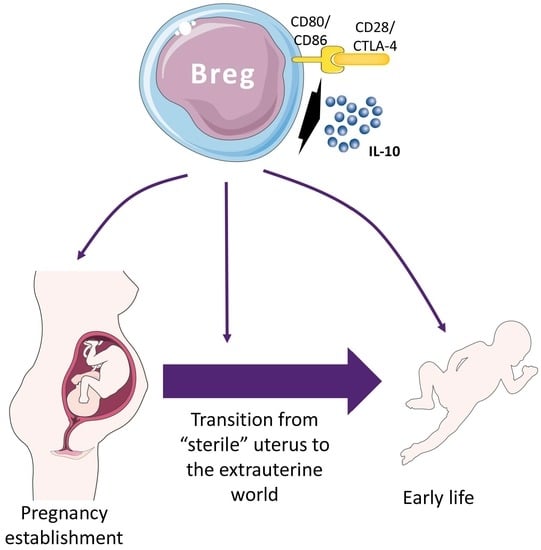
B Regulatory Cells: Players in Pregnancy and Early Life
Abstract
1. The Immune System during Pregnancy and the Neonatal Period
1.1. The Immune System during Pregnancy
1.1.1. Natural Killer Cells
1.1.2. Myeloid-Derived Cells
1.1.3. T Cells
1.2. The Immune System during the Neonatal Period
2. B Cells during Pregnancy and Early Life
2.1. B Cells during Pregnancy
2.2. B Cells in the Neonatal Period
3. The Breg Subpopulation and Its Role in Health and Disease
4. Breg Cells in Pregnancy
5. Breg Cells in Early Life
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCG | Bacille-Calmette Guerin |
| Breg | B regulatory |
| CD | Cluster of diferntiation |
| DC | Dendritic cell |
| FoxP3 | Forkhead box P3 |
| GvHD | Graft versus host disease |
| GZMB | Granzyme B |
| HBV | Hepatitis B virus |
| HIV | Human immunodeficiency virus |
| HLA | Humanl leukocyte antigen |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IgM-ALA | Igm anti-leukocyte autoantibodies |
| IL | Interleukin |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| PD-L | Program death-ligand |
| TGF | Transforming growth factor |
| Th | T helper |
| TIM | T cell immunoglobulin and mucin domain |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| Treg | T regulatory |
References
- Schumacher, A.; Costa, S.-D.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Sappenfield, E.; Jamieson, D.J.; Kourtis, A.P. Pregnancy and Susceptibility to Infectious Diseases. Infect. Dis. Obstet. Gynecol. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.; Westphal, L. Immune Function and Recurrent Pregnancy Loss. Semin. Reprod. Med. 2015, 33, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bonney, E.A. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet. Gynecol. Clin. N. Am. 2016, 43, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Verma, P.; Singh, K. Immune-endocrine crosstalk during pregnancy. Gen. Comp. Endocrinol. 2017, 242, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18, 2166. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Adar, T.; Grisaru-Granovsky, S.; Ben Ya’acov, A.; Goldin, E.; Bar-Gil Shitrit, A.; Ben Ya’acov, A.; Goldin, E.; Bar-Gil Shitrit, A. Pregnancy and the Immune System: General Overview and the Gastroenterological Perspective. Dig. Dis. Sci. 2015, 60, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dunk, C.; Croy, A.B.; Lye, S.J. To serve and to protect: The role of decidual innate immune cells on human pregnancy. Cell Tissue Res. 2016, 363, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Buret, A.; Batey, R.T.; Chen, Q.Y.; Couch, L.; Cripps, A.; Clancy, R. Morphological, phenotypic and functional characteristics of a pure population of CD56+ CD16- CD3- large granular lymphocytes generated from human duodenal mucosa. Immunology 1993, 79, 498–505. [Google Scholar] [PubMed]
- González, I.T.; Barrientos, G.; Freitag, N.; Otto, T.; Thijssen, V.L.J.L.; Moschansky, P.; von Kwiatkowski, P.; Klapp, B.F.; Winterhager, E.; Bauersachs, S.; Blois, S.M. Uterine NK Cells Are Critical in Shaping DC Immunogenic Functions Compatible with Pregnancy Progression. PLoS ONE 2012, 7, e46755. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.-P.; Ding, B.; Zhao, Y.-R.; Chen, Z.-J.; Xu, C.-Y.; Fu, Y.-B.; Wang, X.-T. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J. Obstet. Gynaecol. Res. 2014, 40, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dunk, C.E.; Lye, S.J. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum. Reprod. 2013, 28, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Hazan, A.D.; Smith, S.D.; Jones, R.L.; Whittle, W.; Lye, S.J.; Dunk, C.E. Vascular-leukocyte interactions: Mechanisms of human decidual spiral artery remodeling in vitro. Am. J. Pathol. 2010, 177, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the Fetal-Maternal Interface Express Markers of Alternative Activation and Are Induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two Unique Human Decidual Macrophage Populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Genuino, R.M.; Diener, K.R. Regulatory B cells in pregnancy: Lessons from autoimmunity, graft tolerance, and cancer. Front. Immunol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C. Adaptive Immune Responses During Pregnancy. Am. J. Reprod. Immunol. 