FGF Family: From Drug Development to Clinical Application
Abstract
1. Introduction
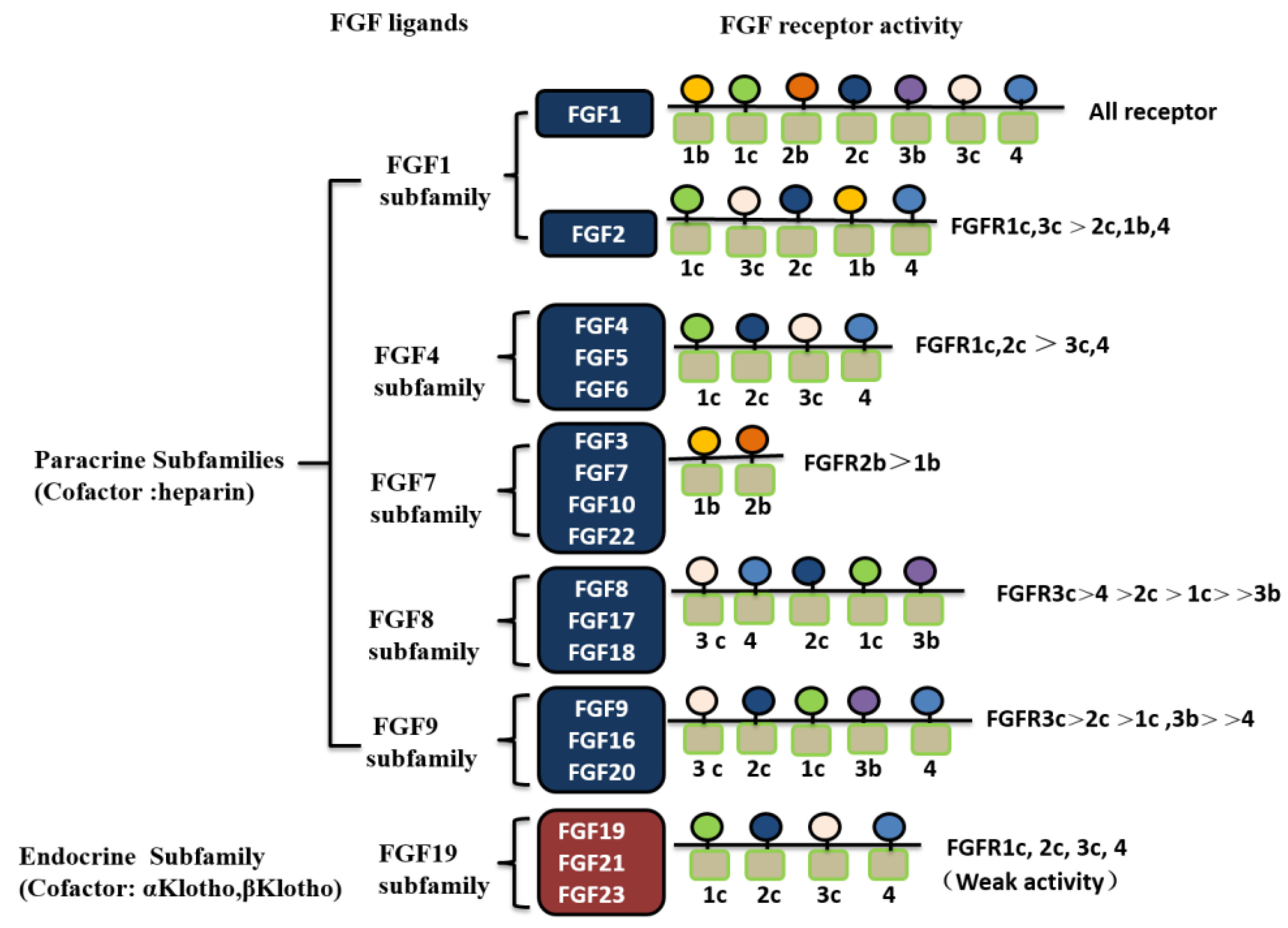
2. FGFs and Their Receptors
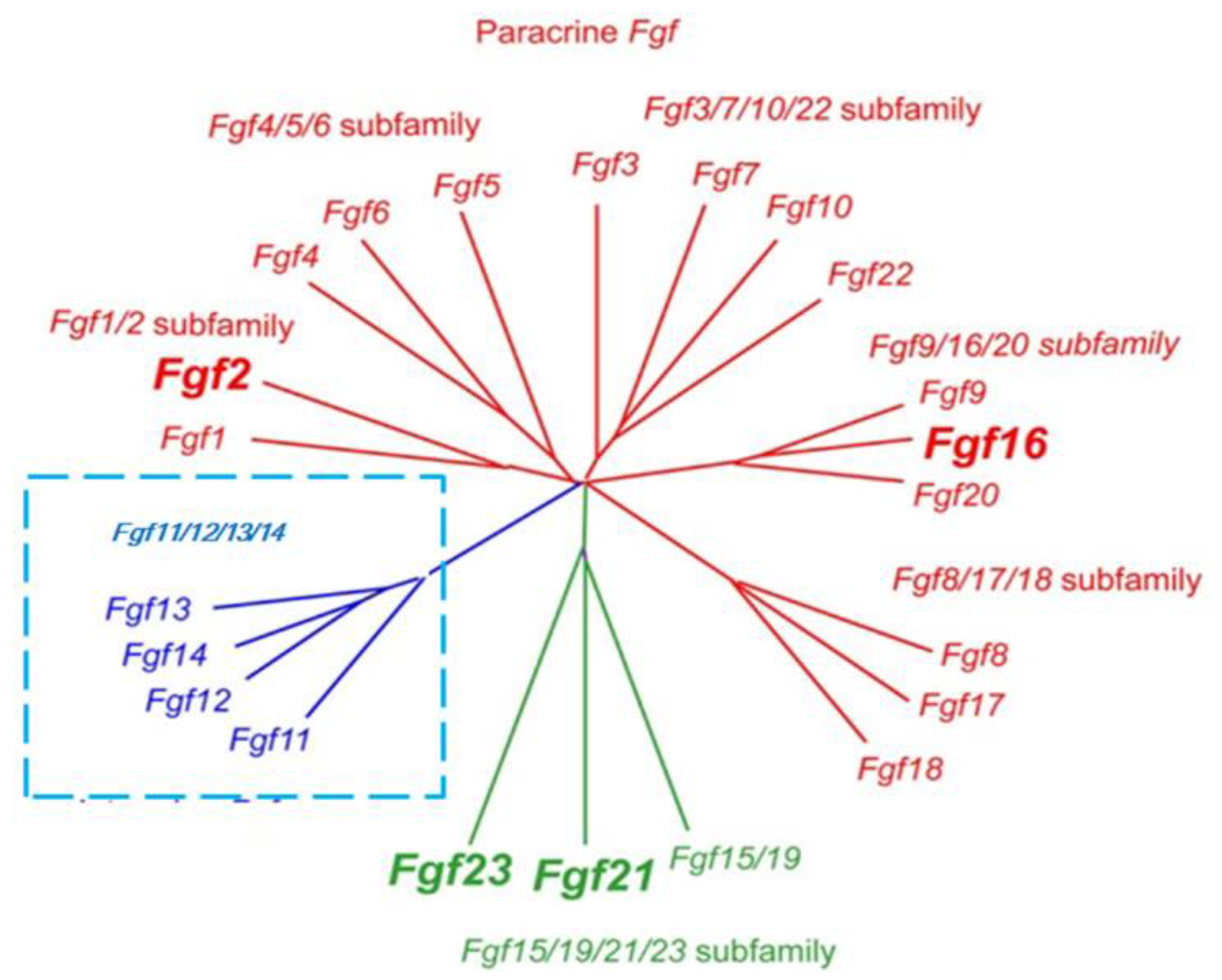
2.1. Classification of FGF Family Members
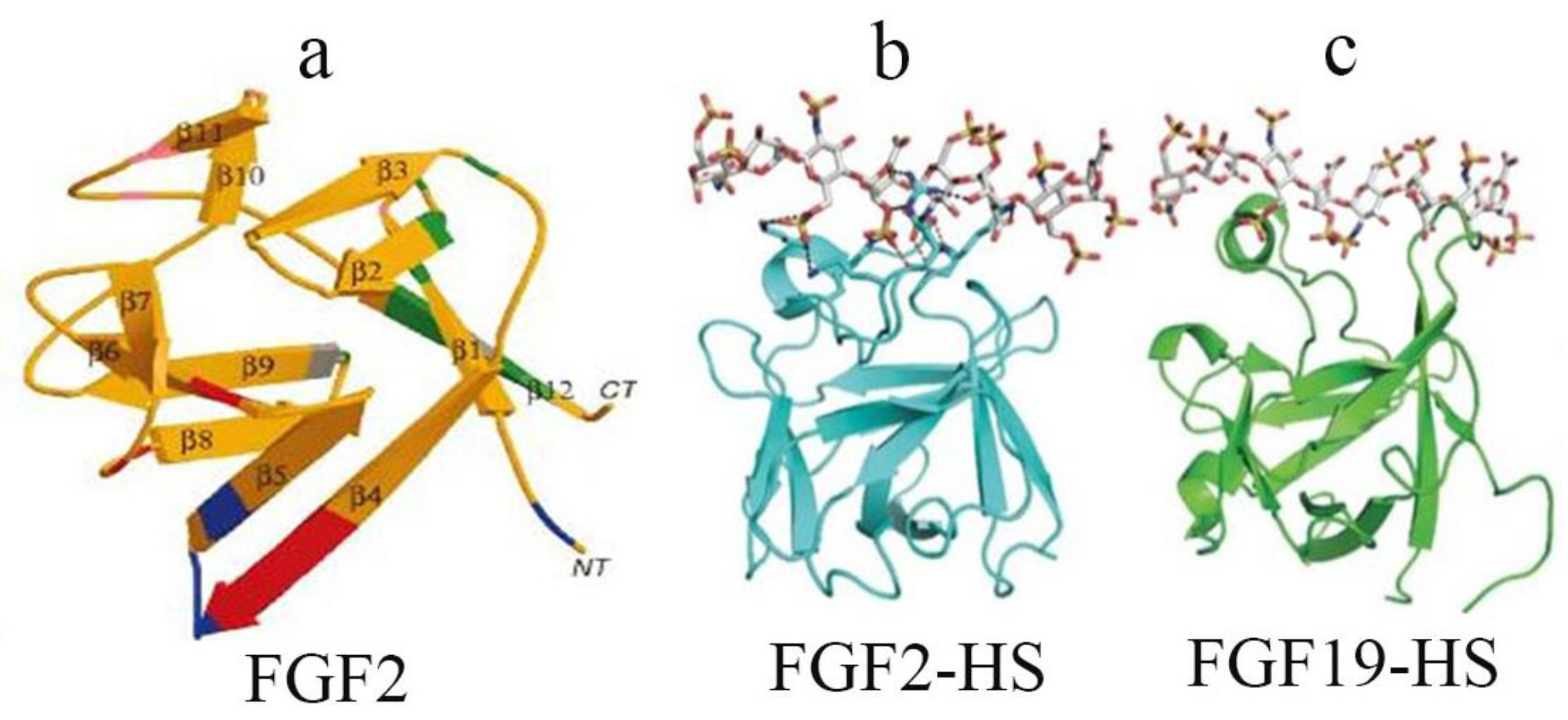
2.2. Structural Characteristics of FGF Family Members and Their Receptors
3. Drug Development and Clinical Application of FGF Family
3.1. New FGF Drugs for Wound Healing and Their Clinical Application
3.2. Prospect of FGF Applications in Endocrine Metabolic Regulation
3.3. Advances in FGFR Inhibitors with Antitumor Activity
3.4. Advances in Basic and Applied FGF Research in China
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Armelin, H.A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc. Natl. Acad. Sci. USA 1973, 70, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 1974, 249, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T. Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: Recent findings and implications for their pharmacological application. Biol. Pharm. Bull. 2014, 37, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, reviews3005.1–reviews3005.12. [Google Scholar] [CrossRef] [PubMed]
- Aimi, F.; Georgiopoulou, S.; Kalus, I.; Lehner, F.; Hegglin, A.; Limani, P.; de Lima, V.G.; Rüegg, M.A.; Hall, M.N.; Lindenblatt, N.; et al. Endothelial Rictor is crucial for midgestational development and sustained and extensive FGF2-induced neovascularization in the adult. Sci. Rep. 2015, 5, 17705. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Sung, M.C.; Chen, P.K.; Chang, B.I.; Lee, F.T.; Cho, C.F.; Hsieh, T.T.; Huang, Y.C.; Li, Y.H.; Shi, G.Y.; et al. FGFR1 mediates recombinant thrombomodulin domain-induced angiogenesis. Cardiovasc. Res. 2015, 105, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocr. Met. 2015, 26, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Herriges, J.C.; Verheyden, J.M.; Zhang, Z.; Sui, P.; Zhang, Y.; Anderson, M.J.; Swing, D.A.; Lewandoski, M.; Sun, X. FGF-Regulated ETV Transcription Factors Control FGF-SHH Feedback Loop in Lung Branching. Dev. Cell 2015, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Blaber, S.I.; Diaz, J.; Blaber, M. Accelerated healing in NONcNZO10/LtJ type 2 diabetic mice by FGF-1. Wound Repair Regen. 2015, 23, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Billings, P.C.; Pacifici, M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: Mechanisms and mysteries. Connect. Tissue Res. 2015, 56, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, K.H.; Park, K.S.; Kong, I.D.; Cha, S.K. Biological Role of Anti-aging Protein Klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Comps-Agrar, L.; Dunshee, D.R.; Eaton, D.L.; Sonoda, J. Unliganded fibroblast growth factor receptor 1 forms density-independent dimers. J. Biol. Chem. 2015, 290, 24166–24177. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Freitas, I.; Owen, B.M. Metabolic roles of endocrine fibroblast growth factors. Curr. Opin. Pharmacol. 2015, 25, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Belov, A.A.; Mohammadi, M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb. Perspect. Biol. 2013, 5, a015958. [Google Scholar] [CrossRef] [PubMed]
