Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing
Abstract
1. Introduction
2. Results
2.1. Inflammatory Response to Fracture in Mice
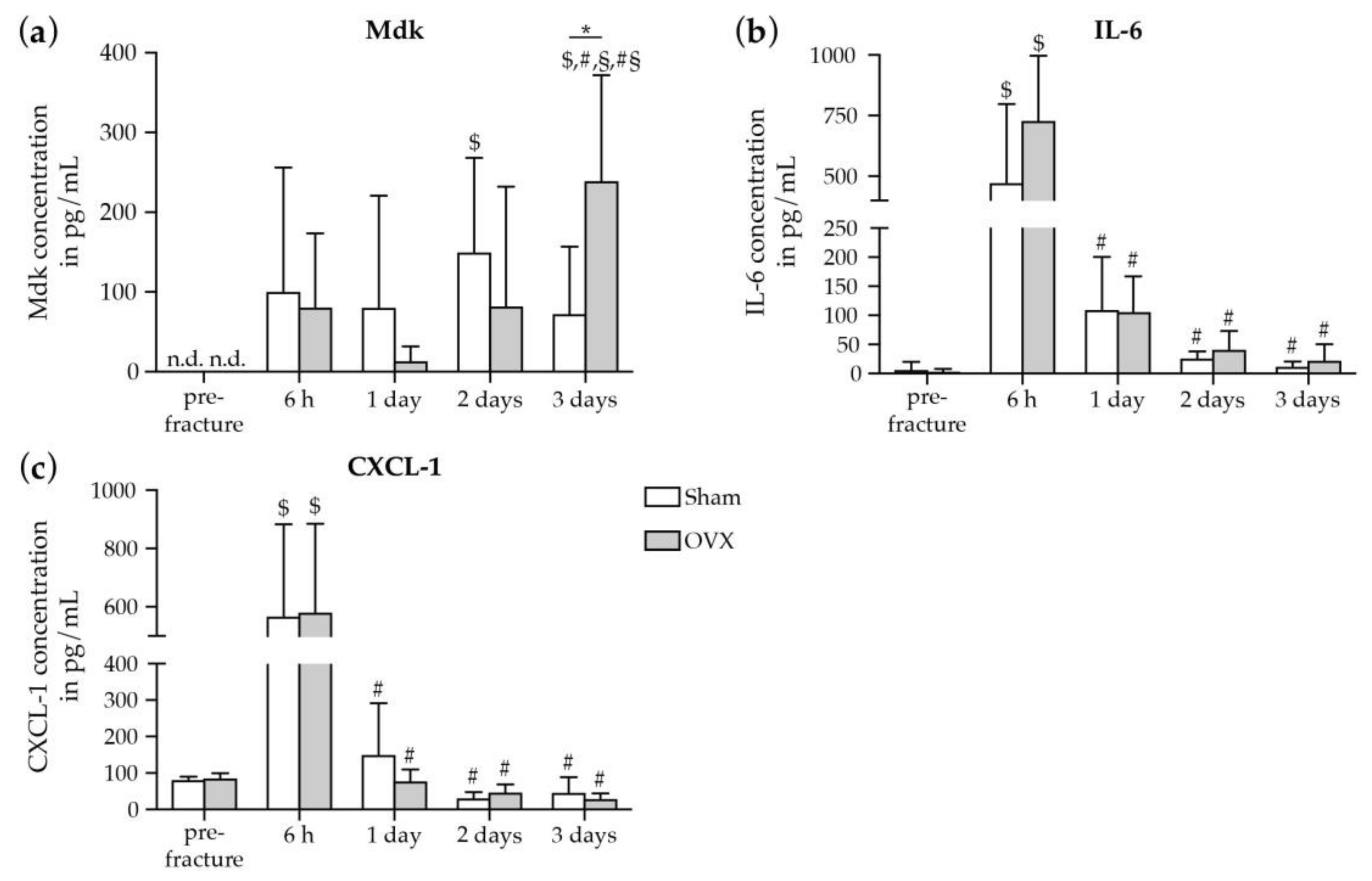
2.1.1. Increase in Systemic Mdk Concentrations in Estrogen-Deficient Mice after Fracture
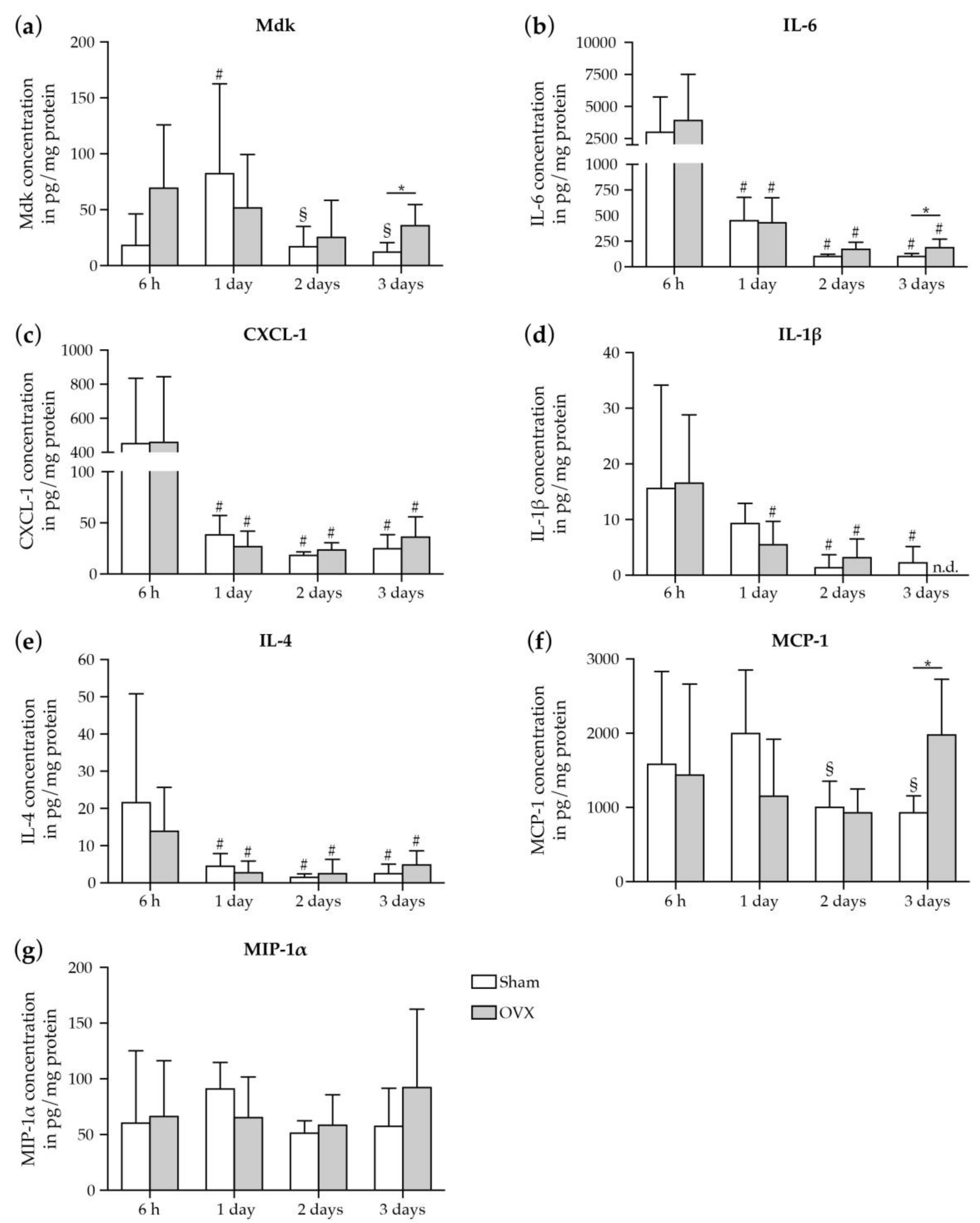
2.1.2. Increase in Mdk and IL-6 Concentrations in The Fracture Hematoma of Estrogen-Deficient Mice after Fracture
2.2. Inflammatory Response to Fracture in Humans
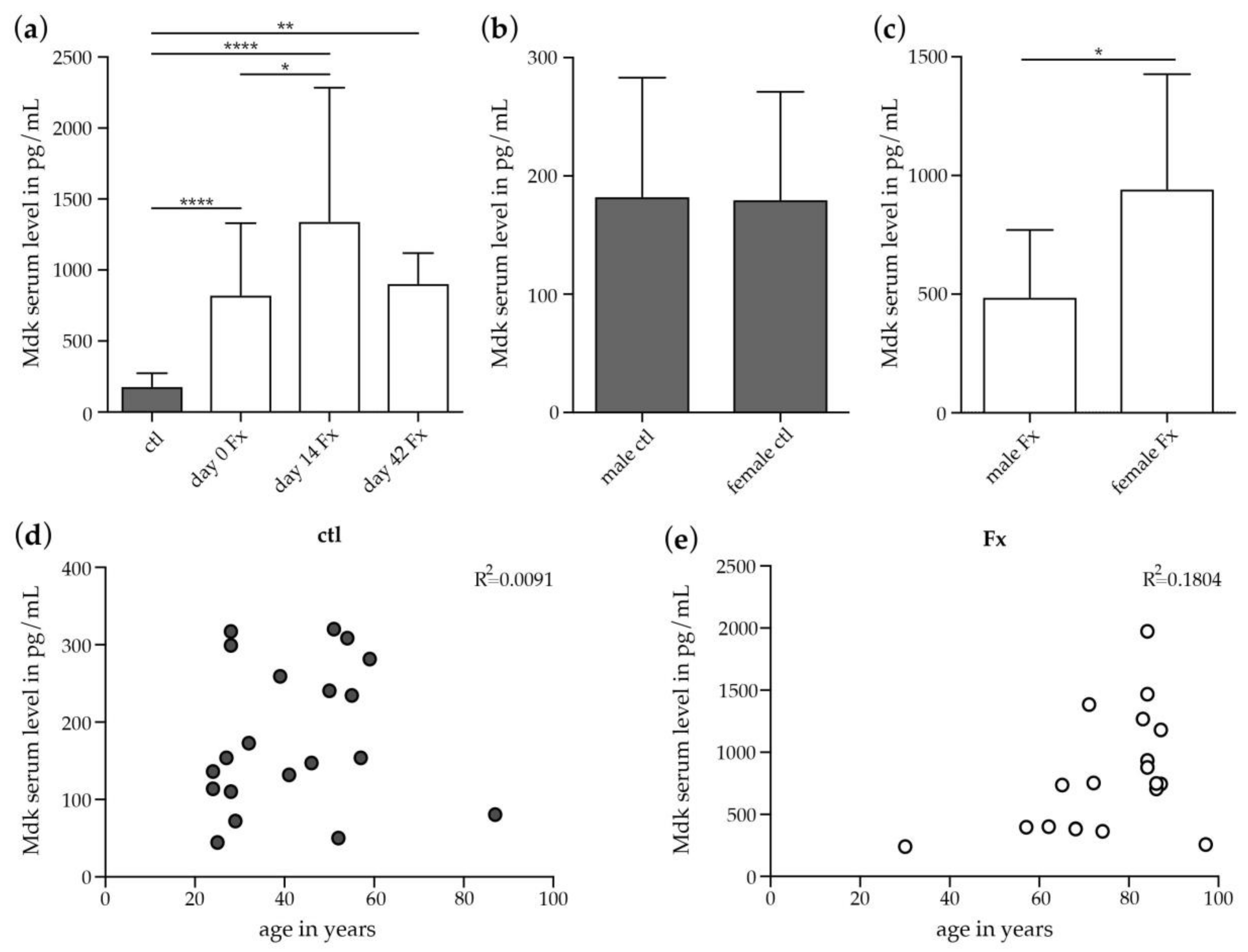
2.2.1. Increase in Systemic Mdk Concentrations in Female Fracture Patients after Menopause
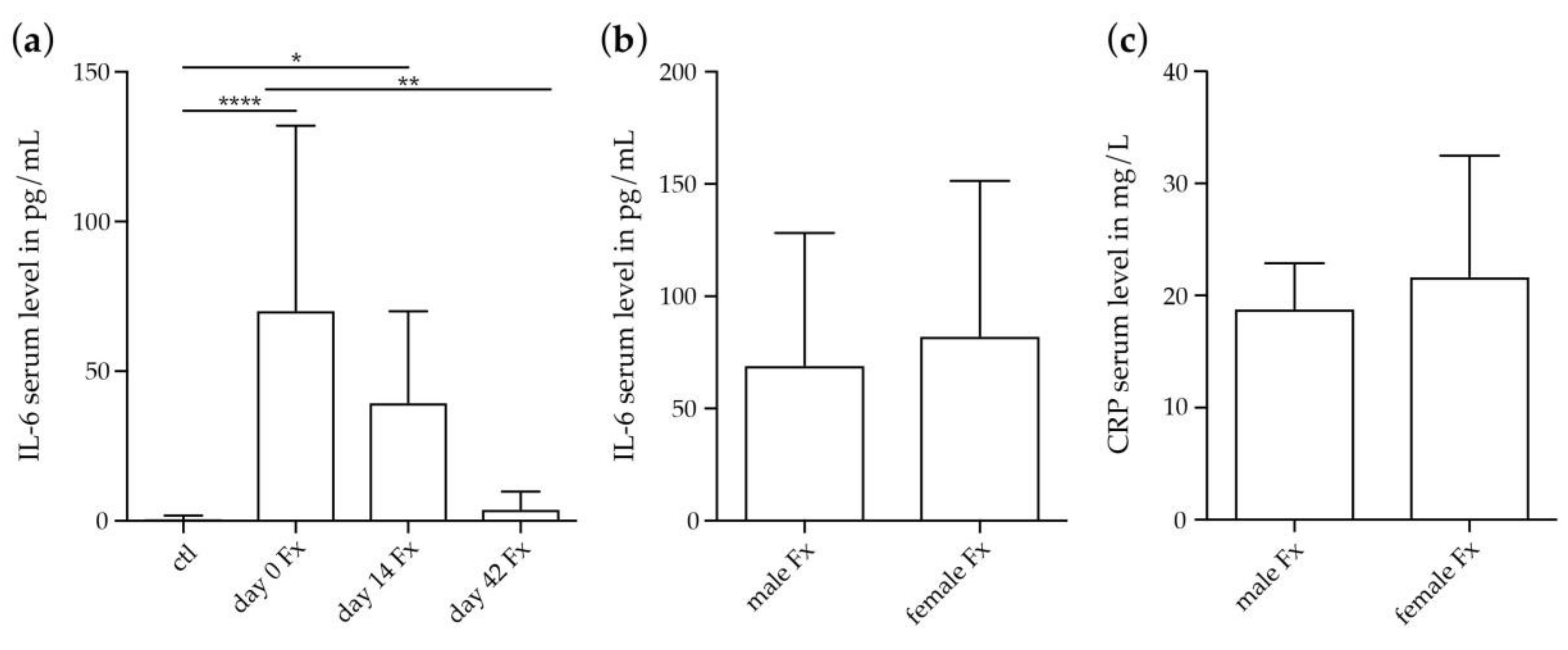
2.2.2. Systemic IL-6 and CRP Concentrations in Female Fracture Patients after Menopause
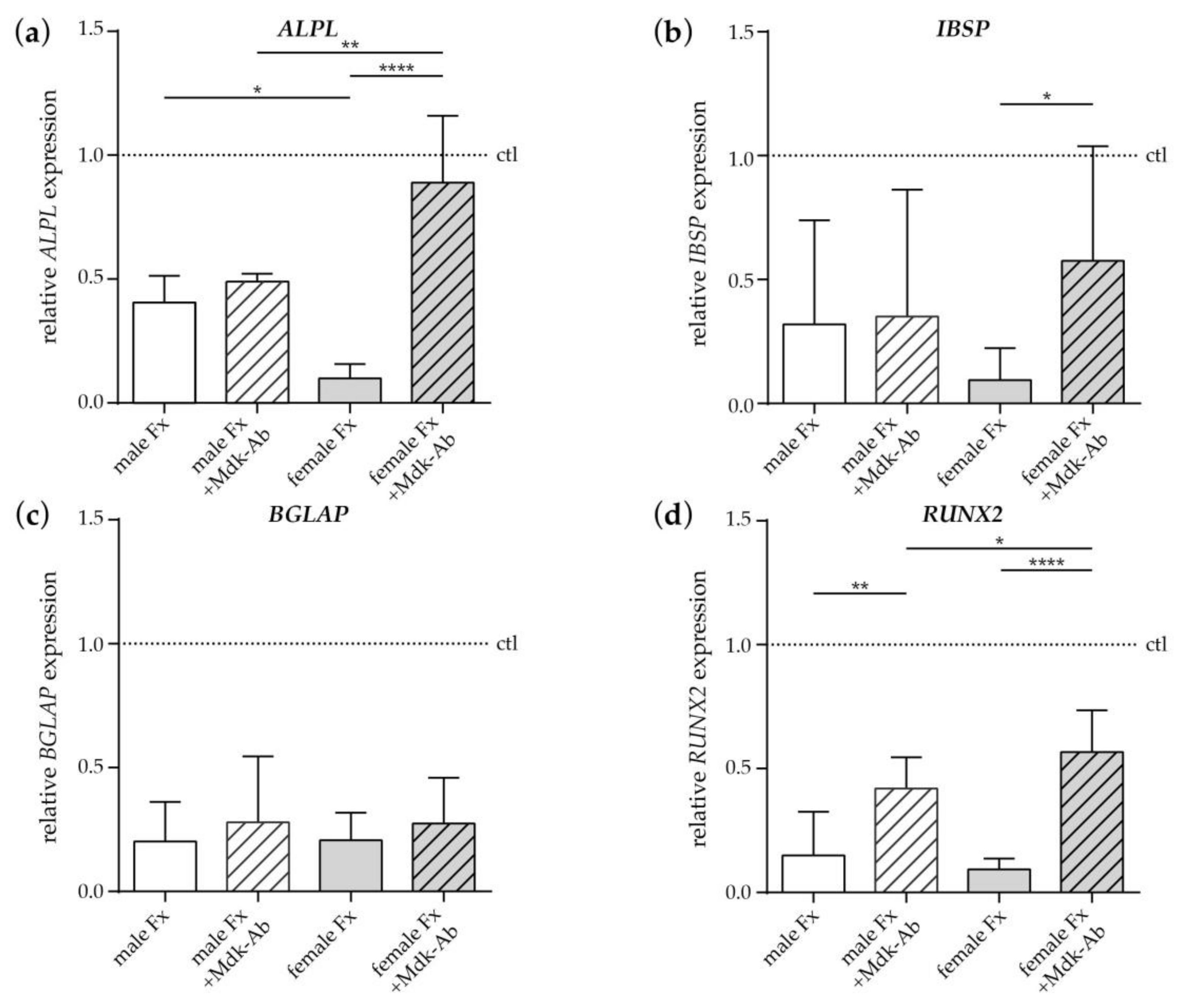
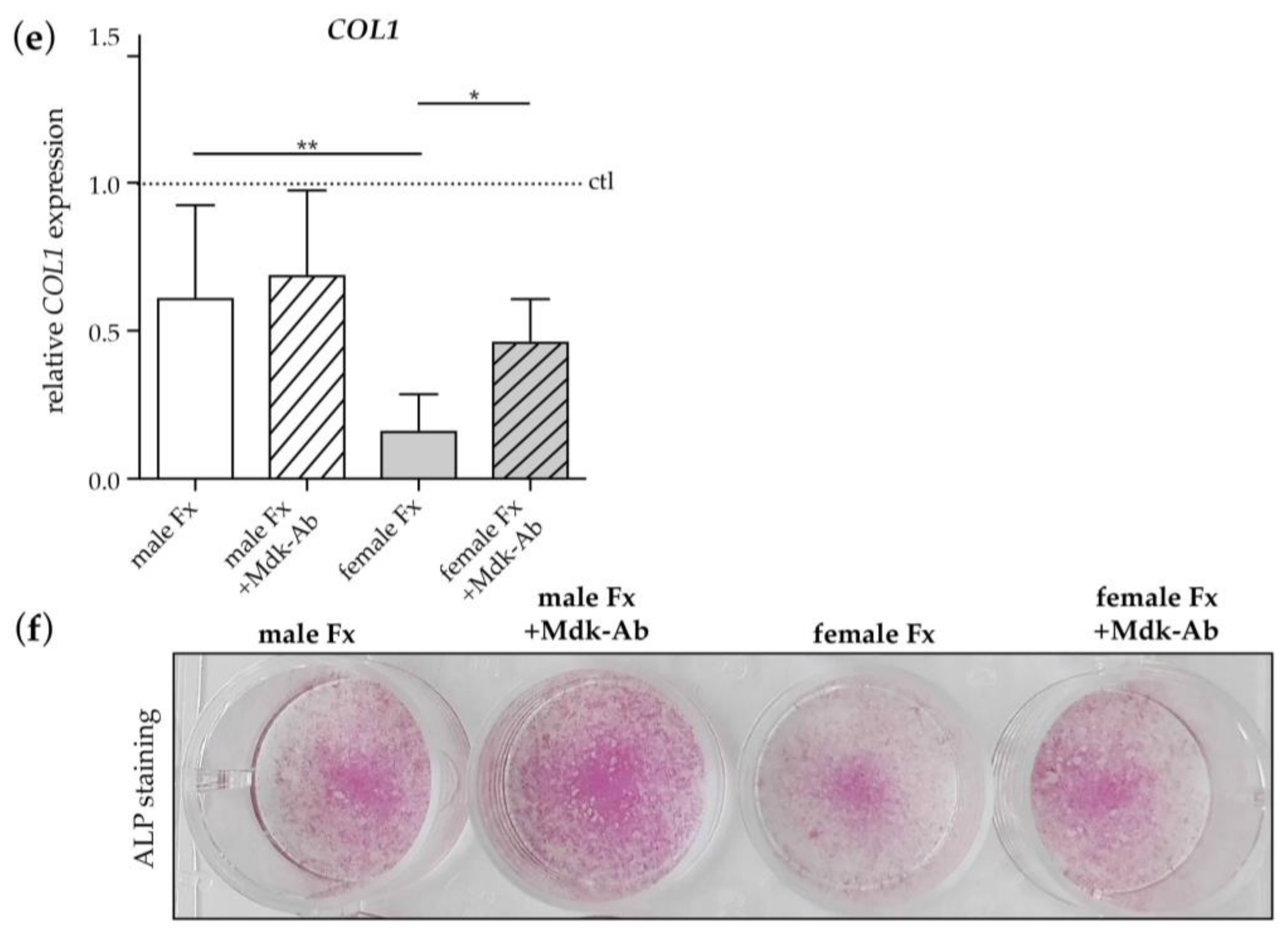
2.2.3. Effects of Fracture Sera on Osteogenic Differentiation of Human MSCs
3. Discussion
4. Materials and Methods
4.1. Evaluation of The Inflammatory Response to Fracture in Mice
4.1.1. Experimental Study Design
4.1.2. Ovariectomy and Osteotomy Surgeries
4.1.3. Cytokine and Chemokine Analyses in Blood Plasma and Fracture Hematoma
4.2. Evaluation of The Inflammatory Response to Fracture in Humans
4.2.1. Clinical Study Design
4.2.2. Blood Samples
4.2.3. ELISAs
4.3. Evaluation of The Effects of Fracture Sera on Human MSCs In Vitro
4.3.1. Cell-Culture Experiments
4.3.2. qPCR
4.4. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ALPL | Alkaline phosphatase |
| BCA | Bicinchoninic acid |
| BGLAP | Bone gamma-carboxyglutamate protein |
| COL1 | Collagen-1 |
| CRP | C-reactive protein |
| Ctl | Control |
| CXCL-1 | Chemokine (C-X-C motif) ligand-1 |
| ELISA | Enzyme-linked immunosorbent assay |
| Fx | Fracture |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IBSP | Integrin-binding sialoprotein |
| IL | Interleukin |
| MCP-1 | Monocyte chemoattractant protein-1 |
| Mdk | Midkine |
| Mdk-Ab | Midkine-antibody |
| MIP-1α | Macrophage inflammatory protein-1α |