2013, 69, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Ertelt, J.M.; Xin, L.; Way, S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012, 490, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, X.; Chen, R.; Liu, J.; Liu, P.; Hu, A.; WU, L.; Hua, H.; Yuan, H. Early Pregnancy Factor Enhances the Generation and Function of CD4+CD25+ Regulatory T Cells. Tohoku J. Exp. Med. 2016, 240, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.; Zygmunt, M.; Jensen, F. The role of pregnancy-associated hormones in the development and function of regulatory B cells. Front. Endocrinol. 2014, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Memarzadeh Tehran, M.; Morell-García, A.; Raeva, Y.; Schumacher, A.; Hartig, R.; Costa, S.-D.; Jensen, F.; Zenclussen, A.C. Cutting Edge: IL-10-Producing Regulatory B Cells in Early Human Pregnancy. Am. J. Reprod. Immunol. 2013, 70, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, G.; Schönlaub, J.; Hammerer-Lercher, A.; Koppelstaetter, C.; Bernhard, D.; Prelog, M. Influence of the delivery modus on subpopulations and replication of lymphocytes in mothers and newborns. Early Hum. Dev. 2015, 91, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.M.; Bhat, B.V. Distinct mechanisms of the newborn innate immunity. Immunol. Lett. 2016, 173, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Allan Walker, W. Initial intestinal colonization in the human infant and immune homeostasis. Ann. Nutr. Metab. 2013, 63, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Maródi, L.; Káposzta, R.; Campbell, D.E.; Polin, R.A.; Csongor, J.; Johnston, R.B. Candidacidal mechanisms in the human neonate. Impaired IFN-gamma activation of macrophages in newborn infants. J. Immunol. 1994, 153, 5643–5649. [Google Scholar] [PubMed]
- Wilson, C.B.; Westall, J.; Johnston, L.; Lewis, D.B.; Dower, S.K.; Alpert, A.R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J. Clin. Investig. 1986, 77, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, M.; Jurstrand, M.; Bodin, L.; Fredlund, H.; Schollin, J. Defective Neutrophil Oxidative Burst in Preterm Newborns on Exposure to Coagulase-Negative Staphylococci. Pediatr. Res. 2004, 55, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Rucker, R.; Bennetts, G.A.; Hicks, D.; Worcester, C.; Amlie, R.; Johnson, S.; Katz, J.; Thorne, G.M. Improved survival of newborns receiving leukocyte transfusions for sepsis. Pediatrics 1984, 74, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ambruso, D.R.; Bentwood, B.; Henson, P.M.; Johnston, R.B. Oxidative Metabolism of Cord Blood Neutrophils: Relationship to Content and Degranulation of Cytoplasmic Granules. Pediatr. Res. 1984, 18, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Carr, R. Neutrophil Production and Function in Newborn Infants. Br. J. Haematol. 2000, 110, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, M.; Toy, T.; Shin, A.; Wagner, M.; Cebon, J.; Maraskovsky, E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: A novel role for the cAMP pathway. Blood 2005, 105, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Vanden Eijnden, S.; Goriely, S.; De Wit, D.; Goldman, M.; Willems, F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 2006, 36, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Angelone, D.F.; Wessels, M.R.; Coughlin, M.; Suter, E.E.; Valentini, P.; Kalish, L.A.; Levy, O. Innate Immunity of the Human Newborn Is Polarized Toward a High Ratio of IL-6/TNF-α Production In Vitro and In Vivo. Pediatr. Res. 2006, 60, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.M.; Bertram, P.; Gahr, M.; Speer, C.P. Reduced secretion of interleukin-1 and tumor necrosis factor-alpha by neonatal monocytes. Biol. Neonate 1993, 63, 157–162. [Google Scholar] [CrossRef] [PubMed]