- Bernuy, J.; Gonzales, G.F. Bone mineral metabolism in patients with chronic kidney disease: Review of its pathophysiology and morbimortality. Rev. Peru. Med. Exp. Salud Publica 2015, 32, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Teven, C.M.; Farina, E.M.; Rivas, J.; Reid, R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Hierro, C.; Rodon, J.; Tabernero, J. Fibroblast Growth Factor (FGF) Receptor/FGF Inhibitors: Novel Targets and Strategies for Optimization of Response of Solid Tumors. Semin. Oncol. 2015, 42, 801–819. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.K.; Garbi, M.; Zampieri, N.; Eliseenkova, A.V.; Ornitz, D.M.; Goldfarb, M.; Mohammadi, M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 2003, 278, 34226–34236. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Ornitz, D.M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 2008, 237, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S. Actions and mode of actions of FGF19 subfamily members. Endocr. J. 2008, 55, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Blaber, M.; DiSalvo, J.; Thomas, K.A. X-ray crystal structure of human acidic fibroblast growth factor. Biochemistry 1996, 35, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Tiong, K.H.; Mah, L.Y.; Leong, C.O. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis 2013, 18, 1447–1468. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, P.; Karavanova, I.; Thesleff, I. Responsiveness of developing dental tissues to fibroblast growth factors: Expression of splicing alternatives of FGFR1, -2, -3, and of FGFR4; and stimulation of cell proliferation by FGF-2, -4, -8, and -9. Dev. Genet. 1998, 22, 374–385. [Google Scholar] [CrossRef]
- Colin, S.; Jeanny, J.C.; Mascarelli, F.; Vienet, R.; Al-Mahmood, S.; Courtois, Y.; Labarre, J. In vivo involvement of heparan sulfate proteoglycan in the bioavailability, internalization, and catabolism of exogenous basic fibroblast growth factor. Mol. Pharmacol. 1999, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kwabi-Addo, B.; Ropiquet, F.; Giri, D.; Ittmann, M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate 2001, 46, 163–172. [Google Scholar] [CrossRef]
- Olsen, S.K.; Ibrahimi, O.A.; Raucci, A.; Zhang, F.; Eliseenkova, A.V.; Yayon, A.; Basilico, C.; Linhardt, R.J.; Schlessinger, J.; Mohammadi, M. Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc. Natl. Acad. Sci. USA 2004, 101, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.N.; Hubbard, S.R.; Schlessinger, J.; Mohammadi, M. Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 2000, 101, 413–424. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Schlessinger, J.; Hubbard, S.R.; Mohammadi, M. Structural basis for FGF receptor dimerization and activation. Cell 1999, 98, 641–650. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lynn Miskus, M.; Lu, H.C. FGF-FGFR Mediates the Activity-Dependent Dendritogenesis of Layer IV Neurons during Barrel Formation. J. Neurosci. 