietnam" International Journal of Molecular Sciences 20, no. 13: 3166. https://doi.org/10.3390/ijms20133166
APA StyleLiem, N. T., Van Phong, N., Kien, N. T., Anh, B. V., Huyen, T. L., Thao, C. T., Tu, N. D., Hiep, D. T., Hoai Thu, D. T., & Nhung, H. T. M. (2019). Phase I Clinical Trial Using Autologous Ex Vivo Expanded NK Cells and Cytotoxic T Lymphocytes for Cancer Treatment in Vietnam. International Journal of Molecular Sciences, 20(13), 3166. https://doi.org/10.3390/ijms20133166





