Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours
Abstract
1. Introduction
2. Results
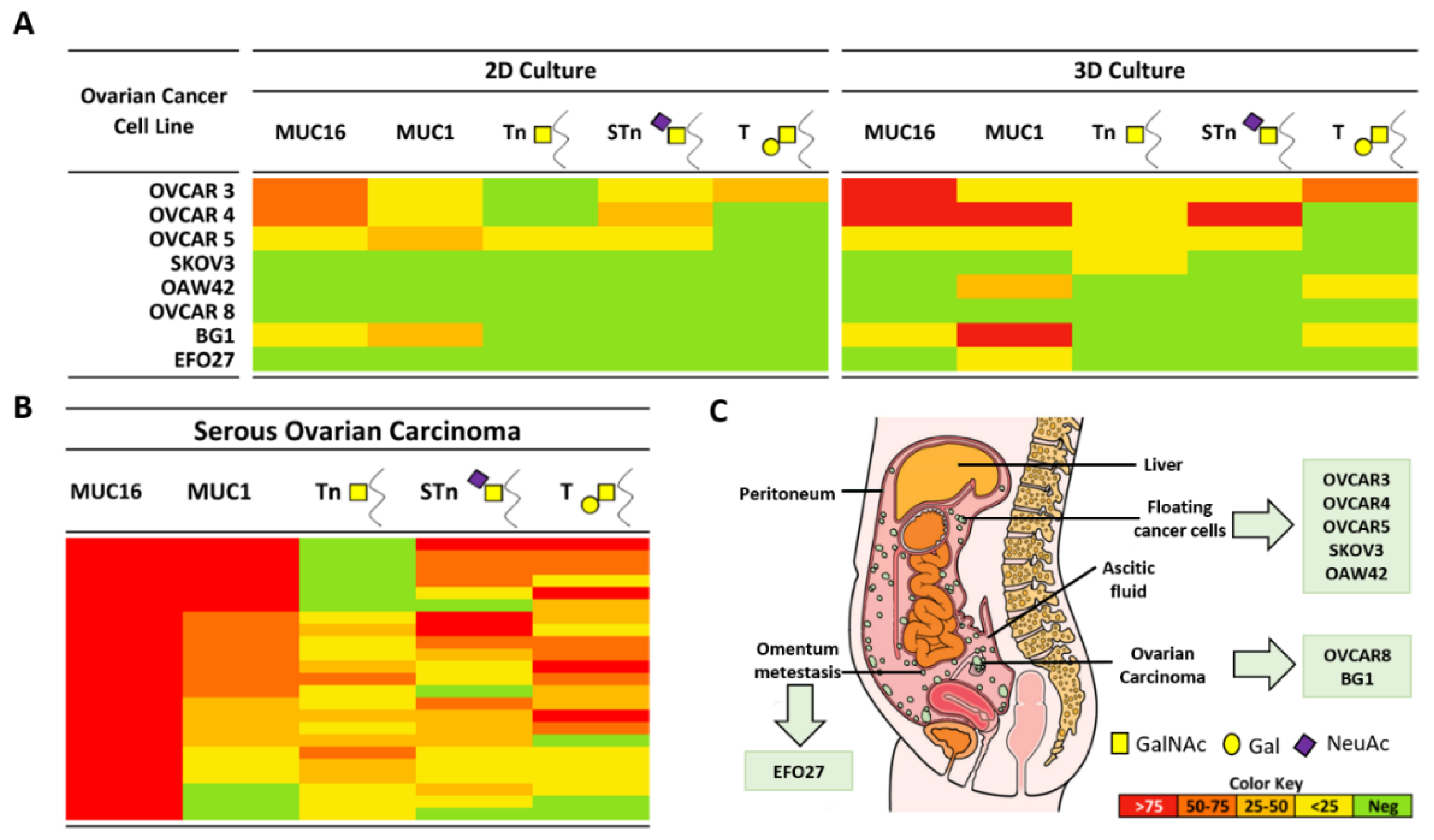
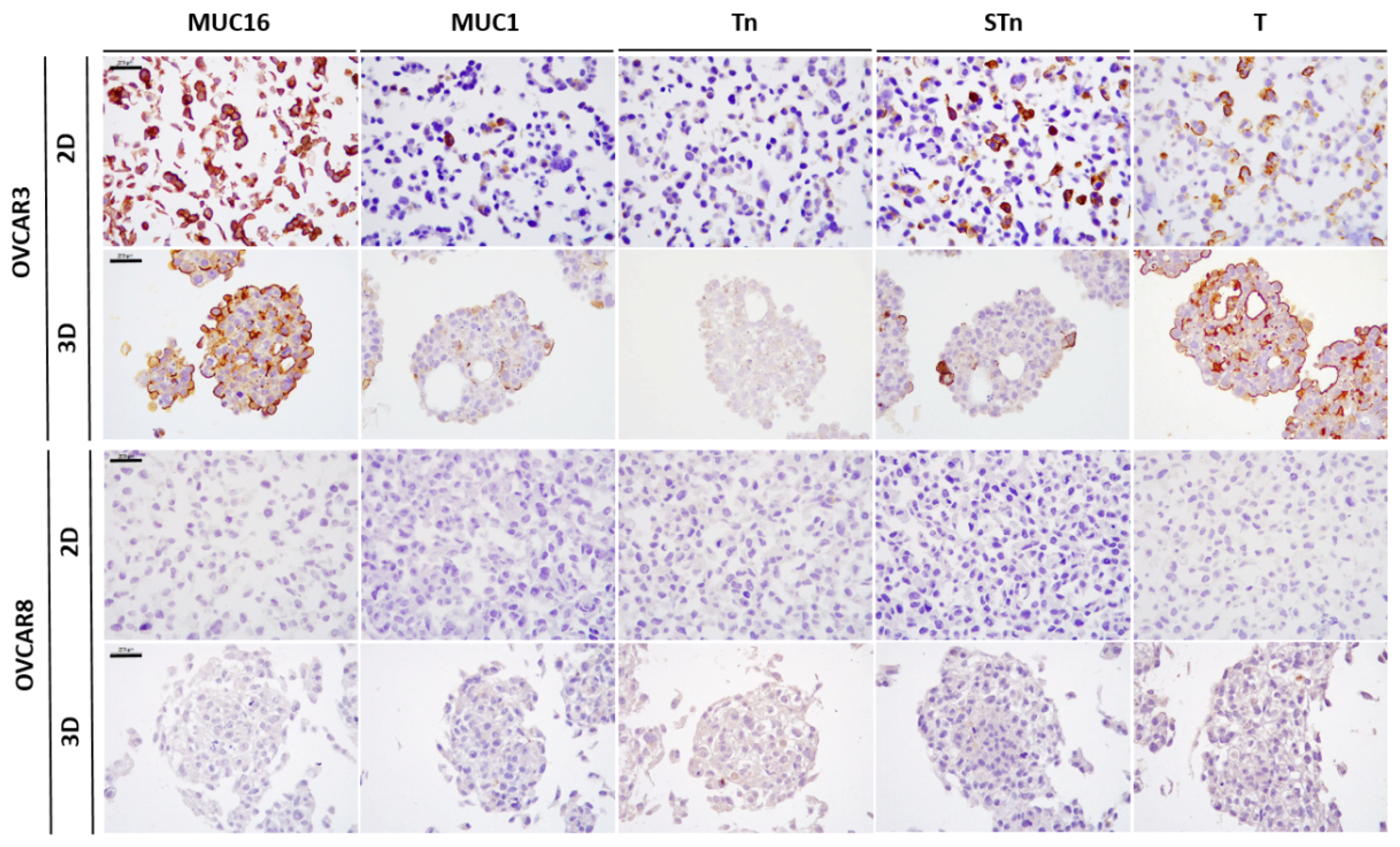
2.1. Expression of MUC16, MUC1, and Truncated O-Glycans in a Panel of Ovarian Cancer Cell Lines
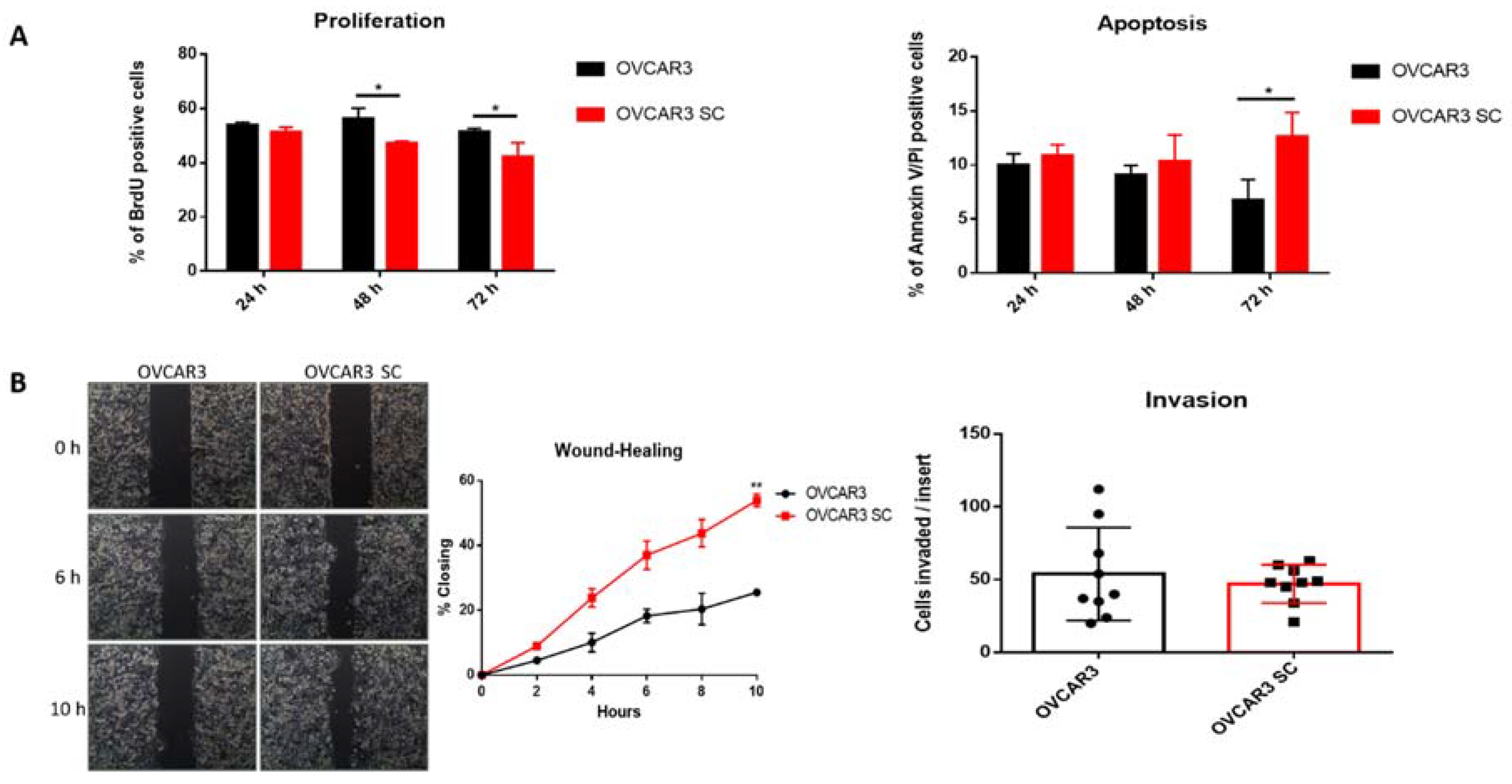
2.2. Truncated O-Glycans Reduce Proliferation while Increasing Apoptosis and Cell Migration
2.3. O-Glycosylation Capacity Affects the Mesothelial Clearance of OVCAR3
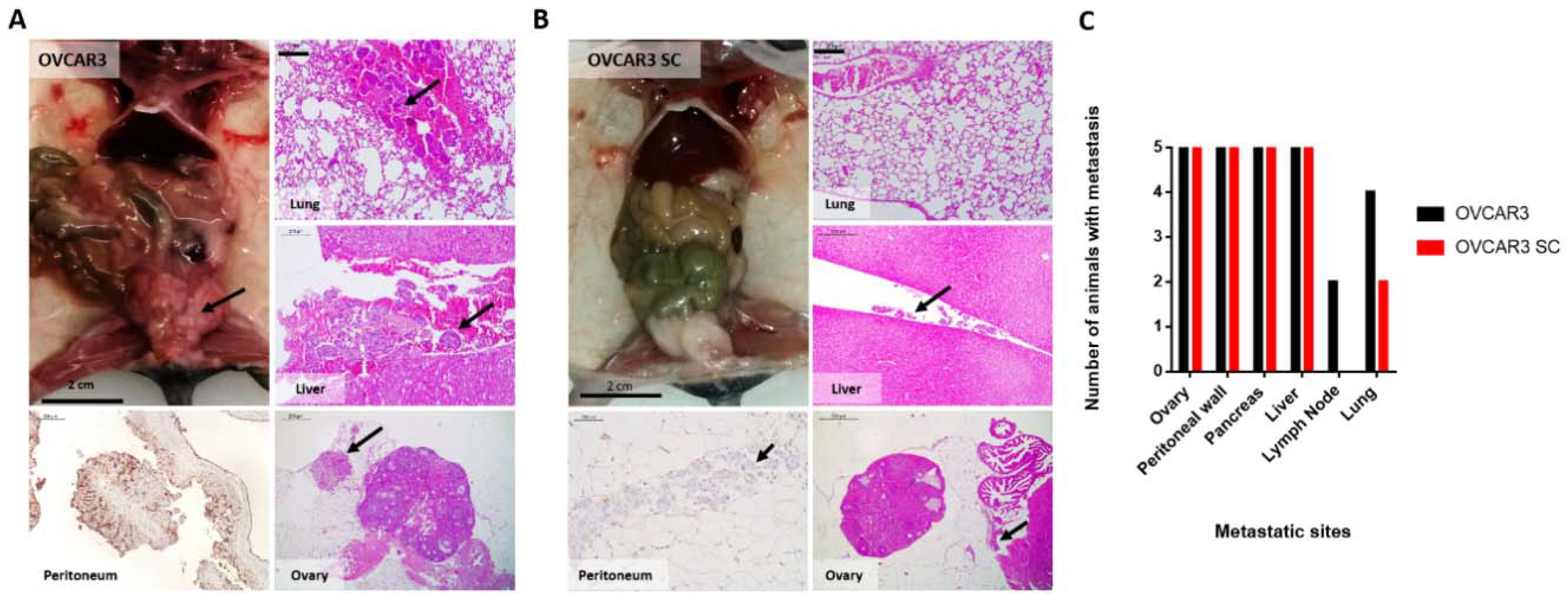
2.4. OVCAR3 Xenografts Formed Larger Tumours than OVCAR3 SC
3. Discussion
4. Materials and Methods
4.1. Cell Lines Culture
4.2. Cell Microarray (CMA) Construction and Immunocytochemistry
4.3. Generation of MeT5A Clones Stable Expressing EGFP Protein
4.4. Apoptosis Assay
4.5. Proliferation Assay
4.6. Wound Healing Assays
4.7. Matrigel Invasion Assays
4.8. Mesothelial Clearance Assay
4.9. Establishment of I.P. Xenograft Models in Nude Mice
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SOC | Serous ovarian carcinoma |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| OVCA | Ovarian cancer |
| SC | Simple cells |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| EGFP | Enhanced green fluorescent protein |
| DAPI | 4′,6-Diamidino-2-Phenylindole, Dihydrochloride |
| FBS | Fetal bovine serum |
| DNA | Deoxyribonucleic acid |
| FITC | Fluorescein isothiocyanate |
| BrdU | 5-Bromo-2′-deoxyuridine |
| G418 | Geneticin |
| CMA | Cell microarray |
| H&E | Haematoxylin and eosin |
| STR | Short tandem repeats |
| EGF | Epidermal growth factor |
| HRP | Horseradish peroxidase |
| C1GalT1 | β1,3-galactosyltransferase |
References
