Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study
Abstract
1. Introduction
2. Results
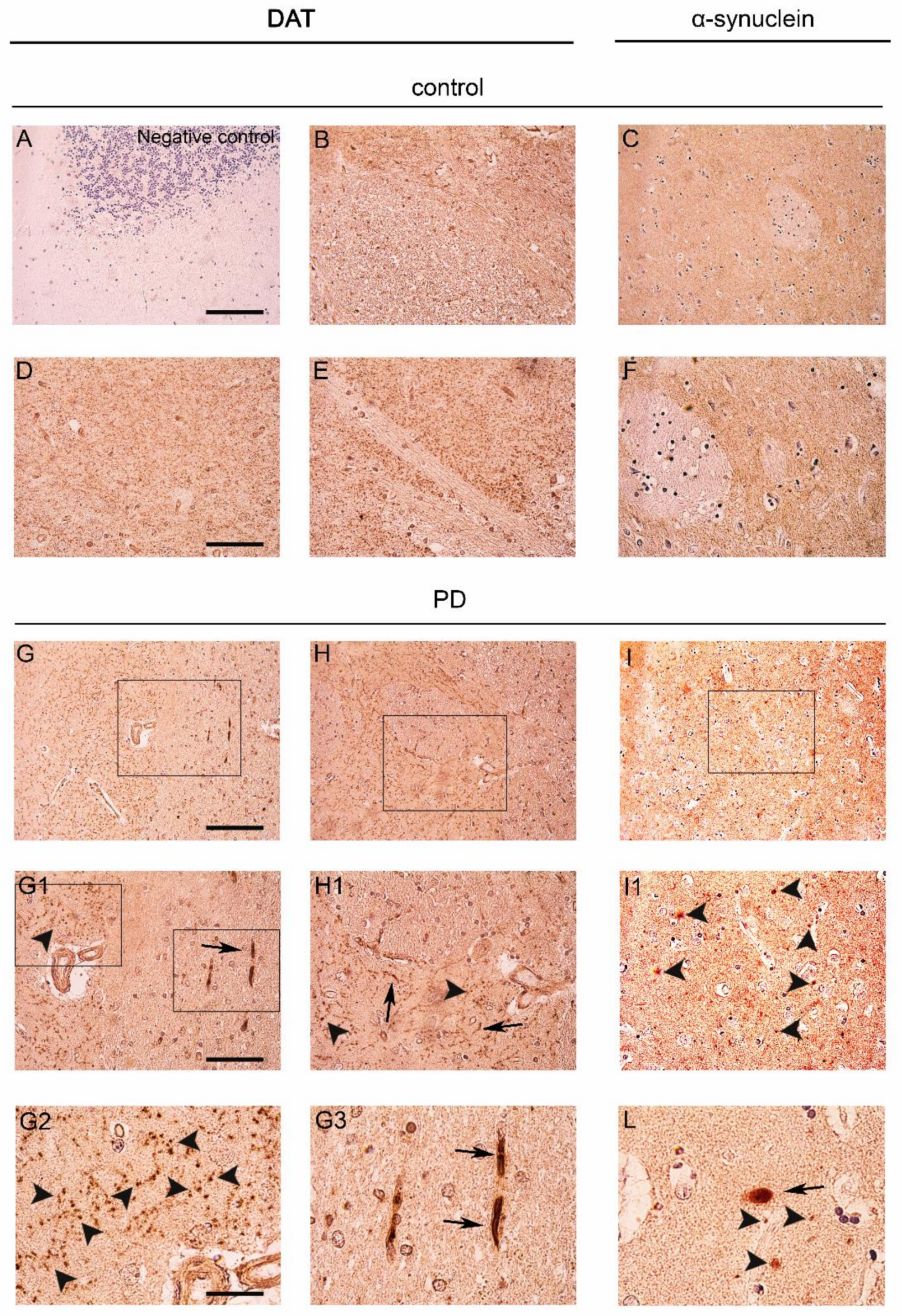
2.1. DAT Redistribution in the Caudate Putamen of Patients Affected by PD
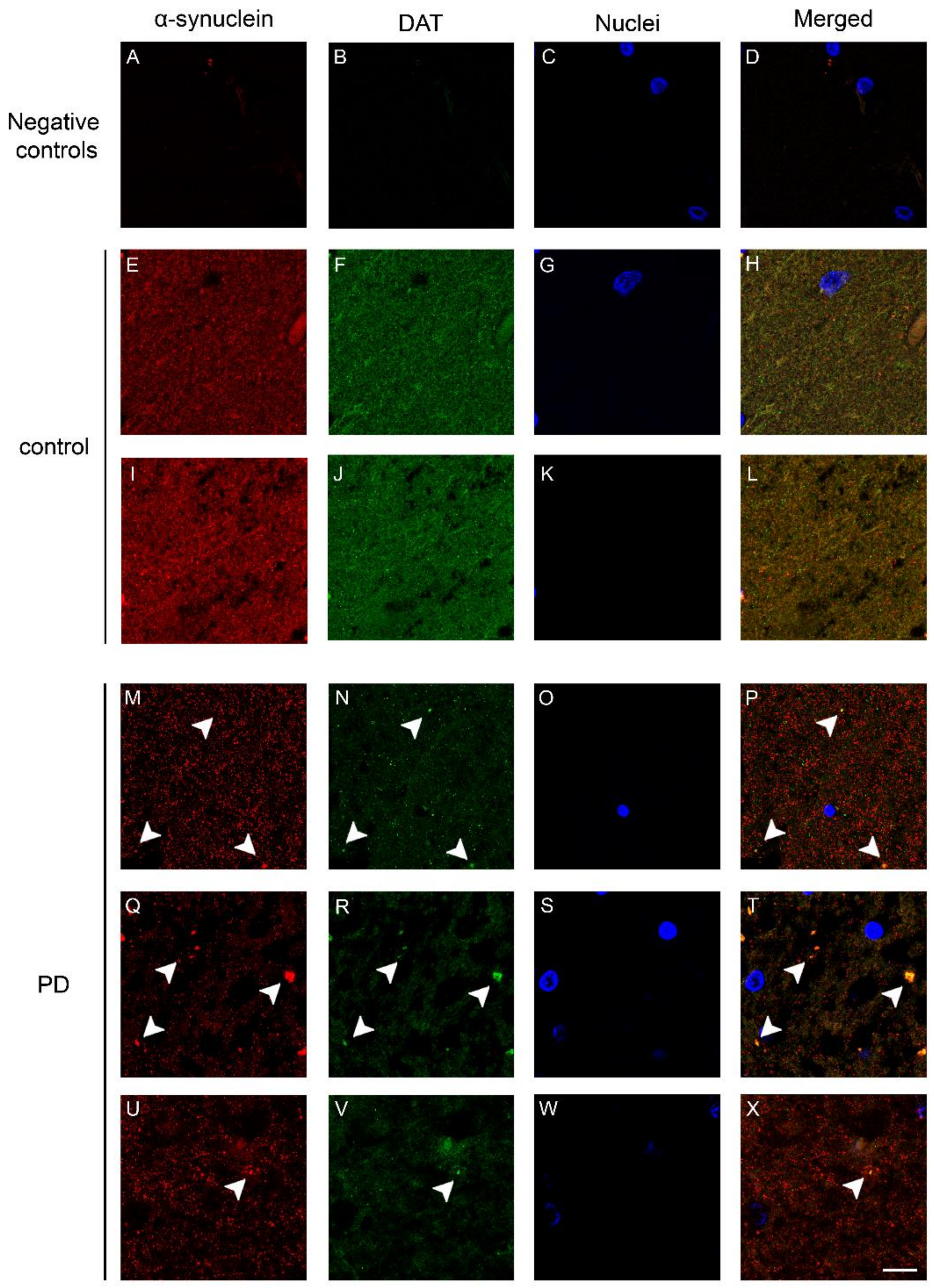
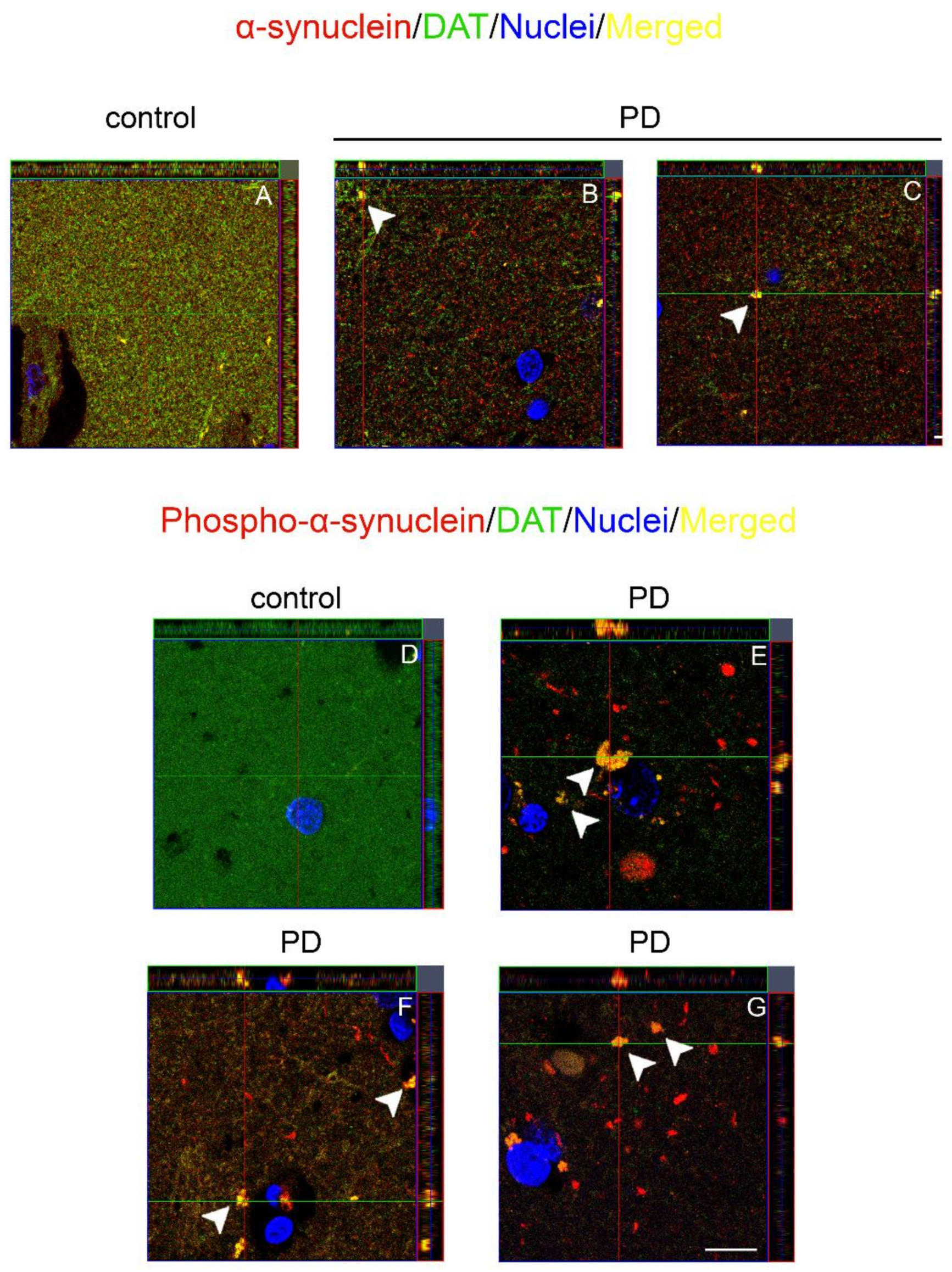
2.2. Co-Localization and Redistribution of DAT and α-Synuclein in the Caudate Putamen of PD Patients and Age Matched Controls
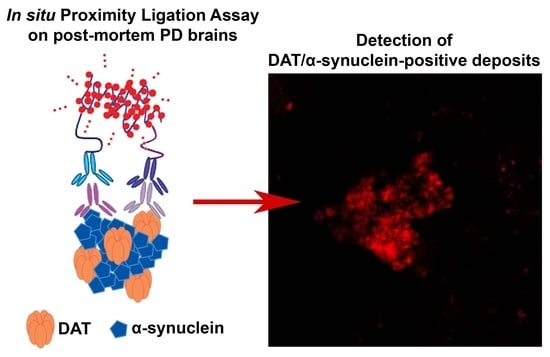
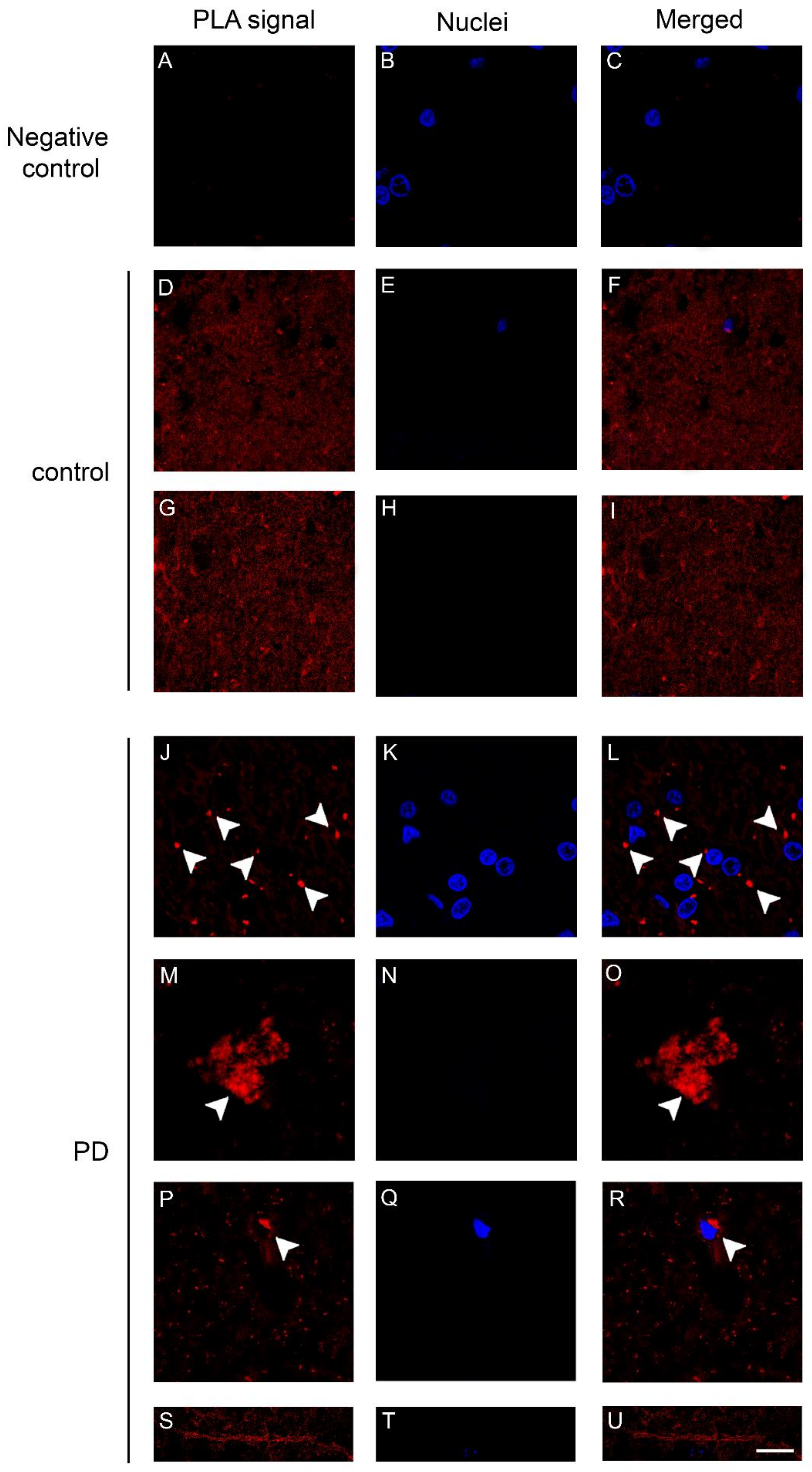
2.3. Dopamine Transporter/α-Synuclein In Situ PLA in the Caudate Putamen of PD and Control Subjects
3. Discussion
4. Material and Methods
4.1. Human Tissues
4.2. Immunohistochemistry
4.3. In Situ PLA
4.4. Bright Field and Confocal Microscopy
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Forno, L.S. Neuropathologic features of Parkinson’s, Huntington’s, and Alzheimer’s diseases. Ann. N. Y. Acad. Sci. 1992, 648, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Mercuri, N.B.; Venneri, A.; Faustini, G.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P. Review: Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016, 42, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Burre, J. The Synaptic Function of alpha-Synuclein. J. Parkinsons Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, A.; Schmitz, Y.; Farinas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef]
- Surguchov, A. Intracellular Dynamics of Synucleins: “Here, There and Everywhere”. Int. Rev. Cell Mol. Biol. 2015, 320, 103–169. [Google Scholar] [PubMed]
