Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability
Abstract
1. Introduction
2. The Unique Microstructure of the VF and Imaging the Structure
3. Histopathology of VF Scarring In Vivo and In Vitro
4. Current Treatment Options and New Injection or Implantation Materials
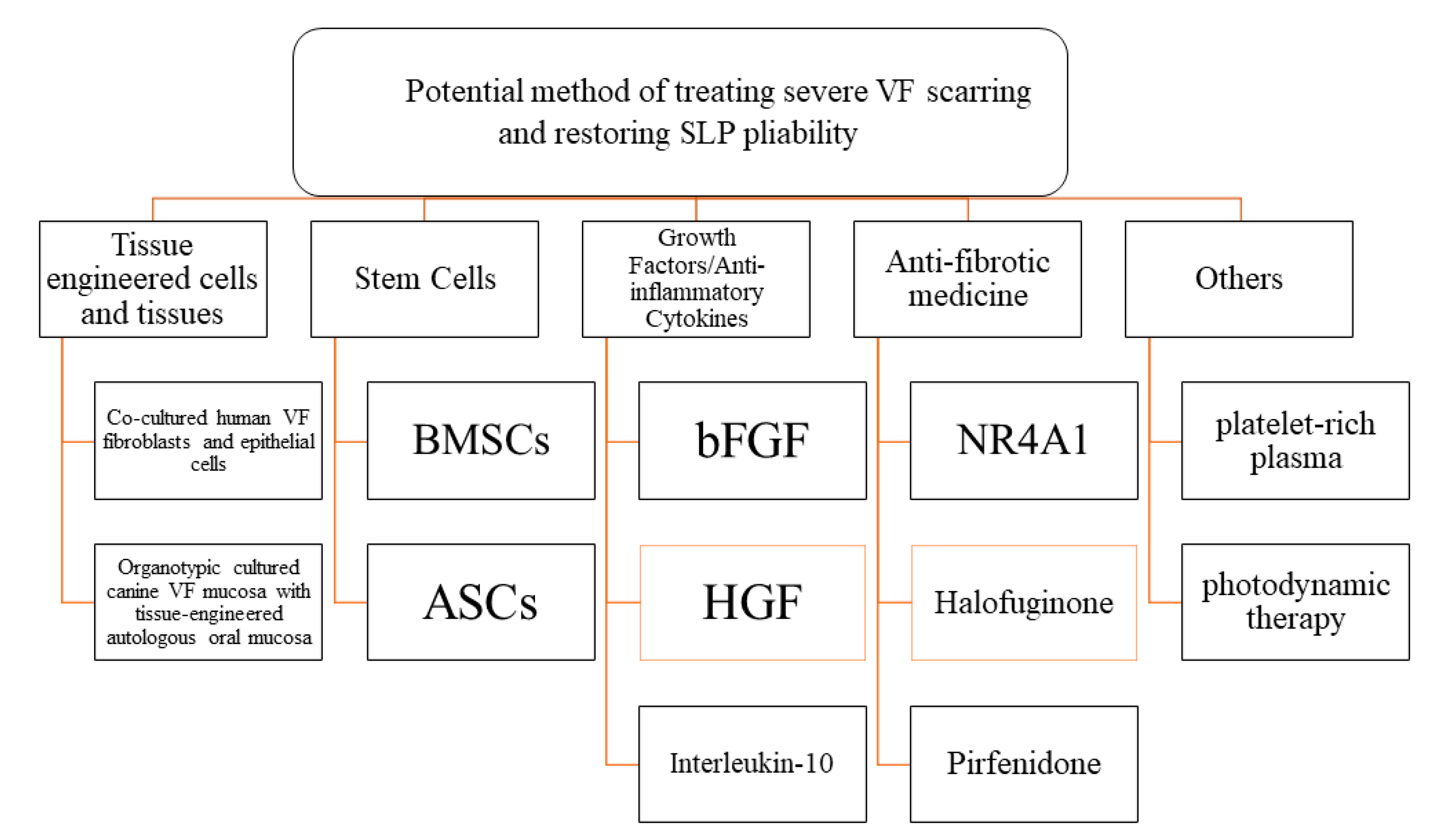
5. New Strategies for VF Scar Including Regenerative Medicine and Tissue Engineering
5.1. Tissue Engineered Cells and Tissues
5.2. Stem-Cell Therapy
5.2.1. BMSCs
5.2.2. ASCs
5.3. Growth Factors and Anti-Inflammatory Cytokines
5.4. Antifibrotic Medicine
5.5. Gene Therapy
5.6. Laser Therapy and Cryotherapy
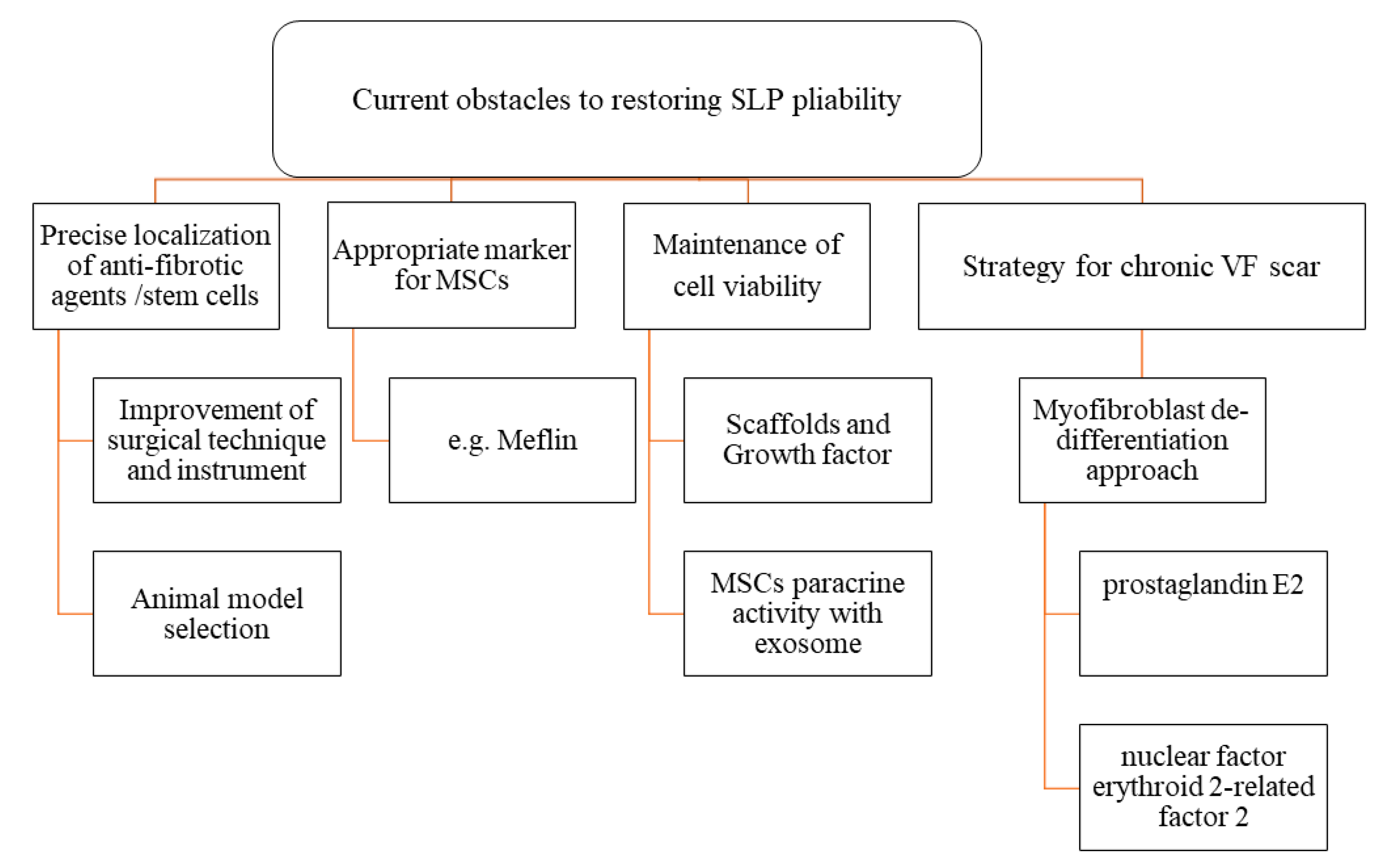
6. Current Obstacles to Restoring SLP Pliability
6.1. Precise Targeting of Antifibrotic Agents and Stem Cells
6.2. Appropriate Markers to Distinguish Normal Fibroblasts from Stem Cells
6.3. Maintenance of Cell Viability and Its Antifibrotic Effect on VF Scars via Paracrine Paradigm
6.4. Strategies for Chronic VF Scarring
7. Conclusions
Funding
Conflicts of Interest
References
- Benninger, M.S.; Alessi, D.; Archer, S.; Bastian, R.; Ford, C.; Koufman, J.; Sataloff, R.T.; Spiegel, J.R.; Woo, P. Vocal fold scarring: Current concepts and management. Otolaryngol. Head Neck Surg. 1996, 115, 474–482. [Google Scholar]
- Thibeault, L.; Gray, S.; Bless, M.; Chan, W.; Ford, C. Histologic and rheologic characterization of vocal fold scarring. J. Voice 2002, 16, 96–104. [Google Scholar] [CrossRef]
- Hirano, S. Current treatment of vocal fold scarring. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 143–147. [Google Scholar] [CrossRef]
- Graupp, M.; Bachna-Rotter, S.; Gerstenberger, C.; Friedrich, G.; Fröhlich-Sorger, E.; Kiesler, K.; Gugatschka, M. The unsolved chapter of vocal fold scars and how tissue engineering could help us solve the problem. Eur. Arch. Otorhinolaryngol. 2016, 273, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, G.; Dikkers, F.G.; Arens, C.; Remacle, M.; Hess, M.; Giovanni, A.; Duflo, S.; Hantzakos, A.; Bachy, V.; Gugatschka, M. European Laryngological Society. Phonosurgery Committee. Vocal fold scars: Current concepts and future directions. Consensus report of the Phonosurgery Committee of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2013, 270, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Magalon, J.; Bertrand, B.; Philandrianos, C.; Veran, J.; Giovanni, A. Cell therapy and vocal fold scarring. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wingstrand, V.L.; Grønhøj Larsen, C.; Jensen, D.H.; Bork, K.; Sebbesen, L.; Balle, J.; Fischer-Nielsen, A.; von Buchwald, C. Mesenchymal Stem Cell Therapy for the Treatment of Vocal Fold Scarring: A Systematic Review of Preclinical Studies. PLoS ONE 2016, 11, e0162349. [Google Scholar] [CrossRef]
- Kumai, Y.; Kobler, J.B.; Herrera, V.L.; Zeitels, S.M. Perspectives on adipose-derived stem/stromal cells as potential treatment for scarred vocal folds: Opportunity and challenges. Curr. Stem Cell Res. Ther. 2010, 5, 175–181. [Google Scholar] [CrossRef]
- Hirano, S.; Mizuta, M.; Kaneko, M.; Tateya, I.; Kanemaru, S.; Ito, J. Regenerative phonosurgical treatments for vocal fold scar and sulcus with basic fibroblast growth factor. Laryngoscope 2013, 123, 2749–2755. [Google Scholar] [CrossRef]
- Ohno, T.; Hirano, S.; Kanemaru, S.; Yamashita, M.; Umeda, H.; Suehiro, A.; Tamura, Y.; Nakamura, T.; Ito, J.; Tabata, Y. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann. Otol. Rhinol. Laryngol. 2007, 116, 762–769. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Hirano, S.; Kitani, Y.; Suehiro, A.; Umeda, H.; Tateya, I.; Kanemaru, S.; Tabata, Y.; Ito, J. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope 2010, 120, 108–113. [Google Scholar] [CrossRef]
- Long, J.L. Tissue engineering for treatment of vocal fold scar. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 521–525. [Google Scholar] [CrossRef]
- Gaston, J.; Thibeault, S.L. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter 2013, 3, e23799. [Google Scholar] [CrossRef]