2017, 37, 12094–12105. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.; Lassalle, G.; Alcouffe, C.; Bono, F. Approaches targeting the FGF-FGFR system: A review of the recent patent literature and associated advanced therapeutic agents. Pharm. Patent Anal. 2014, 3, 585–612. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Miraoui, H.; Severe, N. FGF/FGFR signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Weinstein, M.; Li, C.; Deng, C. Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 1999, 296, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Gillespie, B.; Smith, B.; Smith, W.; Lissmats, A.; Rudebeck, M.; Kullenberg, T.; Olsson, B. Pharmacokinetic and pharmacodynamic interactions between palifermin and heparin. J. Clin. Pharmacol. 2015, 55, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Goldberg, J.D.; Perales, M.A.; Berger, D.P.; van den Brink, M.R. Clinical applications of palifermin: Amelioration of oral mucositis and other potential indications. J. Cell. Mol. Med. 2013, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.D.; Zheng, J.; Castro-Malaspina, H.; Jakubowski, A.A.; Heller, G.; van den Brink, M.R.; Perales, M.A. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone Marrow Transpl. 2013, 48, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Phillips, T.J.; Falanga, V.; Odenheimer, D.J.; Parish, L.C.; Jensen, J.L.; Steed, D.L. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound Repair Regen. 2001, 9, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Freytes, C.O.; Ratanatharathorn, V.; Taylor, C.; Abboud, C.; Chesser, N.; Restrepo, A.; Arango, J.; Odenheimer, D. Phase I/II randomized trial evaluating the safety and clinical effects of repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clin. Cancer Res. 2004, 10, 8318–8324. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Komatsu, S.; Okamoto, K.; Ichikawa, D.; Shiozaki, A.; Fujiwara, H.; Murayama, Y.; Kuriu, Y.; Ikoma, H.; Nakanishi, M.; et al. A novel treatment strategy using trafermin, containing basic fibroblast growth factor, for intractable duodenal fistula following curative gastrectomy for gastric cancer-case report and literature review. Gan to Kagaku Ryoho 2012, 39, 1960–1962. [Google Scholar] [PubMed]
- Richard, J.L.; Parer-Richard, C.; Daures, J.P.; Clouet, S.; Vannereau, D.; Bringer, J.; Rodier, M.; Jacob, C.; Comte-Bardonnet, M. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double-blind, placebo-controlled study. Diabetes Care 1995, 18, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Igarashi, A.; Urabe, K.; Koga, T.; Nakayama, J.; Kawamori, R.; Tamaki, K.; Hirakata, H.; Ohura, T.; Furue, M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur. J. Dermatol. 2009, 19, 461–468. [Google Scholar] [PubMed]
- Fu, X.; Shen, Z.; Chen, Y. Basic fibroblast growth factor (bFGF) and wound healing: A multi-centers and controlled clinical trial in 1024 cases. Chin. J. Repar. Reconstr. Surg. 1998, 12, 209–211. [Google Scholar]