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.M.; Bowtell, D.D. The changing view of high-grade serous ovarian cancer. Cancer Res. 2012, 72, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R. Ovarian cancer update: Lessons from morphology, molecules, and mice. Arch. Pathol. Lab. Med. 2009, 133, 1775–1781. [Google Scholar] [PubMed]
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Nixdorf, S.; Hacker, N.F.; Heinzelmann-Schwarz, V.A. Reliable in vitro studies require appropriate ovarian cancer cell lines. J. Ovarian Res. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Wilson, M.; Ward, J.H.; Rahman, C.V.; Peet, A.C.; Macarthur, D.C.; Rose, F.R.; Grundy, R.G.; Rahman, R. Recapitulation of tumor heterogeneity and molecular signatures in a 3D brain cancer model with decreased sensitivity to histone deacetylase inhibition. PLoS ONE 2012, 7, e52335. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Marcos-Silva, L.; Pereira, D.; Pinto, R.; Almeida, R.; Söderberg, O.; Mandel, U.; Clausen, H.; Felix, A.; Lunet, N.; et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol. Oncol. 2015, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Kong, Y.; Bennett, E.P.; Mandel, U.; Wandall, H.; Levery, S.B.; Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 2011, 8, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Cummings, R.D. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. USA 2002, 99, 16613–16618. [Google Scholar] [CrossRef] [PubMed]
- Kui Wong, N.; Easton, R.L.; Panico, M.; Sutton-Smith, M.; Morrison, J.C.; Lattanzio, F.A.; Morris, H.R.; Clark, G.F.; Dell, A.; et al. Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. J. Biol. Chem. 2003, 278, 28619–28634. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M.; et al. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A.; Monotoya-Rodriguez, I.A.; Schenken, R.S. Whole explants of peritoneum and endometrium: A novel model of the early endometriosis lesion. Fertil. Steril. 1999, 71, 56–60. [Google Scholar] [CrossRef]