- Bellucci, A.; Navarria, L.; Falarti, E.; Zaltieri, M.; Bono, F.; Collo, G.; Spillantini, M.G.; Missale, C.; Spano, P. Redistribution of DAT/alpha-synuclein complexes visualized by “in situ” proximity ligation assay in transgenic mice modelling early Parkinson’s disease. PLoS ONE 2011, 6, e27959. [Google Scholar] [CrossRef] [PubMed]
- Oaks, A.W.; Sidhu, A. Synuclein modulation of monoamine transporters. FEBS Lett. 2011, 585, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.; Jorgensen, T.N.; Gether, U. Regulation of dopamine transporter function by protein-protein interactions: New discoveries and methodological challenges. J. Neurochem. 2010, 113, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Collo, G.; Sarnico, I.; Battistin, L.; Missale, C.; Spano, P. Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J. Neurochem. 2008, 106, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, C.; Sidhu, A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci. Lett. 2003, 340, 189–192. [Google Scholar] [CrossRef]
- Lee, F.J.; Liu, F.; Pristupa, Z.B.; Niznik, H.B. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001, 15, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, E.; Rizzo, F.R.; Federici, M.; Cordella, A.; Ledonne, A.; Latini, L.; Nobili, A.; Viscomi, M.T.; Biamonte, F.; Landrock, K.K.; et al. Functional alterations of the dopaminergic and glutamatergic systems in spontaneous alpha-synuclein overexpressing rats. Exp. Neurol. 2017, 287, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, C.; Prou, D.; Vernier, P.; Sidhu, A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003, 17, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, C.; Sidhu, A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry 2005, 44, 13612–13624. [Google Scholar] [CrossRef] [PubMed]