- Nie, K.; Li, P.; Zeng, X.; Sun, G.; Jin, W.; Wei, Z.; Wang, B.; Qi, J.; Wang, Y.; Wang, D. Clinical observation of basic fibroblast growth factor combined with topical oxygen therapy in enhancing burn wound healing. Chin. J. Repar. Reconstr. Surg. 2010, 24, 643–646. [Google Scholar]
- Hayashida, K.; Fujioka, M.; Morooka, S.; Saijo, H.; Akita, S. Effectiveness of basic fibroblast growth factor for pediatric hand burns. J. Tissue Viabil. 2016, 25, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sachs, M.C.; Hoofnagle, A.N.; Siscovick, D.S.; Ix, J.H.; Robinson-Cohen, C.; Lima, J.A.; Polak, J.F.; Blondon, M.; Ruzinski, J.; et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: The Multi-Ethnic Study of Atherosclerosis. Circ. Heart Fail. 2014, 7, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, R.L. The Roles of Fibroblast Growth Factor (FGF)-23, alpha-Klotho and Furin Protease in Calcium and Phosphate Homeostasis: A Mini-Review. Indian J. Clin. Biochem. 2014, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Ma, H.X. Significant roles of anti-aging protein klotho and fibroblast growth factor23 in cardiovascular disease. J. Geriatr. Cardiol. 2015, 12, 439–447. [Google Scholar] [PubMed]
- Itoh, N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. Front. Endocrinol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, L.; Lin, X.; Cheng, P.; He, L.; Li, X.; Lu, X.; Tan, Y.; Yang, H.; Cai, L.; et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol. Endocrinol. 2015, 29, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Haring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Gavalda-Navarro, A.; Villarroya, F. Fibroblast growth factor-21, energy balance and obesity. Mol. Cell. Endocrinol. 2015, 418 Pt 1, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.; Wolfrum, C. FGF21, energy expenditure and weight loss—How much brown fat do you need? Mol. Metab. 2015, 4, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, S.E.; Vanhorebeek, I.; Derese, I.; Gunst, J.; Van den Berghe, G. FGF21 Response to Critical Illness: Effect of Blood Glucose Control and Relation with Cellular Stress and Survival. J. Clin. Endocrinol. Metab. 2015, 100, E1319–E1327. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Pan, X.; Wu, F.; Ye, D.; Zhang, Y.; Wang, Y.; Jin, L.; Lian, Q.; Huang, Y.; Ding, H.; et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 2015, 131, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Adams, A.C. Inventing new medicines: The FGF21 story. Mol. Metab. 2014, 3, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Veniant, M.M.; Sivits, G.; Helmering, J.; Komorowski, R.; Lee, J.; Fan, W.; Moyer, C.; Lloyd, D.J. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab. 2015, 21, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Januszewski, A.S.; O’Connell, R.; Buizen, L.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Scott, R.S.; Taskinen, M.R.; et al. Relationship of fibroblast growth factor 21 with baseline and new on-study microvascular disease in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia 2015, 58, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yi, X.; Li, X.; Jiang, X. Fibroblast growth factor-21 is positively associated with atrial fibrosis in atrial fibrillation patients with rheumatic heart disease. Int. J. Clin. Exp. Pathol. 2015, 8, 14901–14908. [Google Scholar] [PubMed]