- White, G.P.; Watt, P.M.; Holt, B.J.; Holt, P.G. Differential Patterns of Methylation of the IFN- Promoter at CpG and Non-CpG Sites Underlie Differences in IFN- Gene Expression Between Human Neonatal and Adult CD45RO- T Cells. J. Immunol. 2002, 168, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.; Albarani, V.; Nguyen, M.; Laes, J.-F.; Ruelle, J.-L.; De Wit, D.; Willems, F.; Goldman, M.; Goriely, S. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood 2007, 109, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Coughlin, M.; Cronstein, B.N.; Roy, R.M.; Desai, A.; Wessels, M.R. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006, 177, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- De Wit, D.; Tonon, S.; Olislagers, V.; Goriely, S.; Boutriaux, M.; Goldman, M.; Willems, F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 2003, 21, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Buddle, J.C.; Thrasher, A.J.; Goldblatt, D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 2002, 128, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell. Mol. Immunol. 2007, 4, 377–382. [Google Scholar] [PubMed]
- Dalle, J.-H.; Menezes, J.; Wagner, É.; Blagdon, M.; Champagne, J.; Champagne, M.A.; Duval, M. Characterization of Cord Blood Natural Killer Cells: Implications for Transplantation and Neonatal Infections. Pediatr. Res. 2005, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.G.; Ward, C.M.; Morris, J.M. To B or not to B cells-mediate a healthy start to life. Clin. Exp. Immunol. 2013, 171, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Piątosa, B.; Wolska-Kuśnierz, B.; Pac, M.; Siewiera, K.; Gałkowska, E.; Bernatowska, E. B cell subsets in healthy children: Reference values for evaluation of B cell maturation process in peripheral blood. Cytom. Part B Clin. Cytom. 2010, 78B, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res. Ther. 2013, 15 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- LeBien, T.W. Fates of human B-cell precursors. Blood 2000, 96, 9–23. [Google Scholar] [PubMed]
- Weller, S.; Braun, M.C.; Tan, B.K.; Rosenwald, A.; Cordier, C.; Conley, M.E.; Plebani, A.; Kumararatne, D.S.; Bonnet, D.; Tournilhac, O.; et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004, 104, 3647–3654. [Google Scholar] [CrossRef] [PubMed]
- Fettke, F.; Schumacher, A.; Canellada, A.; Toledo, N.; Bekeredjian-Ding, I.; Bondt, A.; Wuhrer, M.; Costa, S.-D.; Zenclussen, A.C. Maternal and Fetal Mechanisms of B Cell Regulation during Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While Alpha-Fetoprotein Drives Them into Apoptosis. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Fettke, F.; Schumacher, A.; Costa, S.-D.D.; Zenclussen, A.C. B cells: The old new players in reproductive immunology. Front. Immunol. 2014, 5, 285. [Google Scholar] [CrossRef] [PubMed]
- Herse, F.; Staff, A.C.; Hering, L.; Müller, D.N.; Luft, F.C.; Dechend, R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J. Mol. Med. 2008, 86, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Hecher, K.; Solano, M.E. B Cells in Pregnancy: Functional Promiscuity or Tailored Function? Biol. Reprod. 2015, 92, 12. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.O.; Soldati, R.; Ehrhardt, J.; Utpatel, K.; Evert, M.; Zenclussen, A.C.; Zygmunt, M.; Jensen, F. B Cell Development Undergoes Profound Modifications and Adaptations During Pregnancy in Mice. Biol. Reprod. 2014, 91, 115. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Miller, D.; Leng, Y.; Panaitescu, B.; Silva, P.; Faro, J.; Alhousseini, A.; Gill, N.; et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am. J. Reprod. Immunol. 2018, 79, e12827. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Martins, C.; Leandro, M.J.; Nunes, G.; Sousa, M.-J.; Branco, J.C.; Borrego, L.-M. Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: A prospective observational study. BMC Pregnancy Childbirth 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, A.; Paviglianiti, A.; Gluckman, E.; Rocha, V. Impact of HLA in cord blood transplantation outcomes. HLA 2016, 87, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Basar, R.; Mehta, R.S.; Shaim, H.; Muftuoglu, M.; Khoder, A.; Sekine, T.; Gokdemir, E.; Kondo, K.; Marin, D.; et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood 2016, 128, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Lima, J.; Nunes, G.; Borrego, L.M. Pregnancy alters the circulating B cell compartment in atopic asthmatic women, and transitional B cells are positively associated with the development of allergy manifestations in their progeny. Am. J. Reprod. Immunol. 