- German, C.L.; Baladi, M.G.; McFadden, L.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the dopamine and vesicular monoamine transporters: Pharmacological targets and implications for disease. Pharmacol. Rev. 2015, 67, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Butler, B.; Saha, K.; Rana, T.; Becker, J.P.; Sambo, D.; Davari, P.; Goodwin, J.S.; Khoshbouei, H. Dopamine transporter activity is modulated by alpha-synuclein. J. Biol. Chem. 2015, 290, 29542–29554. [Google Scholar] [CrossRef] [PubMed]
- Kisos, H.; Ben-Gedalya, T.; Sharon, R. The clathrin-dependent localization of dopamine transporter to surface membranes is affected by alpha-synuclein. J. Mol. Neurosci. 2014, 52, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Chadchankar, H.; Ihalainen, J.; Tanila, H.; Yavich, L. Decreased reuptake of dopamine in the dorsal striatum in the absence of alpha-synuclein. Brain Res. 2011, 1382, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Arawaka, S.; Sato, H.; Machiya, Y.; Cui, C.; Sasaki, A.; Koyama, S.; Kato, T. Serine 129 phosphorylation of membrane-associated alpha-synuclein modulates dopamine transporter function in a G protein-coupled receptor kinase-dependent manner. Mol. Biol. Cell 2013, 24, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, M.; Decressac, M.; Mattsson, B.; Bjorklund, A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Swant, J.; Goodwin, J.S.; North, A.; Ali, A.A.; Gamble-George, J.; Chirwa, S.; Khoshbouei, H. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J. Biol. Chem. 2011, 286, 43933–43943. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, A.; Kallunki, P.; Yavich, L. Effects of exogenous alpha-synuclein on stimulated dopamine overflow in dorsal striatum. Neurosci. Lett. 2013, 554, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Staley, J.K.; Heilman, C.J.; Perez, J.T.; Mash, D.C.; Rye, D.B.; Levey, A.I. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann. Neurol. 1997, 41, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Longhena, F.; Faustini, G.; Varanita, T.; Zaltieri, M.; Porrini, V.; Tessari, I.; Poliani, P.L.; Missale, C.; Borroni, B.; Padovani, A.; et al. Synapsin III is a key component of alpha-synuclein fibrils in Lewy bodies of PD brains. Brain Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Milenkovic, I.J.; Preusser, M.; Budka, H. Nigral burden of alpha-synuclein correlates with striatal dopamine deficit. Mov. Disord. 2008, 23, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reitbock, P.; Anichtchik, O.; Bellucci, A.; Iovino, M.; Ballini, C.; Fineberg, E.; Ghetti, B.; Della Corte, L.; Spano, P.; Tofaris, G.K.; et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010, 133, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Betzer, C.; Lassen, L.B.; Olsen, A.; Kofoed, R.H.; Reimer, L.; Gregersen, E.; Zheng, J.; Cali, T.; Gai, W.P.; Chen, T.; et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018, 19, e44617. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain 2015, 138, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Fiorentini, C.; Zaltieri, M.; Missale, C.; Spano, P. The “in situ” proximity ligation assay to probe protein-protein interactions in intact tissues. Methods Mol. Biol. 2014, 1174, 397–405. [Google Scholar] [PubMed]
