Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’)
Abstract
1. Introduction
2. Results
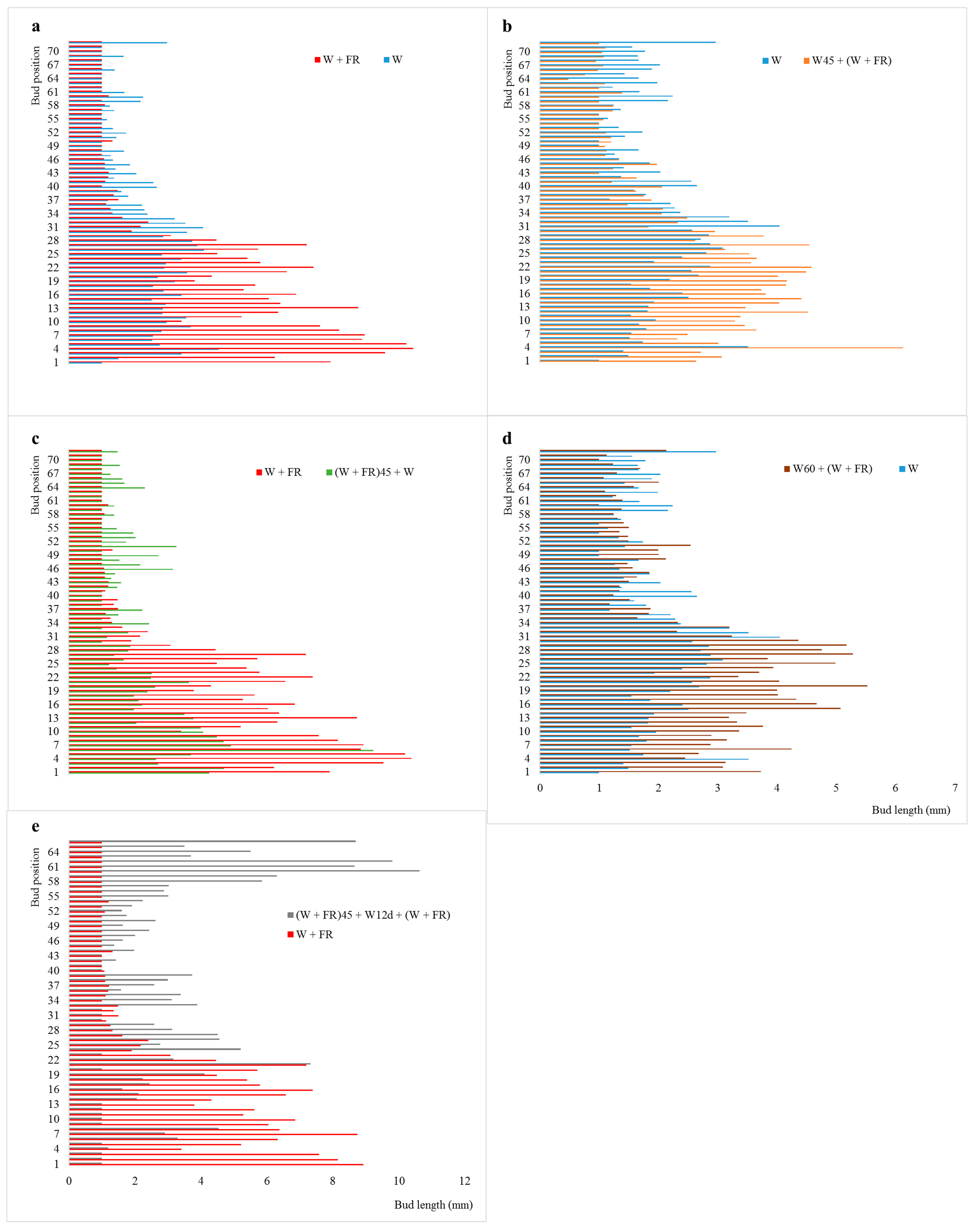
2.1. The Axillary Buds at Specific Positions in Stem Showed Different Response to R:FR
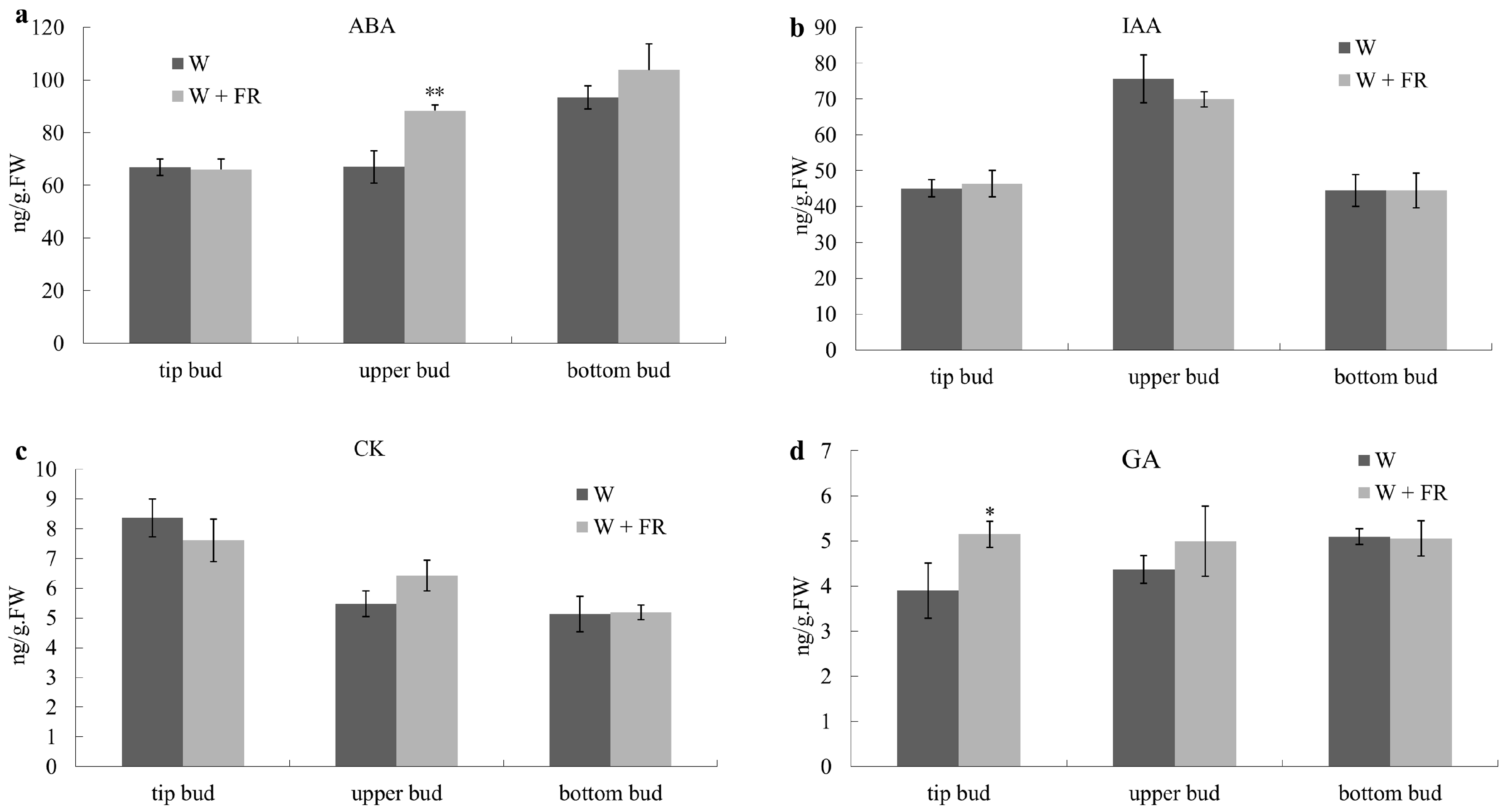
2.2. R:FR Affect Differently the IAA, CK, GA and ABA Deposition in Buds