| MSC | Mesenchymal stem cell |
| ND | Non-detected |
| OVX | Ovariectomized |
| qPCR | Quantitative polymerase chain reaction |
| RNA | Ribonucleic acid |
| RUNX2 | Runt-related transcription factor 2 |
| TNF-α | Tumor necrosis factor-α |
References
- Walsh, N.C.; Gravallese, E.M. Bone loss in inflammatory arthritis: Mechanisms and treatment strategies. Curr. Opin. Rheumatol. 2004, 16, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Klarlund, M.; Hansen, M.; Jensen, K.E.; Skjodt, H.; Hyldstrup, L.; Danisit, T.G. Connective tissue metabolism in patients with unclassified polyarthritis and early rheumatoid arthritis. Relationship to disease activity, bone mineral density, and radiographic outcome. J. Rheumatol. 2004, 31, 1698–1708. [Google Scholar] [PubMed]
- Wu, Y.Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral. Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; De Martinis, M.; Ciccarelli, F.; Saitta, S.; Imbesi, S.; Mannucci, C.; Gangemi, S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Koditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen regulation of immune cell bone interactions. Ann. N. Y. Acad. Sci. 2006, 1068, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Estrogen deficiency T cells and bone loss. Cell. Immunol. 2008, 252, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quinones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Routley, C.E.; Ashcroft, G.S. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen. 2009, 17, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Stubelius, A.; Andersson, A.; Islander, U.; Carlsten, H. Ovarian hormones in innate inflammation. Immunobiology 2017, 222, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Dodsworth, J.; van Boxtel, E.; Tarnuzzer, R.W.; Horan, M.A.; Schultz, G.S.; Ferguson, M.W. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997, 3, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Walters, G.; Pountos, I.; Giannoudis, P.V. The cytokines and micro-environment of fracture haematoma: Current evidence. J. Tissue Eng. Regen. Med. 2018, 12, e1662–e1677. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Nikolaou, V.S.; Efstathopoulos, N.; Kontakis, G.; Kanakaris, N.K.; Giannoudis, P.V. The influence of osteoporosis in femoral fracture healing time. Injury 2009, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Miclau, T.; Chow, S.K.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47, S21–S26. [Google Scholar] [CrossRef]
- Beil, F.T.; Barvencik, F.; Gebauer, M.; Seitz, S.; Rueger, J.M.; Ignatius, A.; Pogoda, P.; Schinke, T.; Amling, M. Effects of estrogen on fracture healing in mice. J. Trauma Acute Care Surg. 2010, 69, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Yu, R.; Zhao, G.F.; Wang, J.W. Early period of fracture healing in ovariectomized rats. Chin. J. Traumatol. 2003, 6, 160–166. [Google Scholar] [PubMed]
- Hatano, H.; Siegel, H.J.; Yamagiwa, H.; Bronk, J.T.; Turner, R.T.; Bolander, M.E.; Sarkar, G. Identification of estrogen-regulated genes during fracture healing, using DNA microarray. J. Bone Miner. Metab. 2004, 22, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Namkung-Matthai, H.; Appleyard, R.; Jansen, J.; Hao Lin, J.; Maastricht, S.; Swain, M.; Mason, R.S.; Murrell, G.A.; Diwan, A.D.; Diamond, T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 2001, 28, 80–86. [Google Scholar] [CrossRef]
- Meyer, R.A., Jr.; Tsahakis, P.J.; Martin, D.F.; Banks, D.M.; Harrow, M.E.; Kiebzak, G.M. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J. Orthop. Res. 2001, 19, 428–435. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, W.; Xu, S.W.; Yang, D.S.; Wang, Y.; Lin, M.; Zhao, G.F. Osteoporosis influences the middle and late periods of fracture healing in a rat osteoporotic model. Chin. J. Traumatol 2005, 8, 111–116. [Google Scholar] [PubMed]
- Hao, Y.J.; Zhang, G.; Wang, Y.S.; Qin, L.; Hung, W.Y.; Leung, K.; Pei, F.X. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone 2007, 41, 631–638. [Google Scholar] [PubMed]
