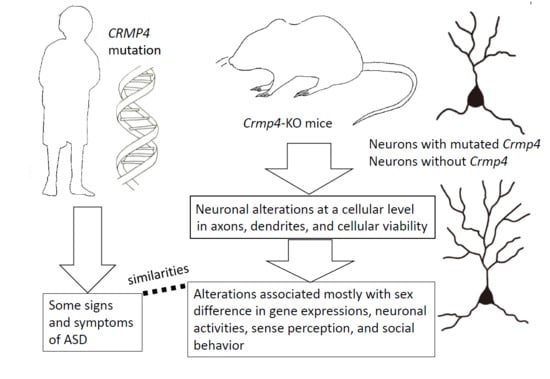
Crmp4-KO Mice as an Animal Model for Investigating Certain Phenotypes of Autism Spectrum Disorders
Abstract
1. Introduction
2. Identification of CRMP4
3. The Regulatory Mechanisms Suggested for CRMP4
4. Potential Involvement of CRMPs Including CRMP4 in Neurodevelopmental Disorders
5. Behavioral and Perceptual Abnormalities Observed in Crmp4-KO Mice
5.1. Impairments in Social Behavior
5.2. Abnormalities in Sensory Perception
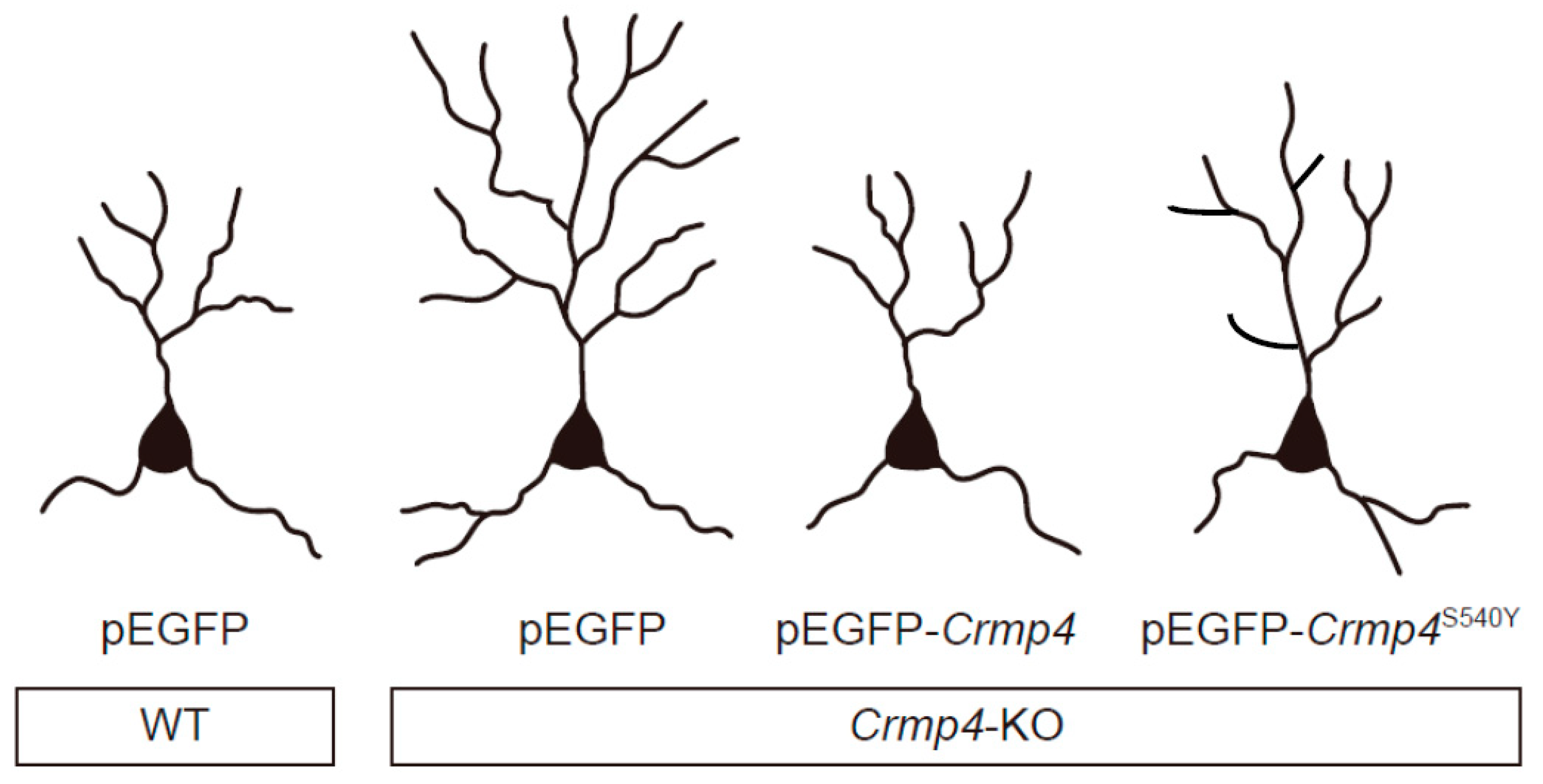
6. Altered Dendritic Arborization in Crmp4-KO Mice and Crmp4-Knockdown (KD) Neurons
7. Altered Expressions of Genes Related to Excitatory and Inhibitory Synaptic Transmission in the Brain of Crmp4-KO Mice
8. Sex-Specific Phenotypes Observed in Crmp4-KO Mice and Other Animal Models of ASD
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | attention-deficit/hyperactivity disorder |
| AMPA | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ASD | autism spectrum disorder |
| AVPV | anteroventral periventricular nucleus |
| CRMP | collapsin response mediator protein |
| DRP | dihydropyrimidase; DPYSL3, dihydropyrimidase-like 3 |
| EA | ethyl acetate |
| EPL | external plexiform layer |
| FMR1 | fragile X mental retardation 1 gene |
| GCL | granule cell layer |
| GL | glomerular layer |
| GluR1 | glutamate receptor 1 |
| GluT1 | glutamate transporter 1 |
| KO | knockout |
| MAI | myelin-associated inhibitor |
| MCL | mitral cell layer |
| NMDA | N-methyl-D-aspartate |
| OB | olfactory bulb |
| RT | room temperature |
| TERT-tg mice | telomerase reverse transcriptase-overexpressing transgenic mice |
| TOAD-64 | turned on after division 64 |
| TUC-4 | TOAD-64/Ulip-1/CRMP4 |
| Ulip-1 | Ulip-1 |
| UV | ultrasonic vocalization |
| vGluT1 | vesicular glutamate transporter 1 |
| WT | wild type |
References
- Charrier, E.; Reibel, S.; Rogemond, V.; Aguera, M.; Thomasset, N.; Honnorat, J. Collapsin response mediator proteins (CRMPs): Involvement in nervous system development and adult neurodegenerative disorders. Mol. Neurol. 2003, 28, 51–64. [Google Scholar] [CrossRef]
- Schmidt, E.F.; Strittmatter, S.M. The CRMP family of proteins and their role in Sema3A signaling. Adv. Exp. Med. Biol. 2007, 600, 1–11. [Google Scholar] [PubMed]
- Quach, T.T.; Honnorat, J.; Kolattukudy, P.E.; Khanna, R.; Duchemin, A.M. CRMPs: Critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol. Psychiatry. 2015, 20, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Venkova, K.; Christov, A.; Gunning, W.; Park, J. Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol. 2011, 43, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Takahashi, A.; Takao, K.; Yamamoto, T.; Kolattukudy, P.; Miyakawa, T.; Goshima, Y. Mice lacking collapsin response mediator protein 1 manifest hyperactivity, impaired learning and memory, and impaired prepulse inhibition. Front. Behav. Neurosci. 2013, 7, 216. [Google Scholar] [CrossRef]
- Lee, H.; Joo, J.; Nah, S.S.; Kim, J.W.; Kim, H.K.; Kwon, J.T.; Lee, H.Y.; Kim, Y.O.; Kim, H.J. Changes in Dpysl2 expression are associated with prenatally stressed rat offspring and susceptibility to schizophrenia in humans. Int. J. Mol. Med. 2015, 35, 1574–1586. [Google Scholar] [CrossRef]