- Lin, Z.; Wu, Z.; Yin, X.; Liu, Y.; Yan, X.; Lin, S.; Xiao, J.; Wang, X.; Feng, W.; Li, X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE 2010, 5, e15534. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Bookout, A.L.; Magomedova, L.; Owen, B.M.; Consiglio, G.P.; Shimizu, M.; Zhang, Y.; Mangelsdorf, D.J.; Kliewer, S.A.; Cummins, C.L. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol. Endocrinol. 2015, 29, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; Gorini, A.; Bellasi, A.; Morrone, L.F.; Rivera, R.; Russo, L.; Santoboni, A.; Russo, D. Fibroblast growth factor 23 and parathyroid hormone predict extent of aortic valve calcifications in patients with mild to moderate chronic kidney disease. Clin. Kidney J. 2015, 8, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.C.; Heilberg, I.P.; Navis, G.; de Borst, M.H. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat. Rev. Nephrol. 2015, 11, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, V.; Shatzen, E.M.; Ward, S.C.; Davis, J.; Stevens, J.; Bi, V.; Renshaw, L.; Hawkins, N.; Wang, W.; Chen, C.; et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J. Clin. Investig. 2012, 122, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Imel, E.A.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Wooddell, M.M.; Kawakami, T.; Ito, T.; Zhang, X.; Humphrey, J.; et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Investig. 2014, 124, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Zhang, X.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Ito, T.; Vergeire, M.; Humphrey, J.S.; Glorieux, F.H.; Portale, A.A.; et al. Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J. Clin. Endocrinol. Metab. 2015, 100, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Imel, E.A.; Ruppe, M.D.; Weber, T.J.; Klausner, M.A.; Ito, T.; Vergeire, M.; Humphrey, J.; Glorieux, F.H.; Portale, A.A.; et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J. Clin. Pharmacol. 2016, 56, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Ciccarese, C.; Santoni, M.; Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Cheng, L. Targeting fibroblast growth factor receptor (FGFR) pathway in renal cell carcinoma. Expert Rev. Anticancer Ther. 2015, 15, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, X.; You, B.; Shan, Y.; Cao, X.; You, Y. High Expression of FGFR4 Enhances Tumor Growth and Metastasis in Nasopharyngeal Carcinoma. J. Cancer 2015, 6, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vida, A.; Saggese, M.; Hughes, S.; Rudman, S.; Chowdhury, S.; Smith, N.R.; Lawrence, P.; Rooney, C.; Dougherty, B.; Landers, D.; et al. Complexity of FGFR signalling in metastatic urothelial cancer. J. Hematol. Oncol. 