- Soderberg, O.; Gullberg, M.; Jarvius, M.; Ridderstrale, K.; Leuchowius, K.J.; Jarvius, J.; Wester, K.; Hydbring, P.; Bahram, F.; Larsson, L.G.; et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 2006, 3, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Fazio, P.; Svenningsson, P.; Cselenyi, Z.; Halldin, C.; Farde, L.; Varrone, A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. 2018, 33, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, S.P.; Presotto, L.; Baroncini, D.; Garibotto, V.; Moresco, R.M.; Gianolli, L.; Volonte, M.A.; Antonini, A.; Perani, D. Axonal damage and loss of connectivity in nigrostriatal and mesolimbic dopamine pathways in early Parkinson’s disease. Neuroimage Clin. 2017, 14, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Zaltieri, M.; Grigoletto, J.; Longhena, F.; Navarria, L.; Favero, G.; Castrezzati, S.; Colivicchi, M.A.; della Corte, L.; Rezzani, R.; Pizzi, M.; et al. alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 2015, 128, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, M.; Chung, K.A.; Zabetian, C.P.; Leverenz, J.B.; Berg, D.; Srulijes, K.; Trojanowski, J.Q.; Lee, V.M.; Siderowf, A.D.; et al. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci. Transl. Med. 2012, 4, 121ra20. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Z.; Zhao, L.; Li, S. Novel strategies for drug discovery based on Intrinsically Disordered Proteins (IDPs). Int. J. Mol. Sci. 2011, 12, 3205–3219. [Google Scholar] [CrossRef] [PubMed]
- Longhena, F.; Spano, P.; Bellucci, A. Targeting of Disordered Proteins by Small Molecules in Neurodegenerative Diseases. Handb. Exp. Pharmacol. 2018, 245, 85–110. [Google Scholar] [PubMed]