- Islam, A.A.; Rasubala, L.; Yoshikawa, H.; Shiratsuchi, Y.; Ohishi, M. Healing of fractures in osteoporotic rat mandible shown by the expression of bone morphogenetic protein-2 and tumour necrosis factor-alpha. Br. J. Oral Maxillofac. Surg. 2005, 43, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Fischer, V.; Prystaz, K.; Liedert, A.; Ignatius, A. The inflammatory phase of fracture healing is influenced by oestrogen status in mice. Eur. J. Med. Res. 2017, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Liedert, A.; Mattausch, L.; Rontgen, V.; Blakytny, R.; Vogele, D.; Pahl, M.; Bindl, R.; Neunaber, C.; Schinke, T.; Harroch, S.; et al. Midkine-deficiency increases the anabolic response of cortical bone to mechanical loading. Bone 2011, 48, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Neunaber, C.; Catala-Lehnen, P.; Beil, F.T.; Marshall, R.P.; Kanbach, V.; Baranowsky, A.; Lehmann, W.; Streichert, T.; Ignatius, A.; Muramatsu, T.; et al. Increased trabecular bone formation in mice lacking the growth factor midkine. J. Bone Miner. Res. 2010, 25, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Heilmann, A.; Rapp, A.E.; Roessler, R.; Schinke, T.; Amling, M.; Ignatius, A.; Liedert, A. Antagonizing midkine accelerates fracture healing in mice by enhanced bone formation in the fracture callus. Br. J. Pharmacol. 2016, 173, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cells Mater. 2016, 32, 152–162. [Google Scholar] [CrossRef]
- Kayal, R.A.; Siqueira, M.; Alblowi, J.; McLean, J.; Krothapalli, N.; Faibish, D.; Einhorn, T.A.; Gerstenfeld, L.C.; Graves, D.T. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J. Bone Miner. Res. 2010, 25, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Glass, G.E.; Ersek, A.; Freidin, A.; Williams, G.A.; Gowers, K.; Espirito Santo, A.I.; Jeffery, R.; Otto, W.R.; Poulsom, R.; et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol. Med. 2015, 7, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Halici, Z.; Albayrak, A.; Polat, B.; Karakus, E.; Yildirim, O.S.; Bayir, Y.; Cadirci, E.; Ayan, A.K.; Aksakal, A.M. Treatment with Carnitine Enhances Bone Fracture Healing under Osteoporotic and/or Inflammatory Conditions. Basic Clin. Pharmacol. Toxicol. 2015, 117, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Inoue, E.; Imai, Y. Female sex hormones ameliorate arthritis in SKG mice. Biochem. Biophys. Res. Commun. 2013, 434, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Kemmler, J.; Heidler, V.; Prystaz, K.; Schinke, T.; Amling, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A.; Liedert, A. Inhibition of Midkine Augments Osteoporotic Fracture Healing. PLoS ONE 2016, 11, e0159278. [Google Scholar] [CrossRef] [PubMed]
- Kroner, J.; Kovtun, A.; Kemmler, J.; Messmann, J.J.; Strauss, G.; Seitz, S.; Schinke, T.; Amling, M.; Kotrba, J.; Froebel, J.; et al. Mast Cells Are Critical Regulators of Bone Fracture-Induced Inflammation and Osteoclast Formation and Activity. J. Bone Miner. Res. 2017, 32, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Chang, M.K.; Maylin, E.R.; Kohler, T.; Muller, R.; Wu, A.C.; Van Rooijen, N.; Sweet, M.J.; Hume, D.A.; Raggatt, L.J.; et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 2011, 26, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, Y.; Ti, Y.; Wang, J.; Zhao, J.; Qian, H. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin. Exp. Pharmacol. Physiol. 2017, 44, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Geissler, S.; Taylor, W.R.; Schmidt-Bleek, K.; Juelke, K.; Schwachmeyer, V.; Dahne, M.; Hartwig, T.; Akyuz, L.; Meisel, C.; et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013, 5, 177ra36. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, J.; Bindl, R.; McCook, O.; Wagner, F.; Groger, M.; Wagner, K.; Scheuerle, A.; Radermacher, P.; Ignatius, A. Exposure to 100% Oxygen Abolishes the Impairment of Fracture Healing after Thoracic Trauma. PLoS ONE 2015, 10, e0131194. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, S.; Bindl, R.; Kurz, J.; Wehner, T.; Ehrnthaller, C.; Knoferl, M.W.; Gebhard, F.; Huber-Lang, M.; Claes, L.; Ignatius, A. Experimental blunt chest trauma impairs fracture healing in rats. J. Orthop. Res. 2011, 29, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.; Halici, Z.; Cadirci, E.; Albayrak, A.; Karakus, E.; Bayir, Y.; Bilen, H.; Sahin, A.; Yuksel, T.N. The effect of alpha-lipoic acid in ovariectomy and inflammation-mediated osteoporosis on the skeletal status of rat bone. Eur. J. Pharmacol. 2013, 718, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Toth, B.; Kitchens, W.C.; Schwacha, M.G.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. Estradiol’s effect on portal response to endothelin-1 after trauma-hemorrhage. J. Surg. Res. 2004, 121, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gatson, J.W.; Maass, D.L.; Simpkins, J.W.; Idris, A.H.; Minei, J.P.; Wigginton, J.G. Estrogen treatment following severe burn injury reduces brain inflammation and apoptotic signaling. J. Neuroinflammation 2009, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, Y.; Wang, P.; Jarrar, D.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann. Surg 2000, 232, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Qi, M.; Fan, Q.W.; Ito, K.; Akiyama, S.; Kasai, Y.; Matsuyama, M.; Muramatsu, T.; Kadomatsu, K. Expression of midkine in the early stage of carcinogenesis in human colorectal cancer. Br. J. Cancer 1999, 79, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Konishi, N.; Nakamura, M.; Nakaoka, S.; Hiasa, Y.; Cho, M.; Uemura, H.; Hirao, Y.; Muramatsu, T.; Kadomatsu, K. Immunohistochemical analysis of midkine expression in human prostate carcinoma. Oncology 1999, 57, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Sakitani, H.; Tsutsumi, M.; Kadomatsu, K.; Ikematsu, S.; Takahama, M.; Iki, K.; Tsujiuchi, T.; Muramatsu, T.; Sakuma, S.; Sakaki, T.; et