- Pham, X.; Song, G.; Lao, S.; Goff, L.; Zhu, H.; Valle, D.; Avramopoulos, D. The DPYSL2 gene connects mTOR and schizophrenia. Transl. Psychiatry 2016, 6, e933. [Google Scholar] [CrossRef]
- Tsutiya, A.; Nakano, Y.; Hansen-Kiss, E.; Kelly, B.; Nishihara, M.; Goshima, Y.; Corsmeier, D.; White, P.; Herman, G.E.; Ohtani-Kaneko, R. Human CRMP4 mutation and disrupted Crmp4 expression in mice are associated with ASD characteristics and sexual dimorphism. Sci. Rep. 2017, 7, 16812. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohshima, T.; Sasaki, Y.; Suzuki, H.; Yanai, S.; Yamashita, N.; Nakamura, F.; Takei, K.; Ihara, Y.; Mikoshiba, K.; et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: Implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 2005, 10, 165–179. [Google Scholar] [CrossRef]
- Toba, J.; Nikkuni, M.; Ishizeki, M.; Yoshii, A.; Watamura, N.; Inoue, T.; Ohshima, T. PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem. Biophys. Res. Commun. 2016, 473, 1039–1044. [Google Scholar] [CrossRef]
- Kim, A.E.; Kang, P.; Bucelli, R.C.; Ferguson, C.J.; Schmidt, R.E.; Varadhachary, A.S.; Day, G.S. Autoimmune encephalitis with multiple autoantibodies: A diagnostic and therapeutic challenge. Neurologist 2018, 23, 55–59. [Google Scholar] [CrossRef]
- Fujisawa, H.; Ohtani-Kaneko, R.; Naiki, M.; Okada, T.; Masuko, K.; Yudoh, K.; Suematsu, N.; Okamoto, K.; Nishioka, K.; Kato, T. Involvement of post-translational modification of neuronal plasticity-related proteins in hyperalgesia revealed by a proteomic analysis. Proteomics 2008, 8, 1706–1719. [Google Scholar] [CrossRef]
- Piekarz, A.D.; Due, M.R.; Khanna, M.; Wang, B.; Ripsch, M.S.; Wang, R.; Meroueh, S.O.; Vasko, M.R.; White, F.A.; Khanna, R. CRMP-2 peptide mediated decrease of high and low voltage-activated calcium channels, attenuation of nociceptor excitability, and anti-nociception in a model of AIDS therapy-induced painful peripheral neuropathy. Mol. Pain 2012, 8, 54. [Google Scholar] [CrossRef]
- Harada, S.; Matsuura, W.; Takano, M.; Tokuyama, S. Proteomic profiling in the spinal cord and sciatic nerve in a global cerebral ischemia-induced mechanical allodynia mouse model. Biol. Pharm. Bull. 2016, 39, 230–238. [Google Scholar] [CrossRef]
- Lawal, M.F.; Olotu, F.A.; Agoni, C.; Soliman, M.E. Exploring the C-Terminal Tail Dynamics: Structural and Molecular Perspectives into the Therapeutic Activities of Novel CRMP-2 Inhibitors, Naringenin and Naringenin-7-O-glucuronide, in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2018, 15, e1800437. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Matthes, D.J.; O’Connor, T.P.; Patel, N.H.; Admon, A.; Bentley, D.; Goodman, C.S. Fasciclin IV: Sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 1992, 9, 831–845. [Google Scholar] [CrossRef]
- Luo, Y.; Raible, D.; Raper, J.A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75, 217–227. [Google Scholar] [CrossRef]
- Raper, J.A. Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol. 2000, 10, 88–94. [Google Scholar] [CrossRef]
- Fenstermaker, V.; Chen, Y.; Ghosh, A.; Yuste, R. Regulation of dendritic length and branching by semaphorin 3A. J. Neurobiol. 2004, 58, 403–412. [Google Scholar] [CrossRef]