2.3. Sucrose Transport is Affected by Extended Application of R:FR
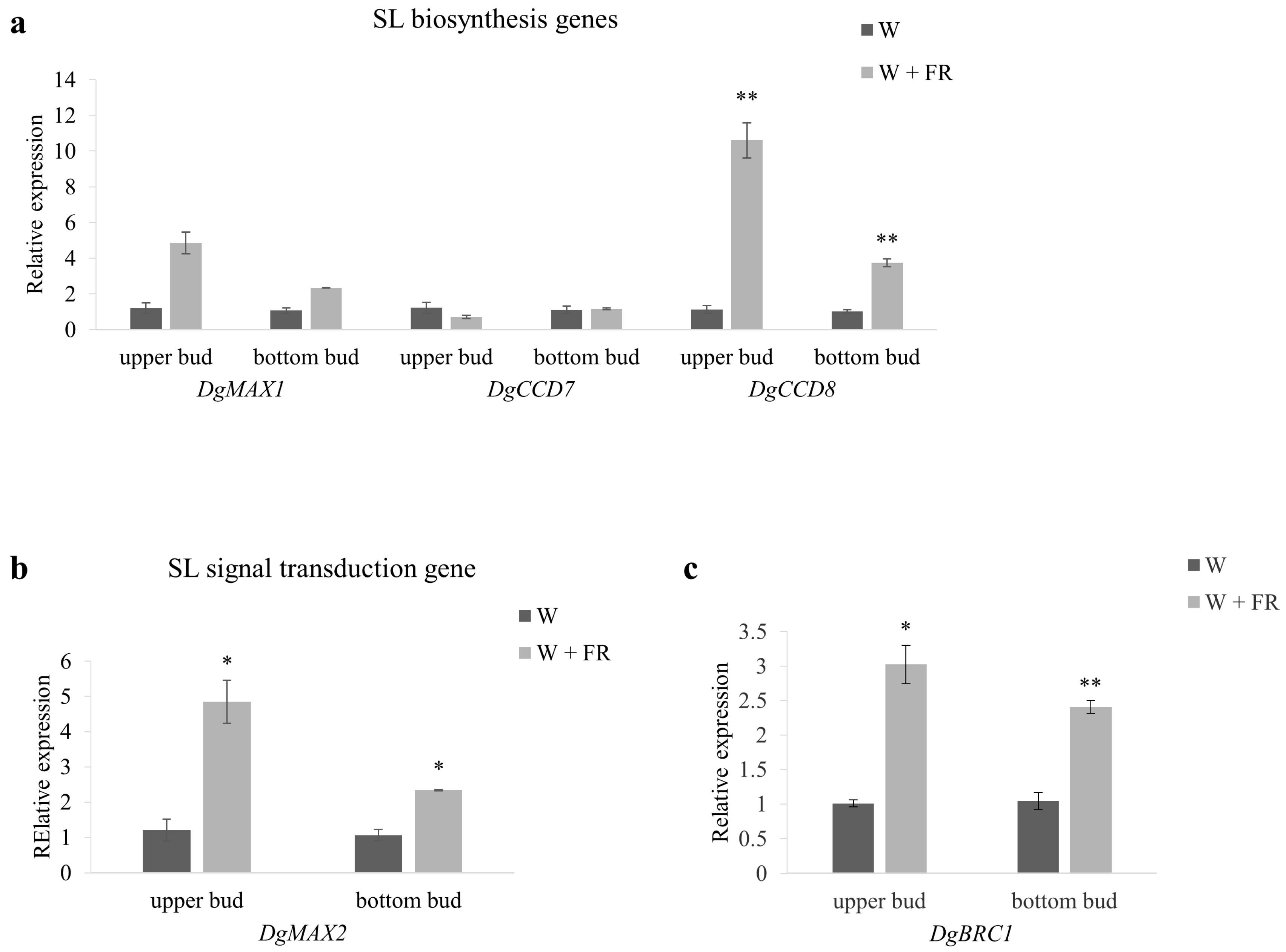
2.4. The Expression of Max-Pathway Related Genes in Response to Low R:FR
2.5. The Expression of ABA-Related Genes in Response to Low R:FR
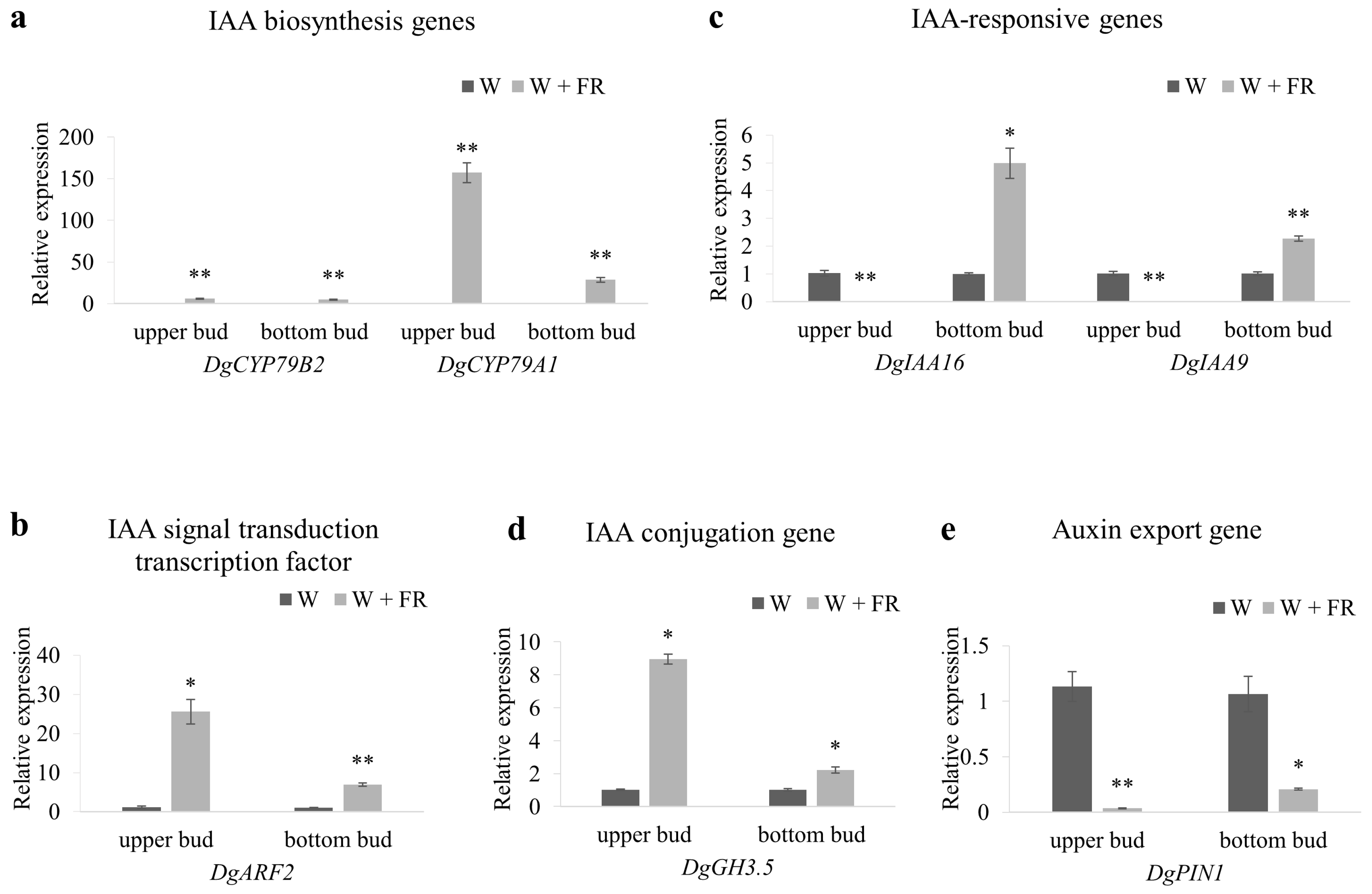
2.6. The Expression of Auxin-Pathway Related Genes in Response to Low R:FR