al. Overexpression of midkine in lung tumors induced by N-nitrosobis(2-hydroxypropyl)amine in rats and its increase with progression. Carcinogenesis 1999, 20, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Ikematsu, S.; Nakagawara, A.; Nakamura, Y.; Ohira, M.; Shinjo, M.; Kishida, S.; Kadomatsu, K. Plasma midkine level is a prognostic factor for human neuroblastoma. Cancer Sci. 2008, 99, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.W.; Guo, J.J.; Guo, L.; Jia, H.L.; Zhu, M.; Zhang, J.B.; Loffredo, C.A.; Forgues, M.; Huang, H.; Xing, X.J.; et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 3944–3954. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, T.; Yuzawa, Y.; Sato, W.; Arata-Kawai, H.; Suzuki, N.; Kato, N.; Matsuo, S.; Kadomatsu, K. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab. Investig. 2007, 87, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Horiba, M.; Kadomatsu, K.; Nakamura, E.; Muramatsu, H.; Ikematsu, S.; Sakuma, S.; Hayashi, K.; Yuzawa, Y.; Matsuo, S.; Kuzuya, M.; et al. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Investig. 2000, 105, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Toriyama, K.; Muramatsu, H.; Song, X.J.; Torii, S.; Muramatsu, T. Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: Chemotactic activity to neutrophils and association with inflammatory synovitis. J. Biochem. 1997, 122, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Mierzchala, M.; Neubauer, K.; Durek, G.; Gamian, A. Midkine, a multifunctional cytokine, in patients with severe sepsis and septic shock: A pilot study. Shock 2011, 35, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Diamond-Stanic, M.K.; Romero-Aleshire, M.J.; Hoyer, P.B.; Greer, K.; Hoying, J.B.; Brooks, H.L. Midkine, a heparin-binding protein, is increased in the diabetic mouse kidney postmenopause. Am. J. Physiol. Renal. Physiol. 2011, 300, F139–F146. [Google Scholar] [CrossRef] [PubMed]