- Pascual, M.; Pozas, E.; Soriano, E. Role of class 3 semaphorins in the development and maturation of the septohippocampal pathway. Hippocampus 2005, 15, 184–202. [Google Scholar] [CrossRef]
- Yoshida, Y. Semaphorin signaling in vertebrate neural circuit assembly. Front. Mol. Neurosci. 2012, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Goshima, Y.; Nakamura, F.; Strittmatter, P.; Strittmatter, S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 1995, 376, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Minturn, J.E.; Fryer, H.J.; Geschwind, D.H.; Hockfield, S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J. Neurosci. 1995, 15, 6757–6766. [Google Scholar] [CrossRef]
- Minturn, J.E.; Geschwind, D.H.; Fryer, H.J.; Hockfield, S. Early postmitotic neurons transiently express TOAD-64, a neural specific protein. J. Comp. Neurol. 1995, 355, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Byk, T.; Dobransky, T.; Cifuentes-Diaz, C.; Sobel, A. Identification and molecular characterization of Unc-33-like phosphoprotein (Ulip), a putative mammalian homolog of the axonal guidance-associated unc-33 gene product. J. Neurosci. 1996, 16, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, N.; Matsuda, K.; Sakata, S.; Tamaki, N.; Sasaki, M.; Nonaka, M. A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene 1996, 180, 157–163. [Google Scholar] [CrossRef]
- Yamashita, N.; Uchida, Y.; Ohshima, T.; Hirai, S.; Nakamura, F.; Taniguchi, M.; Mikoshiba, K.; Honnorat, J.; Kolattukudy, P.; Thomasset, N.; et al. Collapsin response mediator protein 1 mediates reelin signaling in cortical neuronal migration. J. Neurosci. 2006, 26, 13357–13362. [Google Scholar] [CrossRef]
- Alabed, Y.Z.; Poolm, M.; Tone, S.O.; Sutherland, C.; Fournier, A.E. GSK3 beta regulates myelin-dependent axon outgrowth inhibition through CRMP4. J. Neurosci. 2010, 30, 5635–5643. [Google Scholar] [CrossRef] [PubMed]
- Charrier, E.; Mosinger, B.; Meissirel, C.; Aguera, M.; Rogemond, V.; Reibel, S.; Salin, P.; Chounlamountri, N.; Perrot, V.; Belin, M.F.; et al. Transient alterations in granule cell proliferation, apoptosis and migration in postnatal developing cerebellum of CRMP1−/− mice. Genes Cells 2006, 11, 1337–1352. [Google Scholar] [CrossRef]
- Yamashita, N.; Morita, A.; Uchida, Y.; Nakamura, F.; Usui, H.; Ohshima, T.; Taniguchi, M.; Honnorat, J.; Thomasset, N.; Takei, K.; et al. Regulation of spine development by semaphorin3A through cyclin-dependent kinase 5 phosphorylation of collapsin response mediator protein 1. J. Neurosci. 2007, 27, 12546–12554. [Google Scholar] [CrossRef]
- Su, K.Y.; Chien, W.L.; Fu, W.M.; Yu, I.S.; Huang, H.P.; Huang, P.H.; Lin, S.R.; Shih, J.Y.; Lin, Y.L.; Hsueh, Y.P.; et al. Mice deficient in collapsin response mediator protein-1 exhibit impaired long-term potentiation and impaired spatial learning and memory. J. Neurosci. 2007, 27, 2513–2524. [Google Scholar] [CrossRef]
- Yamashita, N.; Goshima, Y. Collapsin response mediator proteins regulate neuronal development and plasticity by switching their phosphorylation status. Mol. Neurobiol. 2012, 45, 234–246. [Google Scholar] [CrossRef]
- Arimura, N.; Inagaki, N.; Chihara, K.; Ménager, C.; Nakamura, N.; Amano, M.; Iwamatsu, A.; Goshima, Y.; Kaibuchi, K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J. Biol. Chem. 2000, 275, 23973–23980. [Google Scholar] [CrossRef]