- Wang, C.T.; Liao, L.J.; Cheng, P.W.; Lo, W.C.; Lai, M.S. Intralesional steroid injection for benign vocal fold disorders: A systematic review and meta-analysis. Laryngoscope 2013, 123, 197–203. [Google Scholar] [CrossRef]
- Mortensen, M. Laryngeal steroid injection for vocal fold scar. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 487–491. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Lopez-Guerra, G.; Park, H.; Kobler, J.B.; Galindo, M.; Aanestad, J.; Mehta, D.D.; Kumai, Y.; Giordano, N.; d’Almeida, A.; et al. Assessment of canine vocal fold function after injection of a new biomaterial designed to treat phonatory mucosal scarring. Ann. Otol. Rhinol. Laryngol. 2011, 120, 175–184. [Google Scholar] [CrossRef]
- Kumai, Y.; Kobler, J.B.; Park, H.; Lopez-Guerra, G.; Karajanagi, S.; Herrera, V.L.; Zeitels, S.M. Crosstalk between adipose-derived stem/stromal cells and vocal fold fibroblasts in vitro. Laryngoscope 2009, 119, 799–805. [Google Scholar] [CrossRef]
- Kumai, Y.; Kobler, J.B.; Park, H.; Galindo, M.; Herrera, V.L.; Zeitels, S.M. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope 2010, 120, 330–337. [Google Scholar] [CrossRef]
- Kodama, H.; Kumai, Y.; Nishimoto, K.; Toya, Y.; Miyamaru, S.; Furushima, S.; Yumoto, E. Potential treatment for vocal fold scar with pirfenidone. Laryngoscope 2018, 128, E171–E177. [Google Scholar] [CrossRef]
- Hirano, M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr. 1974, 26, 89–94. [Google Scholar] [CrossRef]
- Gray, S.D.; Pignatari, S.S.; Harding, P. Morphologic ultrastructure of anchoring fibers in normal vocal fold basement membrane zone. J. Voice 1994, 8, 48–52. [Google Scholar] [CrossRef]
- Story, B.H.; Titze, I.R. Voice simulation with a body-cover model of the vocal folds. J. Acoust. Soc. Am. 1995, 97, 1249–1260. [Google Scholar] [CrossRef]
- Min, Y.B.; Titze, I.R.; Alipour-Haghighi, F. Stress-strain response of the human vocal ligament. Ann. Otol. Rhinol. Laryngol. 1995, 104, 563–569. [Google Scholar] [CrossRef]
- Zeitels, S.M.; Healy, G.B. Laryngology and phonosurgery. N. Engl. J. Med. 2003, 349, 882–892. [Google Scholar] [CrossRef]
- Gray, S.D.; Titze, I.; Chan, R.; Hammond, T. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope 1999, 109, 845–854. [Google Scholar] [CrossRef]
- Gray, S.D.; Titze, I.; Alipour, F.; Hammond, T. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann. Otol. Rhinol. Laryngol. 2000, 109, 77–85. [Google Scholar] [CrossRef]
- Burns, J.A. Optical coherence tomography: Imaging the larynx. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 477–478. [Google Scholar] [CrossRef]
- Nourmahnad, A.; Benboujja, F.; Hartnick, C.J. Using intraoperative optical coherence tomography to image pediatric unilateral vocal fold paralysis. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 72–75. [Google Scholar] [CrossRef]
- Pham, T.T.; Chen, L.; Heidari, A.E.; Chen, J.J.; Zhukhovitskaya, A.; Li, Y.; Patel, U.; Chen, Z.; Wong, B.J.F. Computational analysis of six optical coherence tomography systems for vocal fold imaging: A comparison study. Lasers Surg. Med. 2019. [Google Scholar] [CrossRef]
- Benboujja, F.; Hartnick, C. Clinical and surgical implications of intraoperative optical coherence tomography imaging for benign pediatric vocal fold lesions. Int. J. Pediatr. Otorhinolaryngol. 2018, 114, 111–119. [Google Scholar] [CrossRef]
- Heris, H.K.; Miri, A.K.; Ghattamaneni, N.R.; Li, N.Y.; Thibeault, S.L.; Wiseman, P.W.; Mongeau, L. Microstructural and mechanical characterization of scarred vocal folds. J. Biomech. 2015, 48, 708–711. [Google Scholar] [CrossRef]
- Kishimoto, A.O.; Kishimoto, Y.; Young, D.L.; Zhang, J.; Rowland, I.J.; Welham, N.V. High- and ultrahigh-field magnetic resonance imaging of naïve, injured and scarred vocal fold mucosae in rats. Dis. Model. Mech. 2016, 9, 1397–1403. [Google Scholar] [CrossRef]
- Herrera, V.L.; Viereck, J.C.; Lopez-Guerra, G.; Kumai, Y.; Kobler, J.; Karajanagi, S.; Park, H.; Hillman, R.; Zeitels, S.M. 11.7 Tesla magnetic resonance microimaging of laryngeal tissue architecture. Laryngoscope 2009, 119, 2187–2194. [Google Scholar] [CrossRef]
- Branski, R.C.; Rosen, C.A.; Verdolini, K.; Hebda, P.A. Acute vocal fold wound healing in a rabbit model. Ann. Otol. Rhinol. Laryngol. 2005, 114, 19–24. [Google Scholar] [CrossRef]