2015, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Esposito, A.; De Placido, S.; Curigliano, G. Targeting fibroblast growth factor receptor pathway in breast cancer. Curr. Opin. Oncol. 2015, 27, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Ronca, R.; Giacomini, A.; Di Salle, E.; Coltrini, D.; Pagano, K.; Ragona, L.; Matarazzo, S.; Rezzola, S.; Maiolo, D.; Torrella, R.; et al. Long-Pentraxin 3 Derivative as a Small-Molecule FGF Trap for Cancer Therapy. Cancer Cell 2015, 28, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Tomiguchi, M.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Goto-Yamaguchi, L.; Fujiki, Y.; Fujiwara, S.; Sueta, A.; Hayashi, M.; Takeshita, T.; Inao, T.; et al. Fibroblast growth factor receptor-1 protein expression is associated with prognosis in estrogen receptor-positive/human epidermal growth factor receptor-2-negative primary breast cancer. Cancer Sci. 2016, 107, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Reis, D.; Silva, M.; Alarcao, A.M.; Ladeirinha, A.F.; d’Aguiar, M.J.; Ferreira, T.; Caramujo-Balseiro, S.; Carvalho, L. Amplification of FGFR1 gene and expression of FGFR1 protein is found in different histological types of lung carcinoma. Virchows Arch. 2016, 469, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; Van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.; Hong, M.; Kim, S.T.; Park, S.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; et al. FGFR2 in gastric cancer: Protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod. Pathol. 2016, 29, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Takanashi, M.; Yoshida, N.; Ito, Y.; Kamata, R.; Fukami, K.; Yanagihara, K.; Sakai, R. Saracatinib impairs the peritoneal dissemination of diffuse-type gastric carcinoma cells resistant to Met and fibroblast growth factor receptor inhibitors. Cancer Sci. 2014, 105, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, A.N.; Park, S.Y.; Kim, J.Y.; Park, J.Y.; Yu, J.H.; Ahn, J.H.; Gong, G. Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann. Surg. Oncol. 2014, 21, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Gangat, N.; Knudson, R.A.; Keefe, J.G.; Hanson, C.A.; Pardanani, A.; Ketterling, R.P.; Tefferi, A. Chromosome 8p11.2 translocations: Prevalence, FISH analysis for FGFR1 and MYST3, and clinicopathologic correlates in a consecutive cohort of 13 cases from a single institution. Am. J. Hematol. 2010, 85, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Kalff, A.; Spencer, A. The t(4;14) translocation and FGFR3 overexpression in multiple myeloma: Prognostic implications and current clinical strategies. Blood Cancer J. 2012, 2, e89. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, F.; Wakao, D.; Yokoyama, Y.; Uchida, Y.; Murohashi, I.; Kayano, H.; Taniwaki, M.; Matsuda, A.; Bessho, M. Fusion of ETV6 to fibroblast growth factor receptor 3 in peripheral T-cell lymphoma with a t(4;12)(p16;p13) chromosomal translocation. Cancer Res. 2001, 61, 8371–8374. [Google Scholar] [PubMed]
