Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration
Abstract
1. Introduction
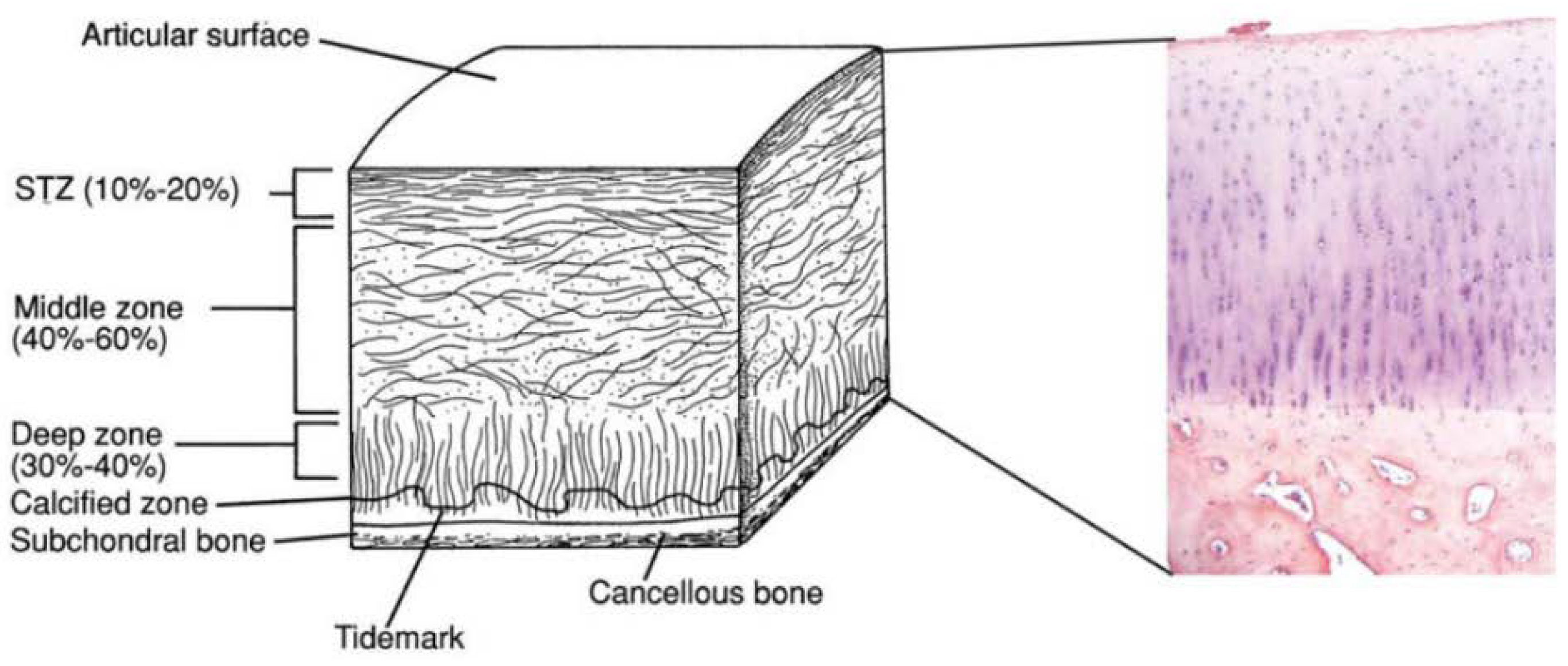
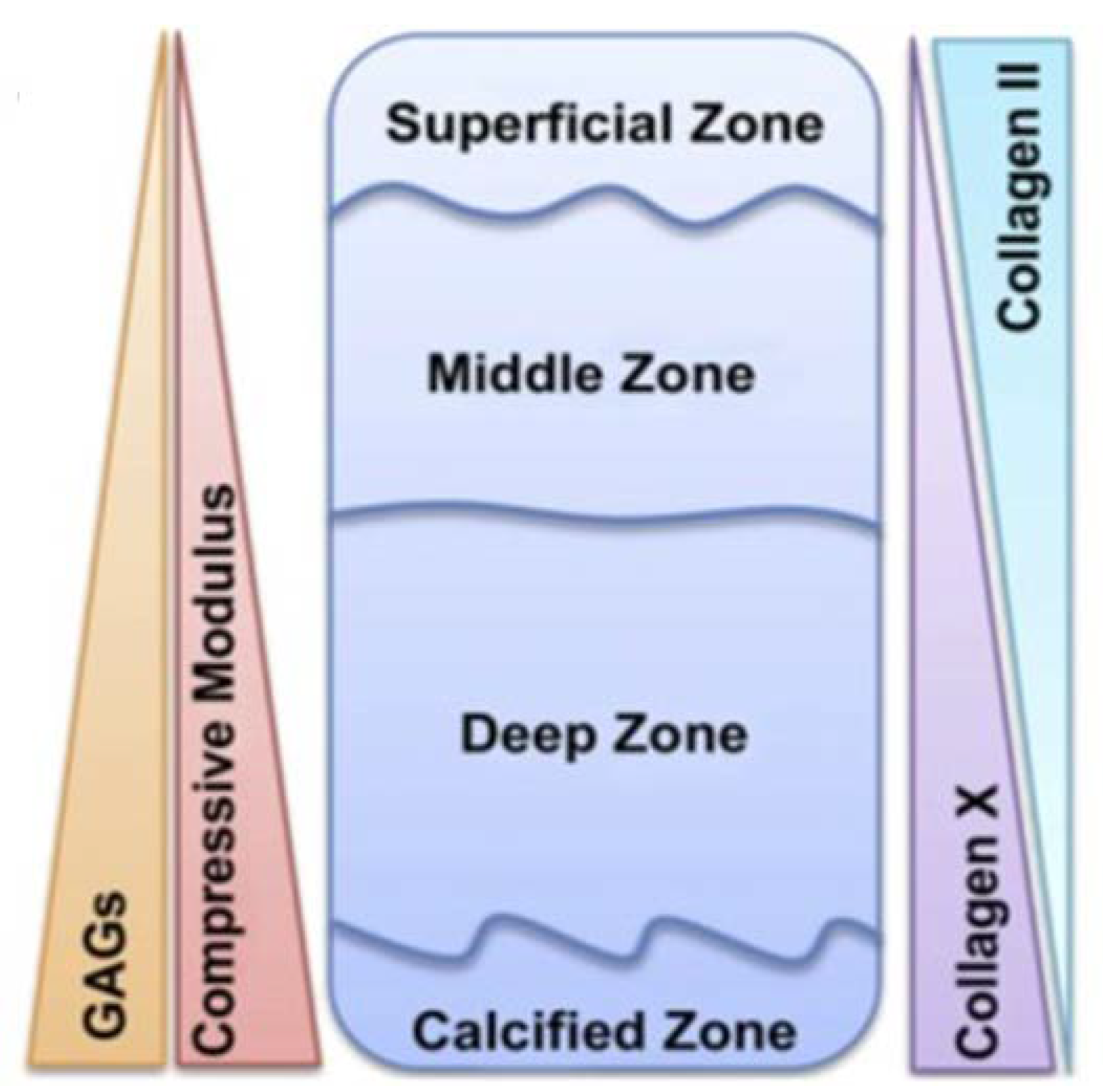
2. Osteochondral Tissue Anatomy
3. Biomimetic Multi-Phasic Structure for Osteochondral Regeneration
3.1. Bi-Phasic Scaffolds
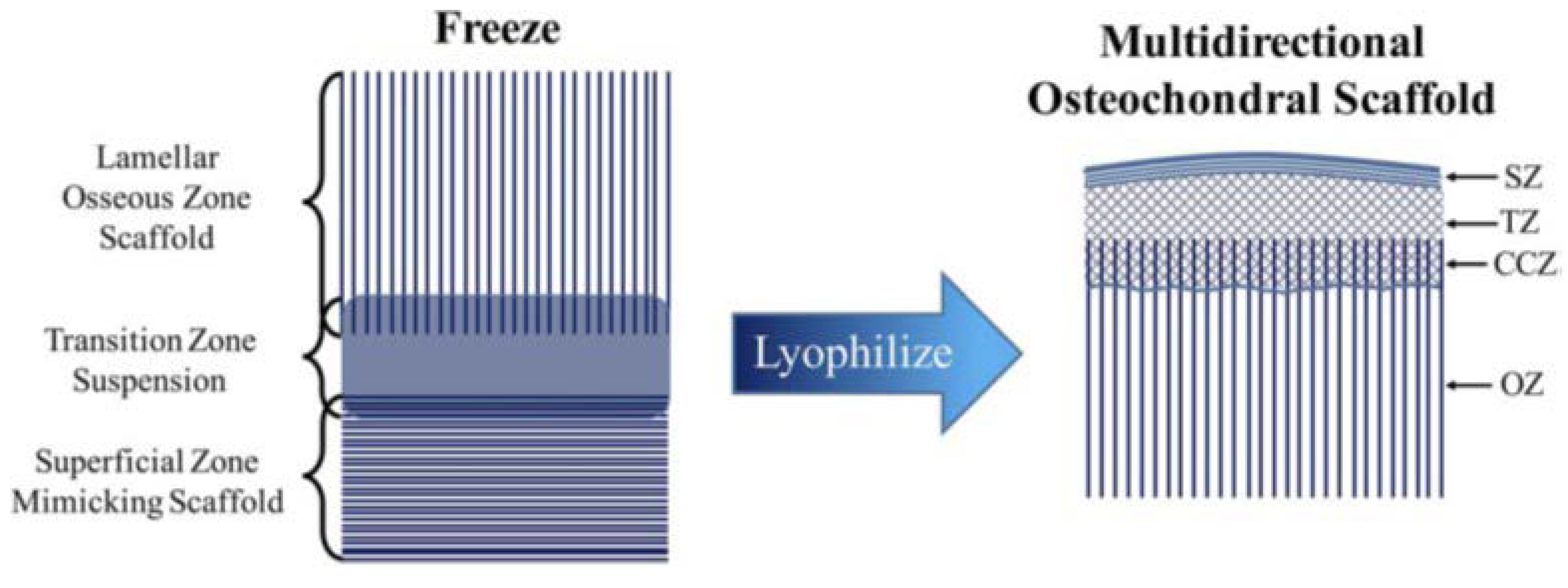
3.2. Tri-Phasic/Multi-Phasic Scaffolds
4. Clinical Progress and Insight into the Still Open Challenges
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphate activity |
| BMSCs | Bone marrow mesenchymal stem |
| β-TCP | Beta-tricalcium phosphate |
| CCZ | Calcified cartilage zone |
| ECM | Extracellular matrix |
| Gel | Gelatin |
| HA | Hydroxyapatite |
| hMSCs | Human mesenchymal stromal/stem cells |
| IKDC | International Knee Documentation Committee |
| Mg-HA | Magnesium-doped hydroxyapatite |
| MRI | Magnetic Resonance Imaging |
| n-HA | Nano-Hydroxyapatite |
| OC | Osteochondral |
| PA6 | Polyamide-6 |
| PEG | Polyethylene glycol |
| PEG-Da | Polyethylene glycol diacrylate |
| PGA | Poly-glycolic acid |
| PLGA | Poly(lactide-co-glycolide) |
| PLCL | Poly-lactide-co-caprolactone |
| PVA | Poly-vinyl alcohol |
| STZ | Superficial tangential zone |
| V | Vanillin |
References
- Yan, L.-P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Current concepts and challenges in osteochondral tissue engineering and regenerative medicine. ACS Biomater. Sci. Eng. 2015, 1, 183–200. [Google Scholar] [CrossRef]
- Yousefi, A.M.; Hoque, M.E.; Prasad, R.G.S.V.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J. Biomed. Mater. Res. Part A 2015, 103, 2460–2481. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.; Van Blitterswijk, C.; Moroni, L. The osteochondral interface as a gradient tissue: From development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Oegema, T.R.; Carpenter, R.J.; Hofmeister, F.; Thompson, R.C. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc. Res. Tech. 1997, 37, 324–332. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Bellows, C.G.; Aubin, J.E.; Heersche, J.N.M.; Antosz, M.E. Mineralized bone nodules formedin vitro from enzymatically released rat calvaria cell populations. Calcif. Tissue Int. 1986, 38, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317. [Google Scholar]
- Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the mineral composition of the human calcified cartilage zone. Int. J. Med. Sci. 2012, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Shibakawa, A.; Yudoh, K.; Masuko-Hongo, K.; Kato, T.; Nishioka, K.; Nakamura, H. The role of subchondral bone resorption pits in osteoarthritis: Mmp production by cells derived from bone marrow. Osteoarthr. Cartil. 2005, 13, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Colonna, C.; Genta, I.; Bruni, G.; Visai, L.; Conti, B. Preparation and characterization of an advanced medical device for bone regeneration. AAPS PharmSciTech 2014, 15, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr. Course Lect. 1998, 47, 487–504. [Google Scholar] [PubMed]
- James, C.-B.; Uhl, T.L. A review of articular cartilage pathology and the use of glucosamine sulfate. J. Athl. Train. 2001, 36, 413. [Google Scholar] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Part I tissue design and chondrocyte-matrix interactions. JBJS 1997, 79, 600–611. [Google Scholar] [CrossRef]
- Chi, S.S.; Rattner, J.B.; Matyas, J.R. Communication between paired chondrocytes in the superficial zone of articular cartilage. J. Anat. 2004, 205, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Dictionary, M.-W. Online Edition, Based on Merriam-Webster’s Collegiate Dictionary; Merriam-Webster: Springfield, MA, USA, 2014. [Google Scholar]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Jung, Y. Bi-layered PLCL/(PLGA/β-TCP) composite scaffold for osteochondral tissue engineering. J. Bioact. Compat. Polym. 2015, 30, 178–187. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, H.; Li, J.; Feng, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Bioactive scaffolds for regeneration of cartilage and subchondral bone interface. Theranostics 2018, 8, 1940. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Gomoll, A.H.; Canseco, J.A.; Far, J.; Lind, M.; Hui, J. Cartilage repair in the degenerative ageing knee: A narrative review and analysis. Acta Orthop. 2016, 87, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.B.F.; Bezemer, J.M.; Pieper, J.S.; Van Blitterswijk, C.A.; Riesle, J. Scaffolds for tissue engineering of cartilage. Crit. Rev. Eukaryot. Gene Expr. 2002, 12. [Google Scholar] [CrossRef]
- Blunk, T.; Sieminski, A.L.; Gooch, K.J.; Courter, D.L.; Hollander, A.P.; Nahir, A.M.; Langer, R.; Vunjak-Novakovic, G.; Freed, L.E. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002, 8, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Bhonde, R.; Gupta, P.K.; Totey, S. Novel biomimetic tripolymer scaffolds consisting of chitosan, collagen type 1, and hyaluronic acid for bone marrow-derived human mesenchymal stem cells-based bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-J.; Mahapatra, C.; Singh, R.K.; Knowles, J.C.; Kim, H.-W. Strategies for osteochondral repair: Focus on scaffolds. J. Tissue Eng. 2014, 5, 2041731414541850. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.Á.; Renaud, A.; Amiaud, J.; Rojewski, M.T.; Schrezenmeier, H.; Heymann, D.; Trichet, V.; Layrolle, P. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res. Ther. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Van Eps, J.; Fernandez-Moure, J.; Cabrera, F.; Wang, X.; Karim, A.; Corradetti, B.; Chan, P.; Dunkin, B.; Tasciotti, E.; Weiner, B. Decreased hernia recurrence using autologous platelet-rich plasma (prp) with strattice™ mesh in a rodent ventral hernia model. Surg. Endosc. 2016, 30, 3239–3249. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioactive Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zuo, Y.; Qu, D.; Liu, Y.; Chen, T.; Jiang, N.; Li, H.; Li, J. Osteogenesis and chondrogenesis of biomimetic integrated porous PVA/gel/v-n-HA/pa6 scaffolds and BMSCs construct in repair of articular osteochondral defect. J. Biomed. Mater. Res. Part A 2015, 103, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Kelly, D.J. A computational model of osteochondral defect repair following implantation of stem cell-laden multiphase scaffolds. Tissue Eng. Part A 2017, 23, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Schwinté, P.; Gomez-Barrena, E.; Arruebo, M.; Benkirane-Jessel, N. Smart implants as a novel strategy to regenerate well-founded cartilage. Trends Biotechnol. 2017, 35, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Lafantaisie-Favreau, C.-H.; Lascau-Coman, V.; Chen, G.; Guzmán-Morales, J. The cartilage-bone interface. J. Knee Surg. 2012, 25, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Jeon, J.E.; Vaquette, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W. Multiphasic construct studied in an ectopic osteochondral defect model. J. R. Soc. Interface 2014, 11, 20140184. [Google Scholar] [CrossRef] [PubMed]
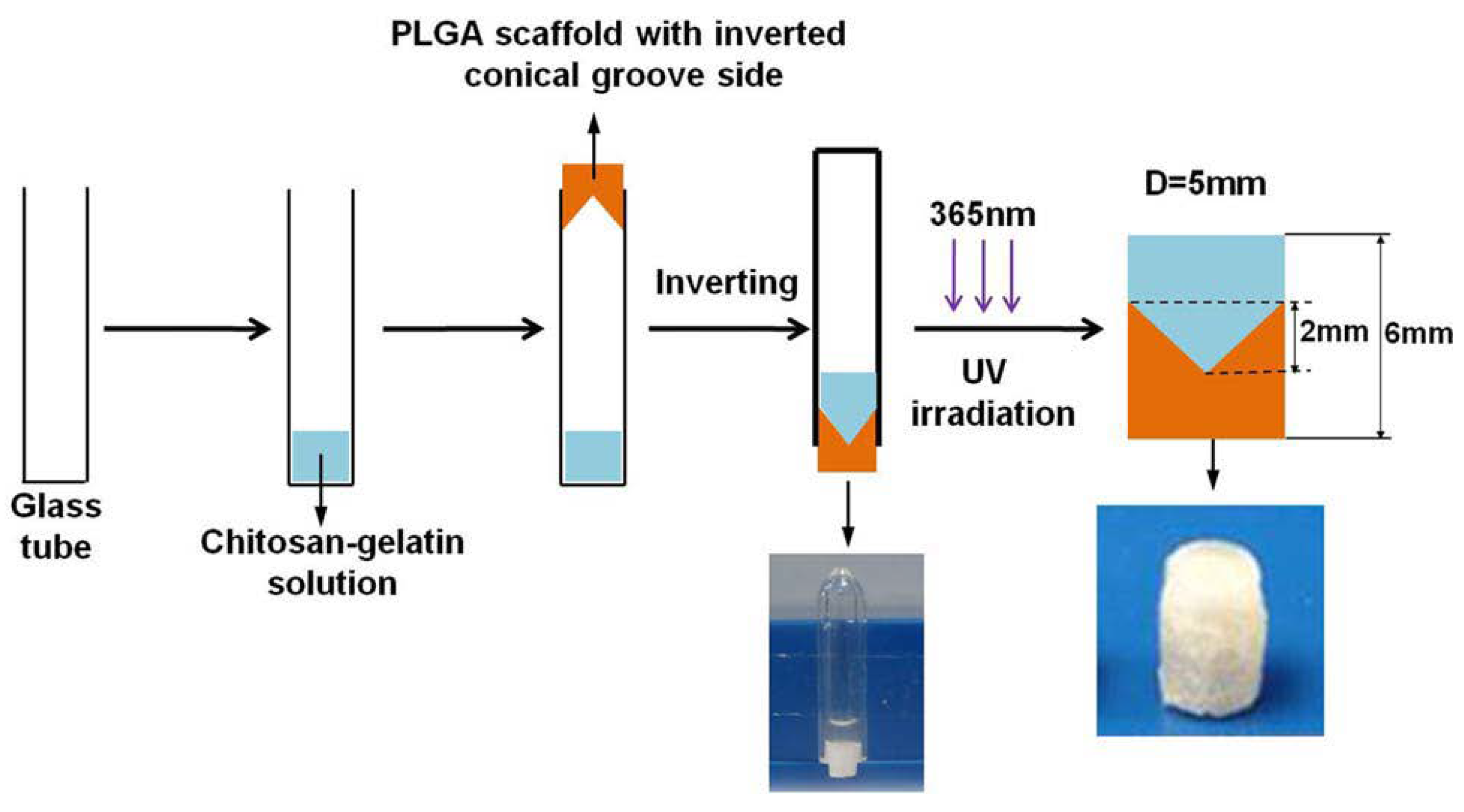
- Han, F.; Zhou, F.; Yang, X.; Zhao, J.; Zhao, Y.; Yuan, X. A pilot study of conically graded chitosan–gelatin hydrogel/PLGA scaffold with dual-delivery of TGF-β1 and BMP-2 for regeneration of cartilage–bone interface. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F.; Cifuentes, A.; Rodríguez, G.; Aguilar, M.R.; González-Gómez, Á.; Solis, R.; García-Honduvilla, N.; Buján, J.; García-Sanmartin, J.; Martínez, A. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents. Acta Biomater. 2015, 23, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, D.; Nguyen, A.; Wei, M. Biomimetic multidirectional scaffolds for zonal osteochondral tissue engineering via a lyophilization bonding approach. J. Biomed. Mater. Res. Part A 2018, 106, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering—Part I: Recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng. Part B Rev. 2013, 19, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering—Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Driesang, I.M.K. Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthr. Cartil. 2003, 11, 320–327. [Google Scholar] [CrossRef]