- Hirano, S.; Minamiguchi, S.; Yamashita, M.; Ohno, T.; Kanemaru, S.I.; Kitamura, M. Histologic characterization of human scarred vocal folds. J. Voice 2009, 23, 399–407. [Google Scholar] [CrossRef]
- Bless, D.M.; Welham, N.V. Characterization of vocal fold scar formation, prophylaxis and treatment using animal models. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 481–486. [Google Scholar] [CrossRef]
- Alipour, F.; Jaiswal, S.; Vigmostad, S. Vocal fold elasticity in the pig, sheep, and cow larynges. J. Voice 2011, 25, 130–136. [Google Scholar] [CrossRef]
- Alipour, F.; Jaiswal, S. Glottal airflow resistance in excised pig, sheep, and cow larynges. J. Voice 2009, 23, 40–50. [Google Scholar] [CrossRef]
- Lim, X.; Tateya, I.; Tateya, T.; Munoz-Del-Rio, A.; Bless, D.M. Immediate inflammatory response and scar formation in wounded vocal folds. Ann. Otol. Rhinol. Laryngol. 2006, 115, 921–929. [Google Scholar] [CrossRef]
- Branski, R.C.; Rosen, C.A.; Verdolini, K.; Hebda, P.A. Biochemical markers associated with acute vocal fold wound healing: A rabbit model. J. Voice 2005, 19, 283–289. [Google Scholar] [CrossRef]
- Hahn, M.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Quantitative and comparative studies of the vocal fold extracellular matrix II: Collagen. Ann. Otol. Rhinol. Laryngol. 2006, 115, 225–232. [Google Scholar] [CrossRef]
- Hahn, M.S.; Kobler, J.B.; Starcher, B.C.; Zeitels, S.M.; Langer, R. Quantitative and comparative studies of the vocal fold extracellular matrix I: Elastic fibers and hyaluronic acid. Ann. Otol. Rhinol. Laryngol. 2006, 115, 156–164. [Google Scholar] [CrossRef]
- Hahn, M.S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Midmembranous vocal fold lamina propria proteoglycans across selected species. Ann. Otol. Rhinol. Laryngol. 2005, 114, 451–462. [Google Scholar] [CrossRef]
- Regner, M.F.; Robitaille, M.J.; Jiang, J.J. Interspecies comparison of mucosal wave properties using high-speed digital imaging. Laryngoscope 2010, 120, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, S.L.; Rousseau, B.; Welham, N.V.; Hirano, S.; Bless, D.M. Hyaluronan levels in acute vocal fold scar. Laryngoscope 2004, 114, 760–764. [Google Scholar] [CrossRef]
- Welham, N.V.; Lim, X.; Tateya, I.; Bless, D.M. Inflammatory factor profiles one hour following vocal fold injury. Ann. Otol. Rhinol. Laryngol. 2008, 117, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Welham, N.V.; Montequin, D.W.; Tateya, I.; Tateya, T.; Choi, S.H.; Bless, D.M. A rat excised larynx model of vocal fold scar. J. Speech Lang. Hear. Res. 2009, 52, 1008–1020. [Google Scholar] [CrossRef]
- Tateya, I.; Tateya, T.; Lim, X.; Sohn, J.H.; Bless, D.M. Cell production in injured vocal folds: A rat study. Ann. Otol. Rhinol. Laryngol. 2006, 115, 135–143. [Google Scholar] [CrossRef]
- Tateya, T.; Tateya, I.; Sohn, J.H.; Bless, D.M. Histologic characterization of rat vocal fold scarring. Ann. Otol. Rhinol. Laryngol. 2005, 114, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tateya, T.; Tateya, I.; Sohn, J.H.; Bless, D.M. Histological study of acute vocal fold injury in a rat model. Ann. Otol. Rhinol. Laryngol. 2006, 115, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Montequin, D.W.; Tateya, I.; Bless, D.M. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann. Otol. Rhinol. Laryngol. 2004, 113, 767–776. [Google Scholar] [CrossRef]
- Yamashita, M.; Bless, D.M.; Welham, N.V. Surgical method to create vocal fold injuries in mice. Ann. Otol. Rhinol. Laryngol. 2009, 118, 131–138. [Google Scholar] [CrossRef]
- Yamashita, M.; Bless, D.M.; Welham, N.V. Morphological and extracellular matrix changes following vocal fold injury in mice. Cells Tissues Organs 2010, 192, 262–271. [Google Scholar] [CrossRef]
- Kodama, H.; Kumai, Y.; Nishimoto, K.; Toya, Y.; Miyamaru, S.; Furushima, S.; Yumoto, E. The Ferret as a Surgical Model for Vocal Fold Scar Creation and Treatment. Ann. Otol. Rhinol. Laryngol. 2018, 127, 146–154. [Google Scholar] [CrossRef]