- Gartside, M.G.; Chen, H.; Ibrahimi, O.A.; Byron, S.A.; Curtis, A.V.; Wellens, C.L.; Bengston, A.; Yudt, L.M.; Eliseenkova, A.V.; Ma, J.; et al. Loss-of-function fibroblast growth factor receptor-2 mutations in melanoma. Mol. Cancer Res. 2009, 7, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Gartside, M.G.; Dejeza, L.C.; Powell, M.A.; Mallon, M.A.; Davies, H.; Mohammadi, M.; Futreal, P.A.; Stratton, M.R.; Trent, J.M.; et al. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene 2007, 26, 7158–7162. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, K.E.; Kompier, L.C.; de Bekker-Grob, E.W.; Zuiverloon, T.C.; Vergouwe, Y.; Zwarthoff, E.C.; Steyerberg, E.W. FGFR3 mutation analysis in voided urine samples to decrease cystoscopies and cost in nonmuscle invasive bladder cancer surveillance: A comparison of 3 strategies. J. Urol. 2013, 189, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Zuiverloon, T.C.; Tjin, S.S.; Busstra, M.; Bangma, C.H.; Boeve, E.R.; Zwarthoff, E.C. Optimization of nonmuscle invasive bladder cancer recurrence detection using a urine based FGFR3 mutation assay. J. Urol. 2011, 186, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.G.T.; Cheuk, A.T.; Tsang, P.S.; Chung, J.Y.; Song, Y.K.; Desai, K.; Yu, Y.; Chen, Q.R.; Shah, K.; Youngblood, V.; et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Investig. 2009, 119, 3395–3407. [Google Scholar] [PubMed]
- Khan, J.; Wei, J.S.; Ringner, M.; Saal, L.H.; Ladanyi, M.; Westermann, F.; Berthold, F.; Schwab, M.; Antonescu, C.R.; Peterson, C.; et al. Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nat. Med. 2001, 7, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Ishii, T.; Imada, T.; Takaba, K.; Sasaki, Y.; Maruyama-Takahashi, K.; Maekawa-Tokuda, Y.; Kusaka, H.; Akinaga, S.; Tanaka, A.; et al. A neutralizing anti-fibroblast growth factor 8 monoclonal antibody shows potent antitumor activity against androgen-dependent mouse mammary tumors in vivo. Clin. Cancer Res. 2005, 11, 3897–3904. [Google Scholar] [CrossRef] [PubMed]
- Krakstad, C.; Birkeland, E.; Seidel, D.; Kusonmano, K.; Petersen, K.; Mjos, S.; Hoivik, E.A.; Wik, E.; Halle, M.K.; Oyan, A.M.; et al. High-throughput mutation profiling of primary and metastatic endometrial cancers identifies KRAS, FGFR2 and PIK3CA to be frequently mutated. PLoS ONE 2012, 7, e52795. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Su, X.; Zhang, L.; Yin, X.; Tang, L.; Zhang, X.; Xu, Y.; Gao, Z.; Liu, K.; Zhou, M.; et al. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin. Cancer Res. 2013, 19, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Arao, T.; Hamaguchi, T.; Shimada, Y.; Kato, K.; Oda, I.; Taniguchi, H.; Koizumi, F.; Yanagihara, K.; Sasaki, H.; et al. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br. J. Cancer 2012, 106, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Maruyama-Takahashi, K.; Shimada, N.; Imada, T.; Maekawa-Tokuda, Y.; Ishii, T.; Ouchi, J.; Kusaka, H.; Miyaji, H.; Akinaga, S.; Tanaka, A.; et al. A neutralizing anti-fibroblast growth factor (FGF) 8 monoclonal antibody shows anti-tumor activity against FGF8b-expressing LNCaP xenografts in androgen-dependent and -independent conditions. Prostate 2008, 68, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef] [PubMed]