2016, 76, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.I.; Parker, D.; Turk, J.L. B-cell suppression of delayed hypersensitivity reactions. Nature 1974, 251, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Jones, C.A. The development of the immune system during pregnancy and early life. Allergy 2000, 55, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Menon, M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Menon, M. The expanding family of regulatory B cells. Int. Immunol. 2015, 27, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Floudas, A.; Amu, S.; Fallon, P.G. New Insights into IL-10 Dependent and IL-10 Independent Mechanisms of Regulatory B Cell Immune Suppression. J. Clin. Immunol. 2016, 36, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B Cells Maintain Regulatory T Cells While Limiting TH1 and TH17 Differentiation. Sci. Transl. Med. 2013, 5, 173ra23. [Google Scholar] [CrossRef] [PubMed]
- Siewe, B.; Stapleton, J.T.; Martinson, J.; Keshavarzian, A.; Kazmi, N.; Demarais, P.M.; French, A.L.; Landay, A. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8+ T cell function in vitro. J. Leukoc. Biol. 2013, 93, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Bosma, A.; Abdel-Gadir, A.; Isenberg, D.A.; Jury, E.C.; Mauri, C. Lipid-Antigen Presentation by CD1d + B Cells Is Essential for the Maintenance of Invariant Natural Killer T Cells. Immunity 2012, 36, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; Dilillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.E.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-induced granzyme b-expressing b cells infiltrate tumors and regulate t cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Saze, Z.; Schuler, P.J.; Hong, C.-S.; Cheng, D.; Jackson, E.K.; Whiteside, T.L. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 2013, 122, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nouël, A.; Pochard, P.; Simon, Q.; Ségalen, I.; Le Meur, Y.; Pers, J.O.; Hillion, S. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J. Autoimmun. 2015, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, W.; Kim, C.J.; Clayton, K.; Zhao, H.; Lee, E.; Cao, J.C.; Ziegler, B.; Gregor, A.; Yue, F.Y.; et al. IL-10-producing B cells are induced early in HIV-1 infection and suppress HIV-1-specific T cell responses. PLoS ONE 2014, 9, e89236. [Google Scholar] [CrossRef] [PubMed]
- Goode, I.; Xu, H.; Ildstad, S.T. Regulatory B Cells: The New “It” Cell. Transplant. Proc. 2014, 46, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; Cheng, K.F.; Al-Abed, Y.; Rothstein, T.L. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J. Immunol. 2014, 193, 5904–5913. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Wang, L.; Dittel, B.N. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int. Immunol. 2015, 27, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Ehrenstein, M.R. The “short” history of regulatory B cells. Trends Immunol. 2008, 29, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Ettinger, R.; Shirota, Y.; Yarboro, C.H.; Illei, G.G.; Lipsky, P.E. Identification and characterization of circulating human transitional B cells. Blood 2016, 105, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Khoder, A.; Sarvaria, A.; Alsuliman, A.; Chew, C.; Sekine, T.; Cooper, N.; Mielke, S.; de Lavallade, H.; Muftuoglu, M.; Fernandez Curbelo, I.; et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014, 124, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Lima, J.; Nunes, G.; Borrego, L.M. Regulatory T and B cells in asthmatic women: Variations from pregnancy to postpartum. J. Investig. Allergol. Clin. Immunol. 2017, 27, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Noh, G.; Lee, S.J.; Lee, J.H.; Kim, A.; Kim, H.S.; Choi, W.S. Tolerogenic effects of interferon-gamma with induction of allergen-specific interleukin-10-producing regulatory B cell (Br1) changes in non-IgE-mediated food allergy. Cell. Immunol. 