- Arimura, N.; Ménager, C.; Kawano, Y.; Yoshimura, T.; Kawabata, S.; Hattori, A.; Fukata, Y.; Amano, M.; Goshima, Y.; Inagaki, M.; et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell Biol. 2005, 25, 9973–9984. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kawano, Y.; Arimura, N.; Kawabata, S.; Kikuchi, A.; Kaibuchi, K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 2005, 120, 137–149. [Google Scholar] [CrossRef]
- Cole, A.R.; Causeret, F.; Yadirgi, G.; Hastie, C.J.; McLauchlan, H.; McManus, E.J.; Hernández, F.; Eickholt, B.J.; Nikolic, M.; Sutherland, C. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J. Biol. Chem. 2006, 281, 16591–16598. [Google Scholar] [CrossRef]
- Alabed, Y.Z.; Pool, M.; Ong Tone, S.; Fournier, A.E. Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J. Neurosci. 2007, 27, 1702–1711. [Google Scholar] [CrossRef]
- Tanaka, H.; Morimura, R.; Ohshima, T. Dpysl2 (CRMP2) and Dpysl3 (CRMP4) phosphorylation by Cdk5 and DYRK2 is required for proper positioning of Rohon-Beard neurons and neural crest cells during neurulation in zebrafish. Dev. Biol. 2012, 370, 223–236. [Google Scholar] [CrossRef]
- Morimura, R.; Nozawa, K.; Tanaka, H.; Ohshima, T. Phosphorylation of Dpsyl2 (CRMP2) and Dpsyl3 (CRMP4) is required for positioning of caudal primary motor neurons in the zebrafish spinal cord. Dev. Neurobiol. 2013, 73, 911–920. [Google Scholar] [CrossRef]
- Kowara, R.; Chen, Q.; Milliken, M.; Chakravarthy, B. Calpain-mediated truncation of dihydropyrimidinase-like 3 protein (DPYSL3) in response to NMDA and H2O2 toxicity. J. Neurochem. 2005, 95, 466–474. [Google Scholar] [CrossRef]
- Kowara, R.; Moraleja, K.L.; Chakravarthy, B. Involvement of nitric oxide synthase and ROS-mediated activation of L-type voltage-gated Ca2+ channels in NMDA-induced DPYSL3 degradation. Brain Res. 2006, 1119, 40–49. [Google Scholar] [CrossRef]
- Kowara, R.; Moraleja, K.L.; Chakravarthy, B. PLA(2) signaling is involved in calpain-mediated degradation of synaptic dihydropyrimidinase-like 3 protein in response to NMDA excitotoxicity. Neurosci. Lett. 2008, 430, 197–202. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.W.; Liu, S.; Hu, K.; Wang, C.; He, Q.; Li, M. Calpain-truncated CRMP-3 and -4 contribute to potassium deprivation-induced apoptosis of cerebellar granule neurons. Proteomics 2009, 9, 3712–3728. [Google Scholar] [CrossRef]
- Quinn, C.C.; Chen, E.; Kinjo, T.G.; Kelly, G.; Bell, A.W.; Elliott, R.C.; McPherson, P.S.; Hockfield, S. TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J. Neurosci. 2003, 23, 2815–2823. [Google Scholar] [CrossRef]
- Yuasa-Kawada, J.; Suzuki, R.; Kano, F.; Ohkawara, T.; Murata, M.; Noda, M. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur. J. Neurosci. 2003, 17, 2329–2343. [Google Scholar] [CrossRef]
- Tan, M.; Cha, C.; Ye, Y.; Zhang, J.; Li, S.; Wu, F.; Gong, S.; Guo, G. CRMP4 and CRMP2 interact to coordinate cytoskeleton dynamics, regulating growth cone development and axon elongation. Neural. Plast. 2015, 947423. [Google Scholar] [CrossRef]
- Seki, T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J. Neurosci. Res. 2002, 70, 327–334. [Google Scholar] [CrossRef]