- Kenny, H.A.; Nieman, K.M.; Mitra, A.K.; Lengyel, E. The first line of intra-abdominal metastatic attack: Breaching the mesothelial cell layer. Cancer Discov. 2011, 1, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.A.; Brugge, J.S. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011, 1, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, R.A.; Selfors, L.M.; Iwanicki, M.P.; Elias, K.M.; Karst, A.; Piao, H.; Ince, T.A.; Drage, M.G.; Dering, J.; Konecny, G.E.; et al. Mesenchymal gene program-expressing ovarian cancer spheroids exhibit enhanced mesothelial clearance. J. Clin. Investig. 2014, 124, 2611–2625. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.W.; Best, C.J.; Bichsel, V.E.; Cole, K.A.; Greenhut, S.F.; Hewitt, S.M.; Ahram, M.; Gathright, Y.B.; Merino, M.J.; Strausberg, R.L.; et al. Evaluation of non-formalin tissue fixation for molecular profiling studies. Am. J. Pathol. 2002, 160, 449–457. [Google Scholar] [CrossRef]
- Taylor, C.R.; Levenson, R.M. Quantification of immunohistochemistry—Issues concerning methods, utility and semiquantitative assessment II. Histopathology 2006, 49, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Stimpfl, M.; Schmid, B.C.; Schiebel, I.; Tong, D.; Leodolter, S.; Obermair, A.; Zeillinger, R. Expression of mucins and cytokeratins in ovarian cancer cell lines. Cancer Lett. 1999, 145, 133–141. [Google Scholar] [CrossRef]
- Reinartz, S.; Failer, S.; Schuell, T.; Wagner, U. CA125 (MUC16) gene silencing suppresses growth properties of ovarian and breast cancer cells. Eur. J. Cancer 2012, 48, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [PubMed]
- Petignat, P.; Joris, F.; Obrist, R. How CA 125 is used in routine clinical practice. Eur. J. Cancer 2000, 36, 1933–1937. [Google Scholar] [CrossRef]