2012, 273, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Amu, S.; Saunders, S.P.; Kronenberg, M.; Mangan, N.E.; Atzberger, A.; Fallon, P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 2010, 125, 1114.e8–1124.e8. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Horikawa, M.; Iwata, Y.; Tedder, T.F. Regulatory B Cells (B10 Cells) and Regulatory T Cells Have Independent Roles in Controlling Experimental Autoimmune Encephalomyelitis Initiation and Late-Phase Immunopathogenesis. J. Immunol. 2010, 185, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Smolders, J.; Thewissen, M.; Peelen, E.; Tervaert, J.W.C.; Hupperts, R.; Damoiseaux, J. Effect of vitamin D 3 supplementation on peripheral B cell differentiation and isotype switching in patients with multiple sclerosis. Mult. Scler. J. 2011, 17, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Saussine, A.; Tazi, A.; Feuillet, S.; Rybojad, M.; Juillard, C.; Bergeron, A.; Dessirier, V.; Bouhidel, F.; Janin, A.; Bensussan, A.; et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS ONE 2012, 7, e43588. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Ishiura, N.; Nakashima, H.; Kuwano, Y.; Okochi, H.; Tamaki, K.; Sato, S.; Tedder, T.F.; Fujimoto, M. Regulatory B Cells (B10 Cells) Have a Suppressive Role in Murine Lupus: CD19 and B10 Cell Deficiency Exacerbates Systemic Autoimmunity. J. Immunol. 2010, 184, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.A.; Rosser, E.C.; Mauri, C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res. Ther. 2012, 14, R32. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.; Lee, J.H. Regulatory B cells and allergic diseases. Allergy Asthma Immunol. Res. 2011, 3, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ellis, G.; Pallant, C.; Lopes, A.R.; Khanna, P.; Peppa, D.; Chen, A.; Blair, P.; Dusheiko, G.; Gill, U.; et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 2012, 189, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, X.; Zhang, J.; Zhu, Y.; Zhu, X.; Liu, H.; Zeng, M.; Graner, M.W.; Zhou, B.; Chen, X. CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell. Immunol. 2012, 274, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Feng, S.; Ren, M.; Ma, E.; Wang, X.; Xu, L.; Xu, M. Glioma cell-derived placental growth factor induces regulatory B cells. Int. J. Biochem. Cell Biol. 2014, 57, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B regulatory cells and the tumor-promoting actions of TNF- during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Lee-Chang, C. A new paradigm for an old story: The role of regulatory B cells in cancer. Front. Immunol. 2012, 3, 206. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, M.; Minard-Colin, V.; Matsushita, T.; Tedder, T.F. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Investig. 2011, 121, 4268–4280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Moore, D.J.; Lee, K.M.; Kim, J.I.; Duff, P.E.; O’Connor, M.R.; Hirohashi, T.; Lei, J.; Yang, M.; Markmann, J.F.; et al. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am. J. Transplant. 2010, 10, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Rowe, V.; Banovic, T.; MacDonald, K.P.; Kuns, R.; Don, A.L.; Morris, E.S.; Burman, A.C.; Bofinger, H.M.; Clouston, A.D.; Hill, G.R. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood 2006, 108, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.A.; Asare, A.; Kirk, A.D.; Gisler, T.D.; Bourcier, K.; Suthanthiran, M.; Burlingham, W.J.; Marks, W.H.; Sanz, I.; Lechler, R.I.; et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Investig. 2010, 120, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Shabir, S.; Girdlestone, J.; Briggs, D.; Kaul, B.; Smith, H.; Daga, S.; Chand, S.; Jham, S.; Navarrete, C.; Harper, L.; et al. Transitional B Lymphocytes Are Associated With Protection From Kidney Allograft Rejection: A Prospective Study. Am. J. Transplant. 2015, 15, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.; Muzzio, D.; Soldati, R.; Fest, S.; Zenclussen, A.C. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol. Reprod. 2013, 89, 90. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Fillatreau, S. Suppressive functions of B cells in infectious diseases. Int. Immunol. 