- Pieri, L.; Madiona, K.; Melki, R. Structural and functional properties of prefibrillar alpha-synuclein oligomers. Sci. Rep. 2016, 6, 24526. [Google Scholar] [CrossRef] [PubMed]
- Borgia, A.; Borgia, M.B.; Bugge, K.; Kissling, V.M.; Heidarsson, P.O.; Fernandes, C.B.; Sottini, A.; Soranno, A.; Buholzer, K.J.; Nettels, D.; et al. Extreme disorder in an ultrahigh-affinity protein complex. Nature 2018, 555, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Reitbock, P.G.; Humby, T.; Lambourne, S.L.; O’Connell, M.; Ghetti, B.; Gossage, H.; Emson, P.C.; Wilkinson, L.S.; Goedert, M.; et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): Implications for Lewy body disorders. J. Neurosci. 2006, 26, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, A.; Antonini, A.; Pizzi, M.; Spano, P. The End Is the Beginning: Parkinson’s Disease in the Light of Brain Imaging. Front. Aging Neurosci. 2017, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.; Kivinen, K.; Gardberg, M.; Joutsa, J.; Noponen, T.; Kaasinen, V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 2017, 88, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Almandoz-Gil, L.; Persson, E.; Lindstrom, V.; Ingelsson, M.; Erlandsson, A.; Bergstrom, J. In Situ Proximity Ligation Assay Reveals Co-Localization of Alpha-Synuclein and SNARE Proteins in Murine Primary Neurons. Front. Neurol. 2018, 9, 180. [Google Scholar] [CrossRef] [PubMed]
| Case | Age | Sex | Onset | Duration | Drugs | PMI | Experiments | |||
|---|---|---|---|---|---|---|---|---|---|---|
| IHC | IF | PLA | ||||||||
| PD | PD020 | 75 | M | 42 | 34 | Sinemet, Artane, Selegiline, Pergolide Domperidone, Apomorphine, Quetiapine Bromocriptine | 2 | X | X | X |