- Reyes, R.; Delgado, A.; Sanchez, E.; Fernandez, A.; Hernandez, A.; Evora, C. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate–PLGA scaffold. J. Tissue Eng. Regen. Med. 2014, 8, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-P.; Silva-Correia, J.; Oliveira, M.B.; Vilela, C.; Pereira, H.; Sousa, R.A.; Mano, J.F.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Bilayered silk/silk-nanocap scaffolds for osteochondral tissue engineering: In vitro and in vivo assessment of biological performance. Acta Biomater. 2015, 12, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Thompson, E.; Matsiko, A.; Schepens, A.; Gleeson, J.P.; O’Brien, F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016, 32, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Coelho, P.G. Engineering Mineralized and Load Bearing Tissues; Springer: Berlin, Germany, 2015; Volume 881. [Google Scholar]
- Shetty, A.A.; Kim, S.-J.; Nakamura, N.; Brittberg, M. Techniques in Cartilage Repair Surgery; Springer: Berlin, Germany, 2014. [Google Scholar]
- Dell’Osso, G.; Bottai, V.; Bugelli, G.; Manisco, T.; Cazzella, N.; Celli, F.; Guido, G.; Giannotti, S. The biphasic bioresorbable scaffold (trufit®) in the osteochondral knee lesions: Long-term clinical and mri assessment in 30 patients. Musculoskelet. Surg. 2016, 100, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Dockery, W.D. A computed tomography scan assessment of synthetic multiphase polymer scaffolds used for osteochondral defect repair. Arthroscopy 2011, 27, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, J.; Clockaerts, S.; Van Osch, G.; Somville, J.; Verdonk, P.; Mertens, P. Trufit plug for repair of osteochondral defects—Where is the evidence? Systematic review of literature. Cartilage 2015, 6, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.W.; Ma, R.; Pais, D.A.; Williams, R.J. Trufit®. In Techniques in Cartilage Repair Surgery; Springer: Berlin, Germany, 2014; pp. 69–80. [Google Scholar]