- Harding, T.C.; Long, L.; Palencia, S.; Zhang, H.; Sadra, A.; Hestir, K.; Patil, N.; Levin, A.; Hsu, A.W.; Charych, D.; et al. Blockade of nonhormonal fibroblast growth factors by FP-1039 inhibits growth of multiple types of cancer. Sci. Transl. Med. 2013, 5, 178ra39. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.E.; Hinz, T.K.; Kono, S.A.; Singleton, K.R.; Bichon, B.; Ware, K.E.; Marek, L.; Frederick, B.A.; Raben, D.; Heasley, L.E. Fibroblast growth factor receptors are components of autocrine signaling networks in head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2011, 17, 5016–5025. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shen, Z.; Chen, Y.; Xie, J.; Guo, Z.; Zhang, M.; Sheng, Z. Randomised placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns. Lancet 1998, 352, 1661–1664. [Google Scholar] [CrossRef]
- Lin, T.; Gong, L. Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: A randomized clinical trial in the People’s Republic of China. Drug Des. Dev. Ther. 2015, 9, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wang, L.Q.; Du, G.P.; Zhang, Y.H.; Ge, M. The effect of recombinant bovine basic fibroblast growth factor on the LASIK-induced neurotrophic epitheliopathy and the recovery of corneal sensation after LASIK. Chin. J. Ophthalmol. 2011, 47, 22–26. [Google Scholar]
- Wang, Z.H.; Wang, X.Y.; Zhang, Y.L.; Zhao, L.; Li, H.X. Treatment of diabetic foot by clearing heat, detoxification, activating blood, and dredging collaterals method. Chin. J. Integr. Tradit. West. Med. 2013, 33, 480–483. [Google Scholar]
- Ma, B.; Cheng, D.S.; Xia, Z.F.; Ben, D.F.; Lu, W.; Cao, Z.F.; Wang, Q.; He, J.; Chai, J.K.; Shen, C.A.; et al. Randomized, multicenter, double-blind, and placebo-controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair Regen. 2007, 15, 795–799. [Google Scholar] [PubMed]
- Wu, J.C.; Huang, W.C.; Chen, Y.C.; Tu, T.H.; Tsai, Y.A.; Huang, S.F.; Huang, H.C.; Cheng, H. Acidic fibroblast growth factor for repair of human spinal cord injury: A clinical trial. J. Neurosurg. Spine 2011, 15, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF Family: From Drug Development to Clinical Application. Int. J. Mol. Sci. 2018, 19, 1875. https://doi.org/10.3390/ijms19071875
Hui Q, Jin Z, Li X, Liu C, Wang X. FGF Family: From Drug Development to Clinical Application. International Journal of Molecular Sciences. 2018; 19(7):1875. https://doi.org/10.3390/ijms19071875
Chicago/Turabian StyleHui, Qi, Zi Jin, Xiaokun Li, Changxiao Liu, and Xiaojie Wang. 2018. "FGF Family: From Drug Development to Clinical Application" International Journal of Molecular Sciences 19, no. 7: 1875. https://doi.org/10.3390/ijms19071875
APA StyleHui, Q., Jin, Z., Li, X., Liu, C., & Wang, X. (2018). FGF Family: From Drug Development to Clinical Application. International Journal of Molecular Sciences, 19(7), 1875. https://doi.org/10.3390/ijms19071875