- Chen, K.; Gentry-Maharaj, A.; Burnell, M.; Steentoft, C.; Marcos-Silva, L.; Mandel, U.; Jacobs, I.; Dawnay, A.; Menon, U.; Blixt, O. Microarray Glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J. Proteome Res. 2013, 12, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.M.; Castro, C.M.; Chung, J.; Miller, N.C.; Ullal, A.V.; Castano, M.D.; Penson, R.T.; Lee, H.; Birrer, M.J.; Weissleder, R. Ascites analysis by a microfluidic chip allows tumor-cell profiling. Proc. Natl. Acad. Sci. USA 2013, 110, E4978–E4986. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, Y.; Chen, D.; Yang, C.; Kai, C.; Wang, X.; Shi, F.; Dou, J. Observation of ovarian cancer stem cell behavior and investigation of potential mechanisms of drug resistance in three-dimensional cell culture. J. Biosci. Bioeng. 2014, 118, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zietarska, M.; Maugard, C.M.; Filali-Mouhim, A.; Alam-Fahmy, M.; Tonin, P.N.; Provencher, D.M.; Mes-Masson, A.M. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Mol. Carcinog. 2007, 46, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Yang, J.; Dickinson, N.; Ogata, S.; Itzkowitz, S.H. Enzymatic basis for sialyl-Tn expression in human colon cancer cells. Glycoconj. J. 1998, 15, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Orntoft, T.F.; Vestergaard, E.M. Clinical aspects of altered glycosylation of glycoproteins in cancer. Electrophoresis 1999, 20, 362–371. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001, 491, 369–402. [Google Scholar] [PubMed]