- Rousseau, B.; Ge, P.J.; Ohno, T.; French, L.C.; Thibeault, S.L. Extracellular matrix gene expression after vocal fold injury in a rabbit model. Ann. Otol. Rhinol. Laryngol. 2008, 117, 598–603. [Google Scholar] [CrossRef]
- Jetté, M.E.; Hayer, S.D.; Thibeault, S.L. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope 2013, 123, 738–745. [Google Scholar] [CrossRef]
- King, S.N.; Berchtold, C.M.; Thibeault, S.L. Lipopolysaccharide responsiveness in vocal fold fibroblasts. J. Inflamm. 2014, 11, 42. [Google Scholar] [CrossRef][Green Version]
- King, S.N.; Chen, F.; Jetté, M.E.; Thibeault, S.L. Vocal fold fibroblasts immunoregulate activated macrophage phenotype. Cytokine 2013, 61, 228–236. [Google Scholar] [CrossRef]
- Hirano, S.; Bless, D.; Rousseau, B. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope 2004, 114, 548–556. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kishimoto, A.O.; Ye, S.; Kendziorski, C.; Welham, N.V. Modeling fibrosis using fibroblasts isolated from scarred rat vocal folds. Lab. Investig. 2016, 96, 807–816. [Google Scholar] [CrossRef]
- Branco, A.; Bartley, S.M.; King, S.N.; Jetté, M.E.; Thibeault, S.L. Vocal fold myofibroblast profile of scarring. Laryngoscope 2016, 126, E110–E117. [Google Scholar] [CrossRef]
- Graupp, M.; Rinner, B.; Frisch, M.T.; Weiss, G.; Fuchs, J.; Sundl, M.; El-Heliebi, A.; Moser, G.; Kamolz, L.P.; Karbiener, M.; Gugatschka, M. Towards an in vitro fibrogenesis model of human vocal fold scarring. Eur. Arch. Otorhinolaryngol. 2018, 275, 1211–1218. [Google Scholar] [CrossRef]
- Woo, P.; Casper, J.; Colton, R.; Brewer, D. Diagnosis and treatment of persistent dysphonia after laryngeal surgery: A retrospective analysis of 62 patients. Laryngoscope 1994, 104, 1084–1091. [Google Scholar] [CrossRef]
- Campagnolo, A.M.; Tsuji, D.H.; Sennes, L.U.; Imamura, R.; Saldiva, P.H. Histologic study of acute vocal fold wound healing after corticosteroid injection in a rabbit model. Ann. Otol. Rhinol. Laryngol. 2010, 119, 133–139. [Google Scholar] [CrossRef]
- Mukudai, S.; Hiwatashi, N.; Bing, R.; Garabedian, M.; Branski, R.C. Phosphorylation of the glucocorticoid receptor alters SMAD signaling in vocal fold fibroblasts. Laryngoscope 2019, 129, E187–E193. [Google Scholar] [CrossRef]
- Yildiz, M.; Yigit, O.; Sünter, A.V.; Edizer, D.T.; Dursun, N.; Okcu, O. Effects of Intracordal Estradiol and Dexamethasone Injection on Wound Healing in Vocal Fold Injuries. J. Voice 2018. [Google Scholar] [CrossRef]
- Ford, C.N.; Bless, D.M. Collagen injection in the scarred vocal fold. J. Voice 1987, 1, 116–118. [Google Scholar] [CrossRef]
- Ford, C.N.; Staskowski, P.A.; Bless, D.M. Autologous collagen vocal fold injection: A preliminary clinical study. Laryngoscope 1995, 105, 944–948. [Google Scholar] [CrossRef]
- Sataloff, R.T. Autologous fat implantation for vocal fold scar. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 503–506. [Google Scholar] [CrossRef]
- Hertegård, S.; Dahlqvist, A.; Goodyer, E. Viscoelastic measurements after vocal fold scarring in rabbits--short-term results after hyaluronan injection. Acta Otolaryngol. 2006, 126, 758–763. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Mendelsohn, A.H. Hyaluronic acid for the treatment of vocal fold scars. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 498–502. [Google Scholar] [CrossRef]
- Coppoolse, J.M.; Van Kooten, T.G.; Heris, H.K.; Mongeau, L.; Li, N.Y.; Thibeault, S.L.; Pitaro, J.; Akinpelu, O.; Daniel, S.J. An in vivo study of composite microgels based on hyaluronic acid and gelatin for the reconstruction of surgically injured rat vocal folds. J. Speech Lang. Hear. Res. 2014, 57, S658–S673. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef]
- Pitman, M.J.; Cabin, J.A.; Iacob, C.E. Small Intestinal Submucosa Implantation for the Possible Treatment of Vocal Fold Scar, Sulcus, and Superficial Lamina Propria Atrophy. Ann. Otol. Rhinol. Laryngol. 2016, 125, 137–144. [Google Scholar] [CrossRef]
- Cobden, S.B.; Oztürk, K.; Duman, S.; Esen, H.; Aktan, T.M.; Avunduk, M.C.; Elsurer, C. Treatment of Acute Vocal Fold Injury with Platelet-Rich Plasma. J. Voice 2016, 30, 731–735. [Google Scholar] [CrossRef]