- Cnops, L.; Hu, T.T.; Burnat, K.; Van der Gucht, E.; Arckens, L. Age-dependent alterations in CRMP2 and CRMP4 protein expression profiles in cat visual cortex. Brain Res. 2006, 1088, 109–119. [Google Scholar] [CrossRef]
- Tsutiya, A.; Ohtani-Kaneko, R. Postnatal alteration of collapsin response mediator protein 4 mRNA expression in the mouse brain. J Anat. 2012, 221, 341–351. [Google Scholar] [CrossRef]
- Koide, T.; Aleksic, B.; Ito, Y.; Usui, H.; Yoshimi, A.; Inada, T.; Suzuki, M.; Hashimoto, R.; Takeda, M.; Iwata, N.; et al. A two-stage case-control association study of the dihydropyrimidinase-like 2 gene (DPYSL2) with schizophrenia in Japanese subjects. J. Hum. Genet. 2010, 55, 469–472. [Google Scholar] [CrossRef][Green Version]
- Bader, V.; Tomppo, L.; Trossbach, S.V.; Bradshaw, N.J.; Prikulis, I.; Leliveld, S.R.; Lin, C.Y.; Ishizuka, K.; Sawa, A.; Ramos, A.; et al. Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits. Hum. Mol. Genet. 2012, 21, 4406–4418. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Cassoli, J.S.; Nascimento, J.M.; Hensley, K.; Guest, P.C.; Pinzon-Velasco, A.M.; Turck, C.W. The protein interactome of collapsin response mediator protein-2 (CRMP2/DPYSL2) reveals novel partner proteins in brain tissue. Proteomics Clin. Appl. 2015, 9, 817–831. [Google Scholar] [CrossRef]
- Liu, Y.; Pham, X.; Zhang, L.; Chen, P.L.; Burzynski, G.; McGaughey, D.M.; He, S.; McGrath, J.A.; Wolyniec, P.; Fallin, M.D.; et al. Functional variants in DPYSL2 sequence increase risk of schizophrenia and suggest a link to mTOR signaling. G3 2014, 5, 61–72. [Google Scholar] [CrossRef]
- Nakamura, H.; Yamashita, N.; Kimura, A.; Kimura, Y.; Hirano, H.; Makihara, H.; Kawamoto, Y.; Jitsuki-Takahashi, A.; Yonezaki, K.; Takase, K.; et al. Comprehensive behavioral study and proteomic analyses of CRMP2-deficient mice. Genes Cells 2016, 21, 1059–1079. [Google Scholar] [CrossRef]
- Nakamura, H.; Takahashi-Jitsuki, A.; Makihara, H.; Asano, T.; Kimura, Y.; Nakabayashi, J.; Yamashita, N.; Kawamoto, Y.; Nakamura, F.; Ohshima, T.; et al. Proteome and behavioral alterations in phosphorylation-deficient mutant Collapsin Response Mediator Protein2 knock-in mice. Neurochem. Int. 2018, 119, 207–217. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, E.; Wang, Y.; Yang, C.; Yu, H.; Wang, Q.; Chen, Z.; Zhang, C.; Christian, K.M.; Song, H.; et al. Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioural impairments in mice. Nat. Commun. 2016, 1, 7. [Google Scholar] [CrossRef]
- Miller, B.H.; Zeier, Z.; Xi, L.; Lanz, T.A.; Deng, S.; Strathmann, J.; Willoughby, D.; Kenny, P.J.; Elsworth, J.D.; Lawrence, M.S.; et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA 2012, 109, 3125–3130. [Google Scholar] [CrossRef]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef]
- Tsutiya, A.; Watanabe, H.; Nakano, Y.; Nishihara, M.; Goshima, Y.; Ohtani-Kaneko, R. Deletion of collapsin response mediator protein 4 results in abnormal layer thickness and elongation of mitral cell apical dendrites in the neonatal olfactory bulb. J. Anat. 2016, 228, 792–804. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-V); American Psychiatric Association: Philadelphia, PA, USA, 2013.