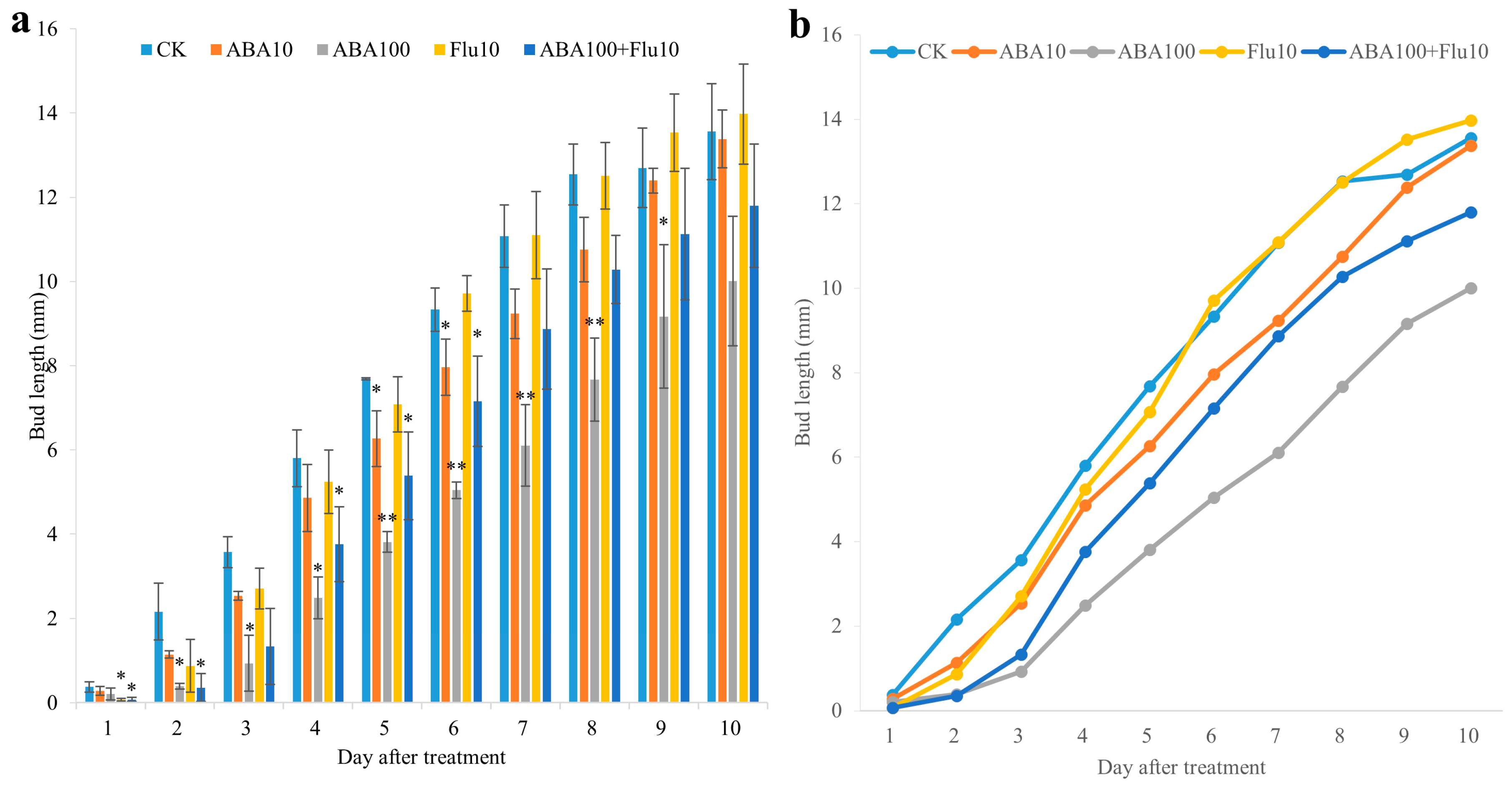
2.7. Exogenous ABA Suppressed Axillary Bud Outgrowth
3. Discussion
3.1. The Axillary Bud in Different Positions of Plant Showed Varied Response to R:FR Change
3.2. Hormones Were Involved in the Regulation of Axillary Bud Outgrowth in Response to Low R:FR
3.3. Sucrose Maybe an Important Regulator of Shoot Branching and Bud in Response to the R:FR
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
- (1)
- Plants were grown in low R:FR throughout the development stage (Low R:FR).
- (2)
- Plants were grown in high R:FR throughout the development stage (High R:FR).
- (3)
- Plants were first grown in low R:FR for 10 days at early seedling stage, then grown in high R:FR (Low R:FR 10 days + High R:FR).
- (4)
- Plants were first grown in high R:FR for 41 days (nearly 45 cm height), then either maintained under high R:FR or provided with low R:FR (High R:FR 45 + Low R:FR).
- (5)
- Plants were first grown in low R:FR for 41 days (nearly 45 cm height), then either maintained under low R:FR or provided with high R:FR (Low R:FR 45 + High R:FR).