- Woo, S.H.; Jeong, H.S.; Kim, J.P.; Koh, E.H.; Lee, S.U.; Jin, S.M.; Kim, D.H.; Sohn, J.H.; Lee, S.H. Favorable vocal fold wound healing induced by platelet-rich plasma injection. Clin. Exp. Otorhinolaryngol. 2014, 7, 47–52. [Google Scholar] [CrossRef]
- Pitman, M.J.; Rubino, S.M.; Cooper, A.L. Temporalis fascia transplant for vocal fold scar and sulcus vocalis. Laryngoscope. 2014, 124, 1653–1658. [Google Scholar] [CrossRef]
- Karle, W.E.; Helman, S.N.; Cooper, A.; Zhang, Y.; Pitman, M.J. Temporalis Fascia Transplantation for Sulcus Vocalis and Vocal Fold Scar: Long-Term Outcomes. Ann. Otol. Rhinol. Laryngol. 2018, 127, 223–228. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Welham, N.V.; Hirano, S. Implantation of atelocollagen sheet for vocal fold scar. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 507–511. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Hirano, S.; Kojima, T.; Kanemaru, S.; Ito, J. Implantation of an atelocollagen sheet for the treatment of vocal fold scarring and sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 2009, 118, 613–620. [Google Scholar] [CrossRef]
- Tse, J.R.; Long, J.L. Microstructure characterization of a decellularized vocal fold scaffold for laryngeal tissue engineering. Laryngoscope 2014, 124, E326–E331. [Google Scholar] [CrossRef]
- Ling, C.; Li, Q.; Brown, M.E.; Kishimoto, Y.; Toya, Y.; Devine, E.E.; Choi, K.O.; Nishimoto, K.; Norman, I.G.; Tsegyal, T.; Jiang, J.J.; et al. Bioengineered vocal fold mucosa for voice restoration. Sci. Transl. Med. 2015, 7, 314ra187. [Google Scholar] [CrossRef]
- Fukahori, M.; Chitose, S.; Sato, K.; Sueyoshi, S.; Kurita, T.; Umeno, H.; Monden, Y.; Yamakawa, R. Regeneration of Vocal Fold Mucosa Using Tissue-Engineered Structures with Oral Mucosal Cells. PLoS ONE 2016, 11, e0146151. [Google Scholar] [CrossRef]
- Kanemaru, S.; Nakamura, T.; Omori, K.; Kojima, H.; Magrufov, A.; Hiratsuka, Y.; Hirano, S.; Ito, J.; Shimizu, Y. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann. Otol. Rhinol. Laryngol. 2003, 112, 915–920. [Google Scholar] [CrossRef]
- Kanemaru, S.; Nakamura, T.; Yamashita, M.; Magrufov, A.; Kita, T.; Tamaki, H.; Tamura, Y.; Iguchi, F.; Kim, T.S.; Kishimoto, M.; et al. Destiny of autologous bone marrow-derived stromal cells implanted in the vocal fold. Ann. Otol. Rhinol. Laryngol. 2005, 114, 907–912. [Google Scholar] [CrossRef]
- Hertegård, S.; Cedervall, J.; Svensson, B.; Forsberg, K.; Maurer, F.H.; Vidovska, D.; Olivius, P.; Ahrlund-Richter, L.; Le Blanc, K. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope 2006, 116, 1248–1254. [Google Scholar] [CrossRef]
- Svensson, B.; Nagubothu, S.R.; Cedervall, J.; Chan, R.W.; Le Blanc, K.; Kimura, M.; Ährlund-Richter, L.; Tolf, A.; Hertegård, S. Injection of human mesenchymal stem cells improves healing of vocal folds after scar excision—A xenograft analysis. Laryngoscope 2011, 121, 2185–2190. [Google Scholar] [CrossRef]
- Ohno, S.; Hirano, S.; Tateya, I.; Kanemaru, S.; Umeda, H.; Suehiro, A.; Kitani, Y.; Kishimoto, Y.; Kojima, T.; Nakamura, T.; Ito, J. Atelocollagen sponge as a stem cell implantation scaffold for the treatment of scarred vocal folds. Ann. Otol. Rhinol. Laryngol. 2009, 118, 805–810. [Google Scholar] [CrossRef]
- Hiwatashi, N.; Hirano, S.; Suzuki, R.; Kawai, Y.; Mizuta, M.; Kishimoto, Y.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Dezawa, M.; Ito, J. Comparison of ASCs and BMSCs combined with atelocollagen for vocal fold scar regeneration. Laryngoscope 2016, 126, 1143–1150. [Google Scholar] [CrossRef]
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R.C. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-β1-stimulated vocal fold fibroblasts. Laryngoscope 2017, 127, E35–E41. [Google Scholar] [CrossRef]
- Nagubothu, S.R.; Sugars, R.V.; Tudzarovski, N.; Andrén, A.T.; Bottai, M.; Davies, L.C.; Hertegård, S.; Le Blanc, K. Mesenchymal stromal cells modulate tissue repair responses within the injured vocal fold. Laryngoscope 2019. [Google Scholar] [CrossRef]