- Pountos, I.; Georgouli, T.; Giannoudis, P.V. The effect of autologous serum obtained after fracture on the proliferation and osteogenic differentiation of mesenchymal stem cells. Cell. Mol. Biol. 2008, 54, 33–39. [Google Scholar] [PubMed]
- Kaspar, D.; Neidlinger-Wilke, C.; Holbein, O.; Claes, L.; Ignatius, A. Mitogens are increased in the systemic circulation during bone callus healing. J. Orthop. Res. 2003, 21, 320–325. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Giannoudis, P.V. Release of growth factors and the effect of age, sex, and severity of injury after long bone fracture. A preliminary report. Acta. Orthop. 2013, 84, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Kovtun, A.; Fischer, V.; Prystaz, K.; Hainzl, A.; Kroeger, C.M.; Krikki, I.; Brinker, T.J.; Ignatius, A.; Gatzka, M. Loss of p53 compensates osteopenia in murine Mysm1 deficiency. FASEB J. 2018, 32, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, V.; Kalbitz, M.; Müller-Graf, F.; Gebhard, F.; Ignatius, A.; Liedert, A.; Haffner-Luntzer, M. Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing. Int. J. Mol. Sci. 2018, 19, 2070. https://doi.org/10.3390/ijms19072070
Fischer V, Kalbitz M, Müller-Graf F, Gebhard F, Ignatius A, Liedert A, Haffner-Luntzer M. Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing. International Journal of Molecular Sciences. 2018; 19(7):2070. https://doi.org/10.3390/ijms19072070
Chicago/Turabian StyleFischer, Verena, Miriam Kalbitz, Fabian Müller-Graf, Florian Gebhard, Anita Ignatius, Astrid Liedert, and Melanie Haffner-Luntzer. 2018. "Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing" International Journal of Molecular Sciences 19, no. 7: 2070. https://doi.org/10.3390/ijms19072070
APA StyleFischer, V., Kalbitz, M., Müller-Graf, F., Gebhard, F., Ignatius, A., Liedert, A., & Haffner-Luntzer, M. (2018). Influence of Menopause on Inflammatory Cytokines during Murine and Human Bone Fracture Healing. International Journal of Molecular Sciences, 19(7), 2070. https://doi.org/10.3390/ijms19072070