- Tsutiya, A.; Nishihara, M.; Goshima, Y.; Ohtani-Kaneko, R. Mouse pups lacking collapsin response mediator protein 4 manifest impaired olfactory function and hyperactivity in the olfactory bulb. Eur. J. Neurosci. 2015, 42, 2335–2345. [Google Scholar] [CrossRef]
- Takarae, Y.; Sablich, S.R.; White, S.P.; Sweeney, J.A. Neurophysiological hyperresponsivity to sensory input in autism spectrum disorders. J. Neurodev. Disord. 2016, 8, 29. [Google Scholar] [CrossRef]
- Takarae, Y.; Sweeney, J. Neural Hyperexcitability in Autism Spectrum Disorders. Brain Sci. 2017, 7, 129. [Google Scholar] [CrossRef]
- Spencer, C.M.; Alekseyenko, O.; Hamilton, S.M.; Thomas, A.M.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Modifying behavioral phenotypes in Fmr1KO mice: Genetic background differences reveal autistic-like responses. Autism Res. 2011, 4, 40–56. [Google Scholar] [CrossRef]
- He, C.X.; Cantu, D.A.; Mantri, S.S.; Zeiger, W.A.; Goel, A.; Portera-Cailliau, C. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J. Neurosci. 2017, 37, 6475–6487. [Google Scholar] [CrossRef]
- Ethridge, L.E.; White, S.P.; Mosconi, M.W.; Wang, J.; Byerly, M.J.; Sweeney, J.A. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl. Psychiatry 2016, 6, e787. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Won, H.; Lee, H.R.; Gee, H.Y.; Mah, W.; Kim, J.I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef]
- Ko, H.G.; Oh, S.B.; Zhuo, M.; Kaang, B.K. Reduced acute nociception and chronic pain in Shank2−/− mice. Mol. Pain 2016, 4, 12. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef]
- Van der Gucht, E.; Clerens, S.; Cromphout, K.; Vandesande, F.; Arckens, L. Differential expression of c-fos in subtypes of GABAergic cells following sensory stimulation in the cat primary visual cortex. Eur. J. Neurosci. 2002, 16, 1620–1626. [Google Scholar] [CrossRef]
- Sullivan, S.L.; Ressler, K.J.; Buck, L.B. Spatial patterning and information coding in the olfactory system. Curr. Opin. Genet. Dev. 1995, 5, 516–523. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an olfactory sensory map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef]
- Salcedo, E.; Zhang, C.; Kronberg, E.; Restrepo, D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem. Senses. 2005, 30, 615–626. [Google Scholar] [CrossRef]
- Pathania, M.; Davenport, E.C.; Muir, J.; Sheehan, D.F.; López-Doménech, G.; Kittler, J.T. The autism and schizophrenia associated gene CYFIP1 is critical for the maintenance of dendritic complexity and the stabilization of mature spines. Transl. Psychiatry. 2014, 4, e374. [Google Scholar] [CrossRef]
- Nagaoka, A.; Takehara, H.; Hayashi-Takagi, A.; Noguchi, J.; Ishii, K.; Shirai, F.; Yagishita, S.; Akagi, T.; Ichiki, T.; Kasai, H. Abnormal intrinsic dynamics of dendritic spines in a fragile X syndrome mouse model in vivo. Sci. Rep. 2016, 6, 26651. [Google Scholar] [CrossRef]
- Cheng, N.; Alshammari, F.; Hughes, E.; Khanbabaei, M.; Rho, J.M. Dendritic overgrowth and elevated ERK signaling during neonatal development in a mouse model of autism. PLoS ONE 2017, 12, e0179409. [Google Scholar] [CrossRef]
- Montani, C.; Ramon-Brossier, M.; Ponzoni, L.; Gritti, L.; Cwetsch, A.W.; Braida, D.; Saillour, Y.; Terragni, B.; Mantegazza, M.; Sala, M.; et al. The X-linked intellectual disability protein IL1RAPL1 regulates dendrite complexity. J. Neurosci. 2017, 37, 6606–6627. [Google Scholar] [CrossRef]
- Niisato, E.; Nagai, J.; Yamashita, N.; Abe, T.; Kiyonari, H.; Goshima, Y.; Ohshima, T. CRMP4 suppresses apical dendrite bifurcation of CA1 pyramidal neurons in the mouse hippocampus. Dev. Neurobiol. 2012, 72, 1447–1457. [Google Scholar] [CrossRef]