- Kim, C.S.; Choi, H.; Park, K.C.; Kim, S.W.; Sun, D.I. The Ability of Human Nasal Inferior Turbinate-Derived Mesenchymal Stem Cells to Repair Vocal Fold Injuries. Otolaryngol. Head Neck Surg. 2018, 159, 335–342. [Google Scholar] [CrossRef]
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R.C. Stem Cell-Mediated Paracrine Signaling Alters Fibroplasia in Human Vocal Fold Fibroblasts in Vitro. Ann. Otol. Rhinol. Laryngol. 2017, 126, 581–588. [Google Scholar] [CrossRef]
- Lee, B.J.; Wang, S.G.; Lee, J.C.; Jung, J.S.; Bae, Y.C.; Jeong, H.J.; Kim, H.W.; Lorenz, R.R. The prevention of vocal fold scarring using autologous adipose tissue-derived stromal cells. Cells Tissues Organs 2006, 184, 198–204. [Google Scholar] [CrossRef]
- Valerie, A.; Vassiliki, K.; Irini, M.; Nikolaos, P.; Karampela, E.; Apostolos, P. Adipose-Derived Mesenchymal Stem Cells in the Regeneration of Vocal Folds: A Study on a Chronic Vocal Fold Scar. Stem Cells Int. 2016. [Google Scholar] [CrossRef]
- King, S.N.; Woo, J.H.; Tang, S.; Thibeault, S.L. Macrophage Response to Allogeneic Adipose Tissue-Derived Stromal Cells in Hyaluronan-Based Hydrogel in a Porcine Vocal Fold Injury Model. Ann. Otol. Rhinol. Laryngol. 2017, 126, 463–477. [Google Scholar] [CrossRef]
- Goel, A.N.; Gowda, B.S.; Veena, M.S.; Shiba, T.L.; Long, J.L. Adipose-Derived Mesenchymal Stromal Cells Persist in Tissue-Engineered Vocal Fold Replacement in Rabbits. Ann. Otol. Rhinol. Laryngol. 2018, 127, 962–968. [Google Scholar] [CrossRef]
- Mattei, A.; Magalon, J.; Bertrand, B.; Grimaud, F.; Revis, J.; Velier, M.; Veran, J.; Dessi, P.; Sabatier, F.; Giovanni, A. Autologous adipose-derived stromal vascular fraction and scarred vocal folds: First clinical case report. Stem Cell Res. Ther. 2018, 9, 202. [Google Scholar] [CrossRef]
- De Bonnecaze, G.; Chaput, B.; Woisard, V.; Uro-Coste, E.; Swider, P.; Vergez, S.; Serrano, E.; Casteilla, L.; Planat-Benard, V. Adipose stromal cells improve healing of vocal fold scar: Morphological and functional evidences. Laryngoscope 2016, 126, E278–E285. [Google Scholar] [CrossRef]
- Morisaki, T.; Kishimoto, Y.; Tateya, I.; Kawai, Y.; Suzuki, R.; Tsuji, T.; Hiwatashi, N.; Nakamura, T.; Omori, K.; Kitano, H.; Takeuchi, H.; Hirano, S. Adipose-derived mesenchymal stromal cells prevented rat vocal fold scarring. Laryngoscope 2018, 128, E33–E40. [Google Scholar] [CrossRef]
- Hirano, S.; Nagai, H.; Tateya, I.; Tateya, T.; Ford, C.N.; Bless, D.M. Regeneration of aged vocal folds with basic fibroblast growth factor in a rat model: A preliminary report. Ann. Otol. Rhinol. Laryngol. 2005, 114, 304–308. [Google Scholar] [CrossRef]
- Hirano, S.; Bless, D.; Heisey, D.; Ford, C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope 2003, 113, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Hirano, S.; Suehiro, A.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2009, 118, 606–611. [Google Scholar] [CrossRef]
- Hirano, S.; Kawamoto, A.; Tateya, I.; Mizuta, M.; Kishimoto, Y.; Hiwatashi, N.; Kawai, Y.; Tsuji, T.; Suzuki, R.; Kaneko, M.; et al. A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus. J. Tissue Eng. Regen. Med. 2018, 12, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, N.; Hirano, S.; Mizuta, M.; Kobayashi, T.; Kawai, Y.; Kanemaru, S.I.; Nakamura, T.; Ito, J.; Kawai, K.; Suzuki, S. The efficacy of a novel collagen-gelatin scaffold with basic fibroblast growth factor for the treatment of vocal fold scar. J. Tissue Eng. Regen. Med. 2017, 11, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Kawai, Y.; Tsuji, T.; Hiwatashi, N.; Kishimoto, Y.; Tateya, I.; Nakamura, T.; Hirano, S. Prevention of vocal fold scarring by local application of basic fibroblast growth factor in a rat vocal fold injury model. Laryngoscope 2017, 127, E67–E74. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.J.; Park, J.H.; Kim, J.W.; Park, K.N.; Lee, J.Y.; Kim, H.K.; Lee, S.W. The Efficacy of Fibroblast Growth Factor for the Treatment of Chronic Vocal Fold Scarring: From Animal Model to Clinical Application. Clin. Exp. Otorhinolaryngol. 2017, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Erndt-Marino, J.; Diaz-Rodriguez, P.; Kulwatno, J.; Jimenez-Vergara, A.C.; Thibeault, S.L.; Hahn, M.S. In vitro evaluation of anti-fibrotic effects of select cytokines for vocal fold scar treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1056–1067. [Google Scholar] [CrossRef]