2015, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.; Antinarelli, L.M.R.; Abramo, C.; Macedo, G.C.; Coimbra, E.S.; Scopel, K.K.G. What do we know about the role of regulatory B cells (Breg) during the course of infection of two major parasitic diseases, malaria and leishmaniasis? Pathog. Glob. Health 2017, 111, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, X.; Zhang, T.; Lijun, S.; Wang, R.; Li, W.; Ji, Y.; Wu, H.; Liu, C. Regulatory B cells correlate with HIV disease progression. Microbiol. Immunol. 2014, 58, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, S.; Wang, C.; Vandenbark, A.A.; Offner, H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur. J. Immunol. 2011, 41, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Benedek, G.; Bodhankar, S.; Lapato, A.; Vandenbark, A.A.; Offner, H. IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. J. Neuroimmunol. 2015, 285, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lapato, A.; Bodhankar, S.; Vandenbark, A.A.; Offner, H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab. Brain Dis. 2015, 30, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Zhang, J.; Bodhankar, S.; Nguyen, H.; Kent, G.; Jordan, K.; Manning, D.; Vandenbark, A.A.; Offner, H. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2016, 293, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Zhang, J.; Nguyen, H.; Kent, G.; Seifert, H.; Vandenbark, A.A.; Offner, H. Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J. Neuroimmunol. 2017, 305, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, E.F.; Murray, E.R.; Kelman, A.; Farmer, P.; Saji, F.; Samejima, Y.; Kamiura, S.; Koyoma, M.; Boothby, L.; Doering, P.; et al. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011, 117, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Damotte, V.; Gelfand, J.M.; Bevan, C.; Cree, B.A.C.; Do, L.; Green, A.J.; Hauser, S.L.; Bove, R. Rituximab before and during pregnancy: A systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e453. [Google Scholar] [CrossRef] [PubMed]
- Happle, C.; Jirmo, A.C.; Meyer-Bahlburg, A.; Habener, A.; Hoymann, H.G.; Hennig, C.; Skuljec, J.; Hansen, G. B cells control maternofetal priming of allergy and tolerance in a murine model of allergic airway inflammation. J. Allergy Clin. Immunol. 2018, 141, 685.e6–696.e6. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Solé, A.; Teixidó, I.; Deyà-Martínez, A.; Yagüe, J.; Plaza-Martín, A.M.; Juan, M.; Alsina, L. Characterization of the Highly Prevalent Regulatory CD24hiCD38hi B-Cell Population in Human Cord Blood. Front. Immunol. 2017, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, F.; Hao, H.; Wang, D.; Gao, Y.; Zhou, J.; Li, F.; Lin, H.C.; Xiao, X.; Zeng, Q. Marked elevation of circulating CD19+CD38hiCD24hitransitional B cells give protection against neonatal sepsis. Pediatr. Neonatol. 2018, 59, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Lobo, P.I.; Bajwa, A.; Schlegel, K.H.; Vengal, J.; Lee, S.J.; Huang, L.; Ye, H.; Deshmukh, U.; Wang, T.; Pei, H.; et al. Natural IgM anti-leukocyte autoantibodies attenuate excess inflammation mediated by innate and adaptive immune mechanisms involving Th-17. J Immunol. 2012, 188, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Lobo, P.I.; Schlegel, K.H.; Spencer, C.E.; Okusa, M.D.; Chisholm, C.; Mchedlishvili, N.; Park, A.; Christ, C.; Burtner, C. Naturally Occurring IgM Anti-Leukocyte Autoantibodies (IgM-ALA) Inhibit T Cell Activation and Chemotaxis. J. Immunol. 2008, 180, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Allman, D.; Pillai, S. Peripheral B cell subsets. Curr. Opin. Immunol. 2008, 20, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.O.; Ziegler, K.B.; Ehrhardt, J.; Zygmunt, M.; Jensen, F. Marginal zone B cells emerge as a critical component of pregnancy well-being. Reproduction 2016, 151, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Yan, D.-C.; Lee, Y.-C.; Hsiao, H.-S.; Lee, P.-T.; Liang, Y.-W.; Kuo, M.-L. Umbilical cord blood immunology: Relevance to stem cell transplantation. Clin. Rev. Allergy Immunol. 2012, 42, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Deriaud, E.; Leclerc, C.; Lo-Man, R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 2005, 22, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.