- Niisato, E.; Nagai, J.; Yamashita, N.; Nakamura, F.; Goshima, Y.; Ohshima, T. Phosphorylation of CRMP2 is involved in proper bifurcation of the apical dendrite of hippocampal CA1 pyramidal neurons. Dev. Neurobiol. 2013, 73, 142–151. [Google Scholar] [CrossRef]
- Cha, C.; Zhang, J.; Ji, Z.; Tan, M.; Li, S.; Wu, F.; Chen, K.; Gong, S.; Guo, G.; Lin, H. CRMP4 regulates dendritic growth and maturation via the interaction with actin cytoskeleton in cultured hippocampal neurons. Brain Res. Bull. 2016, 124, 286–294. [Google Scholar] [CrossRef]
- Takaya, R.; Nagai, J.; Piao, W.; Niisato, E.; Nakabayashi, T.; Yamazaki, Y.; Nakamura, F.; Yamashita, N.; Kolattukudy, P.; Goshima, Y.; et al. CRMP1 and CRMP4 are required for proper orientation of dendrites of cerebral pyramidal neurons in the developing mouse brain. Brain Res. 2017, 1655, 161–167. [Google Scholar] [CrossRef]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef]
- Carlson, C.G. Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012, 100, 850–854. [Google Scholar] [CrossRef]
- Kim, K.C.; Cho, K.S.; Yang, S.M.; Gonzales, E.L.; Valencia, S.; Eun, P.H.; Choi, C.S.; Mabunga, D.F.; Kim, J.W.; Noh, J.K.; et al. Sex differences in autism-like behavioral phenotypes and postsynaptic receptors expression in the prefrontal cortex of TERT transgenic mice. Biomol. Ther. 2017, 25, 374–382. [Google Scholar] [CrossRef]
- Fung, L.K.; Hardan, A.Y. Developing medications targeting glutamatergic dysfunction in autism: Progress to date. CNS Drugs 2015, 29, 453–463. [Google Scholar] [CrossRef]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef]
- Gatto, C.L.; Broadie, K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front. Synaptic. Neurosci. 2010, 2, 4. [Google Scholar] [CrossRef]
- Rubenstein, J.L. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr. Opin. Neurol. 2010, 23, 118–123. [Google Scholar] [CrossRef]
- Jamain, S.; Betancur, C.; Quach, H.; Philippe, A.; Fellous, M.; Giros, B.; Gillberg, C.; Leboyer, M.; Bourgeron, T. Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry 2002, 7, 302–310. [Google Scholar] [CrossRef]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Glennon, J.C.; Franke, B.; Buitelaar, J.K. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: Association to overlapping traits in ADHD and autism. Transl. Psychiatry. 2017, 7, e999. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol. Autism. 2015, 6, 27. [Google Scholar] [CrossRef]
- Rubenstein, E.; Wiggins, L.D.; Lee, L.C. A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. J. Dev. Phys. Disabil. 2015, 27, 119–139. [Google Scholar] [CrossRef]
- Chen, C.; Van Horm, J.D. GENDAAR Research Consortium. Developmental neurogenetics and multimodal neuroimaging of sex differences in autism. Brain Imaging Behav. 2017, 11, 38–61. [Google Scholar] [CrossRef]
- Yu, J.; He, X.; Yao, D.; Li, Z.; Li, H.; Zhao, Z. A sex-specific association of common variants of neuroligin genes (NLGN3 and NLGN4X) with autism spectrum disorders in a Chinese Han cohort. Behav. Brain Funct. 2011, 7, 13. [Google Scholar] [CrossRef]
- Landini, M.; Merelli, I.; Raggi, M.E.; Galluccio, N.; Ciceri, F.; Bonfanti, A.; Camposeo, S.; Massagli, A.; Villa, L.; Salvi, E.; et al. Association Analysis of Noncoding Variants in Neuroligins 3 and 4X Genes with Autism Spectrum Disorder in an Italian Cohort. Int. J. Mol. Sci. 2016, 17, 1765. [Google Scholar] [CrossRef]
- Ey, E.; Torquet, N.; Le Sourd, A.M.; Leblond, C.S.; Boeckers, T.M.; Faure, P.; Bourgeron, T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav. Brain Res. 2013, 256, 677–689. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, P.; Go, H.S.; Choi, C.S.; Park, J.H.; Kim, H.J.; Jeon, S.J.; Dela Pena, I.C.; Han, S.H.; Cheong, J.H.; et al. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J. Neurochem. 2013, 124, 832–843. [Google Scholar] [CrossRef]