- Zhou, H.; Felsen, D.; Sandulache, V.C.; Amin, M.R.; Kraus, D.H.; Branski, R.C. Prostaglandin (PG)E2 exhibits antifibrotic activity in vocal fold fibroblasts. Laryngoscope 2011, 121, 1261–1265. [Google Scholar] [CrossRef]
- Hiwatashi, N.; Mukudai, S.; Bing, R.; Branski, R.C. The effects of cytosporone-B, a novel antifibrotic agent, on vocal fold fibroblasts. Laryngoscope 2018, 128, E425–E428. [Google Scholar] [CrossRef]
- Allen, J. Response of an ovine laryngeal injury model to a novel fibrosis inhibitor. ANZ J. Surg. 2017, 87, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Siavelis, J.C.; Bourdakou, M.M.; Athanasiadis, E.I.; Spyrou, G.M.; Nikita, K.S. Bioinformatics methods in drug repurposing for Alzheimer’s disease. Brief. Bioinform. 2015, 17, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Ma, Z.; Chibale, K. Drug repositioning in the treatment of malaria and TB. Future Med. Chem. 2011, 3, 1413–1426. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef]
- Jahchan, N.S.; Dudley, J.T.; Mazur, P.K.; Flores, N.; Yang, D.; Palmerton, A.; Zmoos, A.F.; Vaka, D.; Tran, K.Q.; Zhou, M.; et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013, 3, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endo toxic shock. Eur. J. Pharm. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Misra, H.P.; Rabideau, C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxylradicals. Mol. Cell. Biochem. 2000, 204, 119–126. [Google Scholar] [CrossRef]
- Kaneko, M.; Inoue, H.; Nakazawa, R.; Azuma, N.; Suzuki, M.; Yamauchi, S.; Margolin, S.B.; Tsubota, K.; Saito, I. Pirfenidone induces intercellular adhesionmolecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin. ExpImmunol. 1998, 113, 72–76. [Google Scholar]
- Iyer, S.N.; Margolin, S.B.; Hyde, D.M.; Giri, S.N. Lung fibrosis is ameliorated by pirfenidone fed in diet after the second doseina three-dose bleomycin-hamster model. Exp. Lung Res. 1998, 24, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef]
- Raghu, G.; Johnson, W.C.; Lockhart, D.; Mageto, Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone:results of a prospective, open-label PhaseII study. Am. J. Respir. Crit. Care Med. 1999, 159, 1061–1069. [Google Scholar] [CrossRef]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef]
- Macías-Barragán, J.; Sandoval-Rodríguez, A.; Navarro-Partida, J.; Armendáriz-Borunda, J. The multifaceted role of pirfenidone and its novel targets. Fibrogenes. Tissue Repair 2010, 3, 16. [Google Scholar]
- Paul, B.C.; Rafii, B.Y.; Gandonu, S.; Bing, R.; Born, H.; Amin, M.R.; Branski, R.C. Smad3: An emerging target for vocal fold fibrosis. Laryngoscope 2014, 124, 2327–2331. [Google Scholar] [CrossRef]
- Branski, R.C.; Bing, R.; Kraja, I.; Amin, M.R. The role of Smad3 in the fibrotic phenotype in human vocal fold fibroblasts. Laryngoscope 2016, 126, 1151–1156. [Google Scholar] [CrossRef]
- Kraja, I.; Bing, R.; Hiwatashi, N.; Rousseau, B.; Nalband, D.; Kirshenbaum, K.; Branski, R.C. Preliminary study of a novel transfection modality for in vivo siRNA delivery to vocal fold fibroblasts. Laryngoscope 2017, 127, E231–E237. [Google Scholar] [CrossRef]
- Hiwatashi, N.; Kraja, I.; Benedict, P.A.; Dion, G.R.; Bing, R.; Rousseau, B.; Amin, M.R.; Nalband, D.M.; Kirshenbaum, K.; Branski, R.C. Nanoparticle delivery of RNA-based therapeutics to alter the vocal fold tissue response to injury. Laryngoscope 2018, 128, E178–E183. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Li, Q.; Yan, H.; Zhou, B.; Gao, Y.; Li, J. Non-Coding RNAs: The New Insight on Hypertrophic Scar. J. Cell Biochem. 2017, 118, 1965–1968. [Google Scholar] [CrossRef]
- Dong, B.S.; Shi, M.J.; Su, S.B.; Zhang, H. Insight into long noncoding competing endogenous RNA networks in hepatic fibrosis: The potential implications for mechanism and therapy. Gene 2019, 687, 255–260. [Google Scholar] [CrossRef]