- Fini, M.; Pagani, S.; Giavaresi, G.; De Mattei, M.; Ongaro, A.; Varani, K.; Vincenzi, F.; Massari, L.; Cadossi, M. Functional tissue engineering in articular cartilage repair: Is there a role for electromagnetic biophysical stimulation? Tissue Eng. Part B Rev. 2013, 19, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Willie, B.M.; Petersen, A.; Schmidt-Bleek, K.; Cipitria, A.; Mehta, M.; Strube, P.; Lienau, J.; Wildemann, B.; Fratzl, P.; Duda, G. Designing biomimetic scaffolds for bone regeneration: Why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter 2010, 6, 4976–4987. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J. Exp. Orthop. 2014, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Perdisa, F.; Di Martino, A.; Busacca, M.; Balboni, F.; Sessa, A.; Marcacci, M. A one-step treatment for chondral and osteochondral knee defects: Clinical results of a biomimetic scaffold implantation at 2 years of follow-up. J. Mater. Sci. Mater. Med. 2014, 25, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.B.; Foldager, C.B.; Jensen, J.; Jensen, N.C.; Lind, M. Poor osteochondral repair by a biomimetic collagen scaffold: 1-to 3-year clinical and radiological follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
| Type of scaffold | Properties | ||
|---|---|---|---|
| Monophasic Scaffold | Acellular |
|  |
| Biological |
|  | |
| Bi-phasic Scaffold | Acellular |
|  |
| Biological |
|  | |
| Tri-phasic or Multi-phasic Scaffolds | Acellular |
| 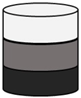 |
| Biological |
| 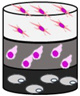 | |
| Scaffold Type and Composition | Manufacturing Process | In Vitro and/or In Vivo Analysis | Reference | |
|---|---|---|---|---|
| Bi-phasic | Acellular scaffold Top: Alginate + TGF-β1-loaded microspheres Bottom: PLGA + BMP2-loaded microspheres | Top: Freeze-drying Bottom: Gas foaming | In vivo implant in cylindrical osteochondral defects (diameter 4.5 mm, deep 4 mm) in adult male New Zealand rabbits for 24 weeks:
| Reyes et al. 2014 [46] |
| Acellular scaffold Top: silk fibroin Bottom: silk fibroin + nano calcium phosphate powder | Salt leaching + freeze-drying | In vivo implant in cylindrical osteochondral defects (diameter 4.5 mm, deep 5 mm) in New Zealand White rabbits (9–11 weeks old) for 4 weeks:
| Yan et al. 2015 [47] | |
| Cellular scaffold (BMSCs seeded on the construct for 3 days before implant) Top: PLCL Bottom: PLGA/ β-TCP | Top: sintering Bottom: gel pressing | In vivo implant in subcutaneous implantation in nude mice (7 week old) for 6 weeks:
| Kim et al. 2015 [21] | |
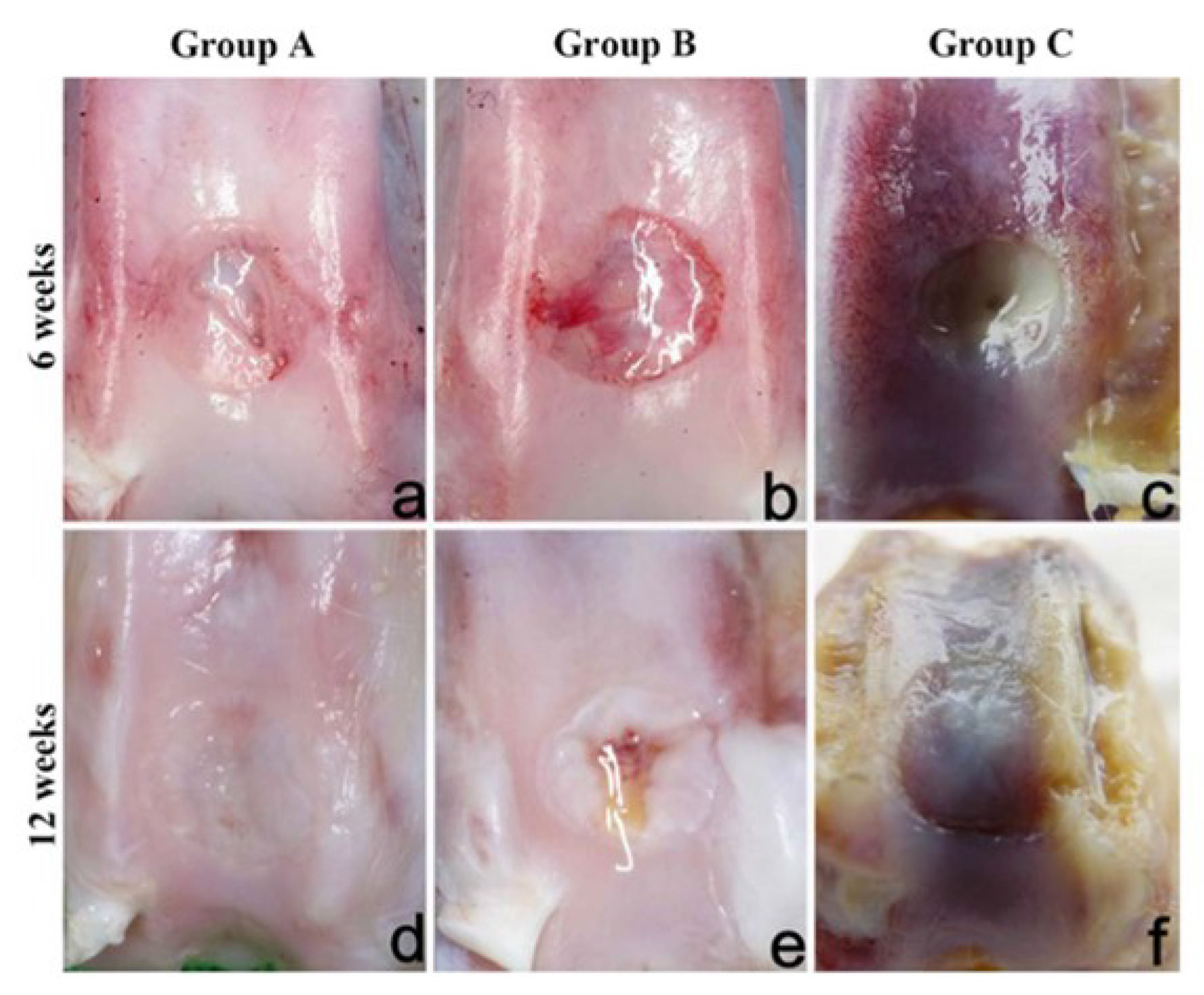
| Cellular scaffold (MSCs seeded on the construct before implant) Top: PVA/Gel/V Bottom: n-HA/PA6 | Freezing-thawing | In vivo implant osteochondral defects (diameter 4 mm, deep 6 mm) in New Zealand rabbits for 12 weeks:
| Li et al. 2015 [32] | |
| Cellular scaffold (hMSCs seeded on the construct before implant) Top: Type I atelocollagen Bottom: Mg-doped HA | Freeze-drying | In vivo subcutaneous implant in mice for 8 weeks:
| Sartori et al. 2017 [20] | |
| Acellular scaffold Top: type I and II collagen and hyaluronic acid Middle: type I and II collagen and HA Bottom: HydroxyCollTM, composed of type I collagen and HA, commercialised by SurgaColl Technologies | Freeze-drying | In vivo implant in cylindrical osteochondral defects (diameter 3 mm, deep 5 mm) in New Zealand White rabbits (9 months old) for 12 weeks:
| Levingstone et al. 2016 [48] | |
| Multi-phasic | Cellular scaffold (MSCs seeded on the construct before implant) Cartilage layer: different layers of CS- glycidyl methacrylate( GMA) and Gel-GMA loaded with TGF-β1 Bone layer: PLGA loaded with BMP-2 | Cartilage layer: hydrogels via UV polymerisation Bone layer: separation/particle leaching method | In vivo implant in cylindrical osteochondral defects (diameter 5 mm, deep 6 mm) in New Zealand White rabbits for 8 weeks:
| Han et al. 2014 [38] |
| Cellular scaffold Cartilage layer: Top: alginate with superficial chondrocytes Bottom: alginate with middle-deep chondrocytes Bone layer: PCL with osteoblasts | Cartilage layer: ionic crosslinked alginate with CaCl2Bone layer: FDM | Ectopic osteochondral model. Bovine osteochondral cores prepared from bovine knees were filled with the construct prior to subcutaneous implantation in nude mice (8 week old) for 12 weeks:
| Jeon et al. 2018 [37] |
| Scaffold | Name and Sponsor | Materials | Plug Size and Depth | References |
|---|---|---|---|---|
 | TruFit CB™, Smith & Nephew | Bi-phasic implant consisting of semi-porous PLGA-PGA (75:25) and Calcium-phosphate | Diam 5–11 mm, 18 mm | [50,51,52,53,54] |
 | Agili-C™, CartiHeal Ltd. | Crystalline aragonite (calcium carbonate based) and hyaluronic acid | Diam 6–18 mm,15 or 20 mm | [4,55]. |
 | Maioregen™, Finceramica | Cartilage layer: equine type I collagenTidemark like layer: type I collagen (60%), Mg-HA (40%) Lower layer: mineralised blend of type I collagen (30%), Mg-HA (70%) | 35 × 35 mm, 6 mm (±2 mm due to the swelling) | [56,57,58] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longley, R.; Ferreira, A.M.; Gentile, P. Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. Int. J. Mol. Sci. 2018, 19, 1755. https://doi.org/10.3390/ijms19061755
Longley R, Ferreira AM, Gentile P. Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. International Journal of Molecular Sciences. 2018; 19(6):1755. https://doi.org/10.3390/ijms19061755
Chicago/Turabian StyleLongley, Ryan, Ana Marina Ferreira, and Piergiorgio Gentile. 2018. "Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration" International Journal of Molecular Sciences 19, no. 6: 1755. https://doi.org/10.3390/ijms19061755
APA StyleLongley, R., Ferreira, A. M., & Gentile, P. (2018). Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. International Journal of Molecular Sciences, 19(6), 1755. https://doi.org/10.3390/ijms19061755