- Schneider, T.; Roman, A.; Basta-Kaim, A.; Kubera, M.; Budziszewska, B.; Schneider, K.; Przewłockia, R. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 2008, 33, 728–740. [Google Scholar] [CrossRef]
- Konopko, M.A.; Densmore, A.L.; Krueger, B.K. Sexually Dimorphic Epigenetic Regulation of Brain-Derived Neurotrophic Factor in Fetal Brain in the Valproic Acid Model of Autism Spectrum Disorder. Dev. Neurosci. 2017, 39, 507–518. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Ju, A.; Hammerschmidt, K.; Tantra, M.; Krueger, D.; Brose, N.; Ehrenreich, H. Juvenile manifestation of ultrasound communication deficits in the neuroligin-4 null mutant mouse model of autism. Behav. Brain Res. 2014, 270, 159–164. [Google Scholar] [CrossRef]
- Iwakura, T.; Sakoh, M.; Tsutiya, A.; Yamashita, N.; Ohtani, A.; Tsuda, M.C.; Ogawa, S.; Tsukahara, S.; Nishihara, M.; Shiga, T.; et al. Collapsin response mediator protein 4 affects the number of tyrosine hydroxylase-immunoreactive neurons in the sexually dimorphic nucleus in female mice. Dev. Neurobiol. 2013, 73, 502–517. [Google Scholar] [CrossRef]
- Sumida, H.; Nishizuka, M.; Kano, Y.; Arai, Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci. Lett. 1993, 151, 41–44. [Google Scholar] [CrossRef]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex differences in autism spectrum disorder: A review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Baron-Cohen, S. Fetal testosterone and sex differences in typical social development and in autism. J. Child. Neurol. 2006, 21, 825–845. [Google Scholar] [CrossRef]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Fetal testosterone and autistic traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef]
- Auyeung, B.; Taylor, K.; Hackett, G.; Baron-Cohen, S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol. Autism. 2010, 1, 11. [Google Scholar] [CrossRef]
- Auyeung, B.; Ahluwalia, J.; Thomson, L.; Taylor, K.; Hackett, G.; O’Donnell, K.J.; Baron-Cohen, S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol. Autism. 2012, 3, 17. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef]
- Cherskov, A.; Pohl, A.; Allison, C.; Zhang, H.; Payne, R.A.; Baron-Cohen, S. Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Transl. Psychiatry 2018, 8, 136. [Google Scholar] [CrossRef]
- Mong, J.A.; Glaser, E.; McCarthy, M.M. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J. Neurosci. 1999, 19, 1464–1472. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef]
- Werling, D.M.; Parikshak, N.N.; Geschwind, D.H. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun. 2016, 7, 10717. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtani-Kaneko, R. Crmp4-KO Mice as an Animal Model for Investigating Certain Phenotypes of Autism Spectrum Disorders. Int. J. Mol. Sci. 2019, 20, 2485. https://doi.org/10.3390/ijms20102485
Ohtani-Kaneko R. Crmp4-KO Mice as an Animal Model for Investigating Certain Phenotypes of Autism Spectrum Disorders. International Journal of Molecular Sciences. 2019; 20(10):2485. https://doi.org/10.3390/ijms20102485
Chicago/Turabian StyleOhtani-Kaneko, Ritsuko. 2019. "Crmp4-KO Mice as an Animal Model for Investigating Certain Phenotypes of Autism Spectrum Disorders" International Journal of Molecular Sciences 20, no. 10: 2485. https://doi.org/10.3390/ijms20102485
APA StyleOhtani-Kaneko, R. (2019). Crmp4-KO Mice as an Animal Model for Investigating Certain Phenotypes of Autism Spectrum Disorders. International Journal of Molecular Sciences, 20(10), 2485. https://doi.org/10.3390/ijms20102485