- Sheu, M.; Sridharan, S.; Paul, B.; Mallur, P.; Gandonu, S.; Bing, R.; Zhou, H.; Branski, R.C.; Amin, M.R. The utility of the potassium titanyl phosphate laser in modulating vocal fold scar in a rat model. Laryngoscope 2013, 123, 2189–2194. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, R.; Wei, C. Potassium titanyl phosphate laser-induced inflammatory response and extracellular matrix turnover in rabbit vocal fold scar. Eur. Arch. Otorhinolaryngol. 2018, 275, 1525–1532. [Google Scholar] [CrossRef]
- Lou, Z.; Zhang, C.; Gong, T.; Xue, C.; Scholp, A.; Jiang, J.J. Wound-healing effects of 635-nm low-level laser therapy on primary human vocal fold epithelial cells: An in vitro study. Lasers Med. Sci. 2019, 34, 547–554. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, C.; Kang, J.; Lou, Z.; Scholp, A.; Jiang, J.J. The effects of cryotherapy on vocal fold healing in a rabbit model. Laryngoscope 2019, 129, E151–E157. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Chou, A.; Gong, T.; Devine, E.E.; Jiang, J.J. Photodynamic therapy induces antifibrotic alterations in primary human vocal fold fibroblasts. Laryngoscope 2018, 128, E323–E331. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Enomoto, A.; Hara, A.; Asai, N.; Kobayashi, T.; Horinouchi, A.; Maruyama, S.; Ishikawa, Y.; Nishiyama, T.; Kiyoi, H.; et al. Identification of Meflin as a Potential Marker for Mesenchymal Stromal Cells. Sci. Rep. 2016, 6, 22288. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.; San-Marina, S.; Oldenburg, M.S.; Ekbom, D.; Madden, B.J.; Charlesworth, M.C.; Janus, J.R. Histone Variants as Stem Cell Biomarkers for Long-Term Injection Medialization Laryngoplasty. Laryngoscope 2018, 128, E402–E408. [Google Scholar] [CrossRef]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Ahrlund-Richter, L.; Svensson, B.; Forsgren, K.; Maurer, F.H.; Vidovska, D.; Hertegård, S. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: Short-term results. Laryngoscope 2007, 117, 2075–2081. [Google Scholar]
- Parekkadan, B.; van Poll, D.; Suganuma, K.; Carter, E.A.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2007, 2, e941. [Google Scholar] [CrossRef]
- Parekkadan, B.; van Poll, D.; Megeed, Z.; Kobayashi, N.; Tilles, A.W.; Berthiaume, F.; Yarmush, M.L. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 363, 247–252. [Google Scholar] [CrossRef]
- Zhao, D.C.; Lei, J.X.; Chen, R.; Yu, W.H.; Zhang, X.M.; Li, S.N.; Xiang, P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J. Gastroenterol. 2005, 11, 3431–3440. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 2196–2203. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Van Poll, D.; Parekkadan, B.; Borel Rinkes, I.H.M.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell therapy for protection and repair of injured vital organs. Cell. Mol. Bioeng. 2008, 1, 42–50. [Google Scholar] [CrossRef]
- Wettlaufer, S.H.; Scott, J.P.; McEachin, R.C.; Peters-Golden, M.; Huang, S.K. Reversal of the Transcriptome by Prostaglandin E2 during Myofibroblast Dedifferentiation. Am. J. Respir. Cell Mol. Biol. 2016, 54, 114–127. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Liu, T.; Chen, X. Reversal of myofibroblast differentiation: A review. Eur. J. Pharmacol. 2014, 734, 83–90. [Google Scholar] [CrossRef]
- Artaud-Macari, E.; Goven, D.; Brayer, S.; Hamimi, A.; Besnard, V.; Marchal-Somme, J.; Ali, Z.E.; Crestani, B.; Kerdine-Römer, S.; Boutten, A.; et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 2013, 18, 66–79. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumai, Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. Int. J. Mol. Sci. 2019, 20, 2551. https://doi.org/10.3390/ijms20102551
Kumai Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. International Journal of Molecular Sciences. 2019; 20(10):2551. https://doi.org/10.3390/ijms20102551
Chicago/Turabian StyleKumai, Yoshihiko. 2019. "Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability" International Journal of Molecular Sciences 20, no. 10: 2551. https://doi.org/10.3390/ijms20102551
APA StyleKumai, Y. (2019). Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. International Journal of Molecular Sciences, 20(10), 2551. https://doi.org/10.3390/ijms20102551