- Davidson, B.; Berner, A.; Nesland, J.M.; Risberg, B.; Kristensen, G.B.; Trope, C.G.; Bryne, M. Carbohydrate antigen expression in primary tumors, metastatic lesions, and serous effusions from patients diagnosed with epithelial ovarian carcinoma: Evidence of up-regulated Tn and Sialyl Tn antigen expression in effusions. Hum. Pathol. 2000, 31, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F. T and Tn, general carcinoma autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Huang, M.J.; Liao, Y.Y.; Chen, C.H.; Huang, M.C. C1GALT1 Seems to Promote In Vitro Disease Progression in Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Gotlieb, W.H.; Ben-Baruch, G.; Kopolovic, J.; Goldberg, I.; Nesland, J.M.; Berner, A.; Bjamer, A.; Bryne, M. Expression of carbohydrate antigens in advanced-stage ovarian carcinomas and their metastases-A clinicopathologic study. Gynecol. Oncol. 2000, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Silva, L.; Ricardo, S.; Chen, K.; Blixt, O.; Arigi, E.; Pereira, D.; Høgdall, E.; Mandel, U.; Bennett, E.P.; Vakhrushev, S.Y.; et al. A novel monoclonal antibody to a defined peptide epitope in MUC16. Glycobiology 2015, 25, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.W.; Kim, K.J.; Munoz, P.A.; Evans, C.B.; Asofsky, R. Monoclonal antibodies to stage-specific, species-specific, and cross-reactive antigens of the rodent malarial parasite, Plasmodium yoelii. Infect. Immun. 1981, 32, 563–570. [Google Scholar] [PubMed]
- Mandel, U.; Petersen, O.W.; Sorensen, H.; Vedtofte, P.; Hakomori, S.; Clausen, H.; Dabelsteen, E. Simple mucin-type carbohydrates in oral stratified squamous and salivary gland epithelia. J. Investig. Dermatol. 1991, 97, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, T.; Clausen, H.; Hirohashi, S.; Ogawa, T.; Iijima, H.; Hakomori, S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2—6 α-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988, 48, 2214–2220. [Google Scholar] [PubMed]