-O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.-A.; Hervé, P.-L.; Dériaud, E.; et al. Respiratory Syncytial Virus Infects Regulatory B Cells in Human Neonates via Chemokine Receptor CX3CR1 and Promotes Lung Disease Severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Watkins, M.L.V.; Semple, P.L.; Abel, B.; Hanekom, W.A.; Kaplan, G.; Ress, S.R. Exposure of cord blood to Mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin. Vaccine Immunol. 2008, 15, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.G.; Correia-Silva, J.D.F.; Silva, T.A.; Xavier, S.G.; Bittencourt, H.; Gomez, R.S.; Abreu, M.H.N.G. Saliva and blood interferon gamma levels and IFNG genotypes in acute graft-versus-host disease. Oral Dis. 2012, 18, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Rottman, M.; Soudais, C.; Vogt, G.; Renia, L.; Emile, J.-F.; Decaluwe, H.; Gaillard, J.-L.; Casanova, J.-L. IFN-γ Mediates the Rejection of Haematopoietic Stem Cells in IFN-γR1-Deficient Hosts. PLoS Med. 2008, 5, e26. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Boisson-Dupuis, S.S.S.; Abel, L.; Casanova, J.-L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFNgamma immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef] [PubMed]
- Fieschi, C.; Dupuis, S.; Picard, C.; Smith, C.I.; Holland, S.M.; Casanova, J.L. High levels of interferon gamma in the plasma of children with complete interferon gamma receptor deficiency. Pediatrics 2001, 107, E48. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, P.; Martínez-Saavedra, M.T.; Perez-Hurtado, J.M.; Sanchez, C.; Sanchez, B.; Deswarte, C.; Obando, I.; Casanova, J.-L.; Speckmann, C.; Bustamante, J.; et al. Diagnostic and therapeutic challenges in a child with complete Interferon-γ Receptor 1 deficiency. Pediatr. Blood Cancer 2015, 62, 2036–2039. [Google Scholar] [CrossRef] [PubMed]


| Name | Phenotypic Markers | Function | References |
|---|---|---|---|
| Immature B-cells | CD24hiCD38hi | perform their action on CD4 and CD8 T cells, plasmacitoid DCs, and invariant NK T cells by IL-10 secretion, and PD-L1, CD80, CD86, and CD1d ligation | [66,71,72,73,74] |
| B10 cells | CD24hiCD27hi | produce IL-10 and regulate monocytes and effector CD4 T cells | [75] |
| Granzyme B+ (GZMB) cells | CD38+CD1d+IgM+CD147+ | regulatory function on CD4 T cells by IL10, indoleamine 2,3-dioxygenase (IDO), and GZMB | [76] |
| Br1 | CD25hiCD71hiCD73low | produce IL-10 and allergen-specific IgG4, thus suppressing allergen-specific CD4 cells and maintaining allergen tolerance | [77] |
| Plasmablasts | CD27intCD38hi | produce IL-10, but their target cell type is not known yet | [78] |
| - | CD39+CD73+ | regulate CD4 and CD8 T cells by adenosine formation, thus reducing inflammation by adenosine triphosphate | [79] |
| Induced Breg cells | - | produce TGF-ß and IDO to suppress CD4 T cells. These cells are developed after T cell cytotoxic T-lymphocyte antigen 4 interaction | [80] |
| - | Express TIM1 | inhibit CD8 and CD4 T cells by producing IL-10 | [81] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteve-Solé, A.; Luo, Y.; Vlagea, A.; Deyà-Martínez, Á.; Yagüe, J.; Plaza-Martín, A.M.; Juan, M.; Alsina, L. B Regulatory Cells: Players in Pregnancy and Early Life. Int. J. Mol. Sci. 2018, 19, 2099. https://doi.org/10.3390/ijms19072099
Esteve-Solé A, Luo Y, Vlagea A, Deyà-Martínez Á, Yagüe J, Plaza-Martín AM, Juan M, Alsina L. B Regulatory Cells: Players in Pregnancy and Early Life. International Journal of Molecular Sciences. 2018; 19(7):2099. https://doi.org/10.3390/ijms19072099
Chicago/Turabian StyleEsteve-Solé, Ana, Yiyi Luo, Alexandru Vlagea, Ángela Deyà-Martínez, Jordi Yagüe, Ana María Plaza-Martín, Manel Juan, and Laia Alsina. 2018. "B Regulatory Cells: Players in Pregnancy and Early Life" International Journal of Molecular Sciences 19, no. 7: 2099. https://doi.org/10.3390/ijms19072099
APA StyleEsteve-Solé, A., Luo, Y., Vlagea, A., Deyà-Martínez, Á., Yagüe, J., Plaza-Martín, A. M., Juan, M., & Alsina, L. (2018). B Regulatory Cells: Players in Pregnancy and Early Life. International Journal of Molecular Sciences, 19(7), 2099. https://doi.org/10.3390/ijms19072099