- (6)
- Plants were first grown in high R:FR for 64 days (nearly 60 cm height), then either maintained under high R:FR or provided with low R:FR (High R:FR 60 + Low R:FR).
- (7)
- Plants were first grown in high R:FR for 41 days (nearly 45 cm height), then 1/2 plants were maintained in high R:FR and another 1/2 provided with low R:FR for 12 days, then re-grown in high R:FR (High R:FR 45 + Low R:FR 12 days + High R:FR).
- (8)
- Plants were first grown in low R:FR for 41 days (nearly 45 cm height), then 1/2 plants were maintained in low R:FR and another 1/2 provided with high R:FR for 12 days, then re-grown in low R:FR (Low R:FR 45 + High R:FR 12 days + Low R:FR).
4.2. Determination of Hormone Content
4.3. Determination of Sucrose Content
4.4. Quantitative Real-Time RT-PCR Analysis
4.5. ABA Treatments
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| R:FR | Red light to far-red light ratio |
| ABA | Abscisic acid |
| SL | Strigolactones |
| CK | Cytokinin |
| IAA | Indole-3-acetic acid |
| GA | Gibberellin |
| MAX | More axillary branching |
| PAT | Polar auxin transport |
| PIN1 | PIN-FORMED 1 |
| phyB | Phytochrome B |
| SAS | Shade avoidance syndrome |
| CCD | Carotenoid cleavage dioxygenase |
References
- Holalu, S.; Finlayson, S.A. The ratio of red light to far red light alters Arabidopsis axillary bud growth and abscisic acid signalling before stem auxin changes. J. Exp. Bot. 2017, 68, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.A.; Ottoline, L. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.J.; Drummond, R.S.; Snowden, K.C. Regulation of axillary shoot development. Curr. Opin. Plant Biol. 2014, 17, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rameau, C.; Bertheloot, J.; Leduc, N.; Andrieu, B.; Foucher, F.; Sakr, S. Multiple pathways regulate shoot branching. Front. Plant Sci. 2015, 5, 741. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.D.; Feldman, L.J. Bi-directional inflorescence development in Arabidopsis thaliana: Acropetal initiation of flowers and basipetal initiation of paraclades. Planta 1994, 192, 276–286. [Google Scholar] [CrossRef]
- Finlayson, S.A.; Krishnareddy, S.R.; Kebrom, T.H.; Casal, J.J. Phytochrome regulation of branching in Arabidopsis. Plant Physiol. 2010, 152, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Finlayson, S. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol. 2014, 164, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Holalu, S.V.; Casal, J.J.; Finlayson, S.A. Abscisic acid regulates Arabidopsis axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 2013, 163, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Holalu, S.V.; Casal, J.J.; Finlayson, S.A. The timing of low R:FR exposure profoundly affects Arabidopsis branching responses. Plant Signal. Behav. 2014, 9, e28668. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.; Schmitt, J. The genetic architecture of plasticity to density in Impatiens capensis. Evolution 1999, 53, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Brutnell, T.; Finlayson, S.A. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ. 2010, 33, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.H.; Hay, M.J.M.; Newton, P.C.D.; Greer, D.H. Effect of light quality (red:far-red ratio) at the apical bud of the main stolon on morphogenesis of Trifolium repens L. Ann. Bot. 1994, 74, 119–123. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. In The Arabidopsis Book; The American Society of Plant Biologists: Rockville, MD, USA, 2012; Volume 10, p. e0157. [Google Scholar]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Ljung, K.; Lopez-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; Xenarios, I.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Pacín, M.; Semmoloni, M.; Legris, M.; Finlayson, S.A.; Casal, J.J. Convergence of constitutive photomorphogenesis 1 and phytochrome interacting factor signalling during shade avoidance. New Phytol. 2016, 211, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed]
- González-Grandío, E.; Pozacarrion, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 2013, 25, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Cline, M. Concepts and terminology of apical dominance. Am. J. Bot. 1997, 84, 1064. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.E.; Cox, M.C.H.; Ross, J.J.; Krisantini, S.; Beveridge, C.A. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 2005, 138, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Dun, E.A.; Gui, R.; Mason, M.G.; Beveridge, C.A. Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol. 2015, 168, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; Hanan, J.; Beveridge, C.A. Computational modeling and molecular physiology experiments reveal new insights into shoot branching in pea. Plant Cell 2009, 21, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Q.; Xi, L.; Kou, Y.P.; Zhao, Y.; Zhao, L.J. Current perspectives on shoot branching regulation. Front. Agric. Sci. Eng. 2015, 2, 38–52. [Google Scholar] [CrossRef]