| PD045 | 80 | M | 60 | 19 | Sinemet, Ropinirole Selegiline, Entacapone Tolcapone, Cabergoline | 16 | X | X | X | |
| PD050 | 82 | F | 68 | 14 | Sinemet, Selegiline Entacapone, Pergolide | 18 | X | X | X | |
| PD081 | 73 | M | 65 | 9 | Madopar, Amantadine Amitriptyline, Haloperidol | 19 | X | X | ||
| PD093 | 81 | F | 67 | 14 | Madopar, Cabergoline, Sulpiride, Selegiline, Olanzapine, Amantadine | 22 | X | X | X | |
| PD099 | 82 | M | 72 | 11 | Pramipexole, Benzhexol, Sinemet, Madopar | 10 | X | X | ||
| Controls | PDC022 | 65 | M | 12 | X | X | X | |||
| PDC028 | 84 | F | 11 | X | X | X | ||||
| PDC029 | 82 | M | 48 | X | X | |||||
| PDC034 | 90 | M | 12 | X | X | |||||
| C026 | 78 | F | 33 | X | X | X | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhena, F.; Faustini, G.; Missale, C.; Pizzi, M.; Bellucci, A. Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study. Int. J. Mol. Sci. 2018, 19, 1611. https://doi.org/10.3390/ijms19061611
Longhena F, Faustini G, Missale C, Pizzi M, Bellucci A. Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study. International Journal of Molecular Sciences. 2018; 19(6):1611. https://doi.org/10.3390/ijms19061611
Chicago/Turabian StyleLonghena, Francesca, Gaia Faustini, Cristina Missale, Marina Pizzi, and Arianna Bellucci. 2018. "Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study" International Journal of Molecular Sciences 19, no. 6: 1611. https://doi.org/10.3390/ijms19061611
APA StyleLonghena, F., Faustini, G., Missale, C., Pizzi, M., & Bellucci, A. (2018). Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study. International Journal of Molecular Sciences, 19(6), 1611. https://doi.org/10.3390/ijms19061611