- Zen, K.; Notarfrancesco, K.; Oorschot, V.; Slot, J.W.; Fisher, A.B.; Shuman, H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am. J. Physiol. 1998, 275 Pt 1, L172–L183. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, R.A.; Iwanicki, M.P.; Brugge, J.S. In vitro mesothelial clearance assay that models the early steps of ovarian cancer metastasis. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Azar, H.A.; Hansen, C.T.; Costa, J. N:NIH(S)-nu/nu mice with combined immunodeficiency: A new model for human tumor heterotransplantation. J. Natl. Cancer Inst. 1980, 65, 421–430. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, R.; Marcos-Silva, L.; Mendes, N.; Pereira, D.; Brito, C.; Jacob, F.; Steentoft, C.; Mandel, U.; Clausen, H.; David, L.; et al. Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours. Int. J. Mol. Sci. 2018, 19, 2045. https://doi.org/10.3390/ijms19072045
Coelho R, Marcos-Silva L, Mendes N, Pereira D, Brito C, Jacob F, Steentoft C, Mandel U, Clausen H, David L, et al. Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours. International Journal of Molecular Sciences. 2018; 19(7):2045. https://doi.org/10.3390/ijms19072045
Chicago/Turabian StyleCoelho, Ricardo, Lara Marcos-Silva, Nuno Mendes, Daniela Pereira, Catarina Brito, Francis Jacob, Catharina Steentoft, Ulla Mandel, Henrik Clausen, Leonor David, and et al. 2018. "Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours" International Journal of Molecular Sciences 19, no. 7: 2045. https://doi.org/10.3390/ijms19072045
APA StyleCoelho, R., Marcos-Silva, L., Mendes, N., Pereira, D., Brito, C., Jacob, F., Steentoft, C., Mandel, U., Clausen, H., David, L., & Ricardo, S. (2018). Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours. International Journal of Molecular Sciences, 19(7), 2045. https://doi.org/10.3390/ijms19072045