- Balla, J.; Kalousek, P.; Reinöhl, V.; Friml, J.; Procházka, S. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 2011, 65, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; Hines, G.; van Rongen, M.; Waldie, T.; Sawchuk, M.G.; Scarpella, E.; Ljung, K.; Leyser, O. Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol. 2016, 14, e1002446. [Google Scholar] [CrossRef] [PubMed]
- Prusinkiewicz, P.; Crawford, S.; Smith, R.S.; Ljung, K.; Bennett, T.; Ongaro, V.; Leyser, O. Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA 2009, 106, 17431–17436. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Peron, T.; Lecerf, M.; Perez-Garcia, M.D.; Barriere, Q.; Rolcik, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2010, 30, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Decourteix, M.; Alves, G.; Bonhomme, M.; Peuch, M.; Baaziz, K.B.; Brunel, N.; Guilliot, A.; Rageau, R.; Ameglio, T.; Sakr, G.P.S. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells: Their potential role in early events of growth resumption. Tree Physiol. 2008, 28, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Rabot, A.; Laoi, M.; Mortreau, E.; Sigogne, M.; Leduc, N.; Lemoine, R.; Sakr, S.; Vian, A.; Pelleschi-Travier, S. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ. 2011, 34, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Marquat, C.; Vandamme, M.; Gendraud, M.; Gendraud, G. Dormancy in vegetative buds of peach: Relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Sci. Hortic. 1999, 79, 151–162. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Baaziz, K.B.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Gourrierec, S.P.J.L.; et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Girault, T.; Abidi, F.; Sigogne, M.; Pelleschi-Travier, S.; Boumaza, R.; Sakr, S.; Leduc, N. Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ. 2010, 33, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Wareing, P.F. Apical dominance in Phaseolus vulgaris L.: The possible roles of abscisic acid and indole-3-acetic acid. J. Exp. Bot. 1984, 35, 239–244. [Google Scholar] [CrossRef]
- Mader, J.C.; Emery, R.J.N.; Turnbull, C.G.N. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiol. Plant. 2003, 119, 295–308. [Google Scholar] [CrossRef]
- Tamas, I.A.; Ozbun, J.L.; Wallace, D.H. Effect of Fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol. 1979, 64, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Gocal, G.F.W.; Pharis, R.P.; Yeung, E.C.; Pearce, D. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol. 1991, 95, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.J. Effects of far-red light on lateral bud outgrowth in decapitated tomato plants and associated changes in levels of auxin and abscisic acid. Plant Sci. Lett. 1977, 8, 339–344. [Google Scholar] [CrossRef]
- Tucker, D.J.; Mansfield, T. Effects of light quality on apical dominance in Xanthium strumarium and the associated changes in endogenous levels of abscisic acid and cytokinins. Planta 1972, 102, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000, 24, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G.; Oh, C. A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann. Bot. 2006, 98, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Le Bris, M.; Michaux-Ferriere, N.; Jacob, Y.; Poupet, A.; Barthe, P.; Guigonis, J.M.; Le Page-Degivry, M.T. Regulation of bud dormancy by manipulation of ABA in isolated buds of Rosa hybrida cultured in vitro. Aust. J. Plant Physiol. 1999, 26, 273–281. [Google Scholar] [CrossRef]
- Arend, M.; Schnitzler, J.P.; Ehlting, B.; Hänsch, R.; Lange, T.; Rennenberg, H.; Himmelbach, A.; Grill, E.; Fromm, J. Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiol. 2009, 151, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Morea, F.A.; Vicentini, R.; Silva, G.F.F.; Silva, E.M.; Carrer, H.; Rodrigues, A.P.; Nogueira, F.T. Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. J. Exp. Bot. 2013, 64, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Mullet, J.E. Transcriptome profiling of tiller buds provides new insights into PhyB regulation of tillering and indeterminate growth in sorghum. Plant Physiol. 2016, 170, 2232–2250. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Finlayson, S.A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; de Saint Germain, A.; Pillot, J.P.; Boutet-Mercey, S.; Dalmais, M.; Antoniadi, I.; Li, X.; Maia-Grondard, A.; Le Signor, C.; Bouteiller, N.; et al. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 2012, 158, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhou, X.Y.; Xi, L.; Li, J.X.; Zhao, R.Y.; Ma, N.; Zhao, L.J. Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema x grandiflora cv. Jinba). PLoS ONE 2013, 8, e61717. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.A.; De, S.G.A.; Rameau, C.; Beveridge, C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012, 158, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Ross, J.H.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, S.A. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol. 2007, 48, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martínez, J.A.; Pozacarrion, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H.; Burson, B.L.; Finlayson, S.A. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 2006, 140, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Dierck, R.; Dhooghe, E.; Huylenbroeck, J.V.; Riek, J.D.; Keyser, E.D.; Straeten, D.V.D. Response to strigolactone treatment in chrysanthemum axillary buds is influenced by auxin transport inhibition and sucrose availability. Acta Physiol. Plant 2016, 38, 271. [Google Scholar] [CrossRef]
- Dierck, R.; Keyser, E.D.; Riek, J.D.; Dhooghe, E.; Huylenbroeck, J.V.; Prinsen, E.; Straeten, D.V.D. Change in auxin and cytokinin levels coincides with altered expression of branching genes during axillary bud outgrowth in Chrysanthemum. PLoS ONE 2016, 11, e0161732. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.L.; Ishak, A.; Yu, J.; Zhao, R.Y.; Zhao, L.J. Identification and functional analysis of three MAX2 orthologs in Chrysanthemum. J. Integr. Plant Biol. 2013, 55, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Chen, S.; Jiang, J.; Zhang, S.; Chen, F.; Fang, W. Changes of endogenous hormones in lateral buds of chrysanthemum during their outgrowth. Russian J. Plant Physiol. 2012, 59, 356–363. [Google Scholar] [CrossRef]
- Liang, J.L.; Zhao, L.J.; Challis, R.; Leyser, O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J. Exp. Bot. 2010, 61, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L.; Casal, J.J.; Ghersa, C.M. Light signals perceived by crop and weed plants. Field Crop. Res 2000, 67, 149–160. [Google Scholar] [CrossRef]
- Casal, J.J.; Deregibus, V.A.; Sánchez, R.A. Variations in tiller dynamics and morphology in Lolium multiflorum Lam. Vegetative and reproductive plants as affected by differences in red/far-red irradiation. Ann. Bot. 1985, 56, 553–559. [Google Scholar] [CrossRef]
- Girault, T.B.; Bergougnoux, V.; Combes, D.; Viemont, J.D.; Leduc, N. Light controls shoot meristem organogenic activity and leaf primordia growth during bud burst in Rosa sp. Plant Cell Environ. 2008, 31, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Arenashuertero, F.; Arroyo, A.; Li, Z.; Sheen, J.; Leon, P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000, 14, 2085–2096. [Google Scholar]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–78. [Google Scholar] [CrossRef]
- Gazzarrini, S.; McCourt, P. Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr. Opin. Plant Biol. 2001, 4, 387–391. [Google Scholar] [CrossRef]
- Gibson, S.I. Plant sugar-response pathways: Part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.C.; Sheen, J. Sugar sensing in higher plants. Trends Plant Sci. 1997, 2, 208–214. [Google Scholar] [CrossRef]
- Kurata, T.; Yamamoto, K.T. Petit1, a conditional growth mutant of Arabidopsis defective in sucrose-dependent elongation growth. Plant Physiol. 1998, 118, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Matsukura, C.; Vernieri, P.; Yamaguchi, J. Sugar repression of a gibberellin-dependent signaling pathway in barley embryos. Plant Cell 1997, 9, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S. Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 49–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Lee, Y.C.; Fang, S.C.; Chan, M.T.; Hwa, S.F.; Liu, L.F. Sugars act as signal molecules and osmotica to regulate the expression of α-amylase genes and metabolic activities in germinating cereal grains. Plant Mol. Biol. 1996, 30, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef]
- Stulke, J.; Hillen, W. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 1999, 2, 195–201. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 1998, 95, 4784–4788. [Google Scholar] [CrossRef] [PubMed]
- Rook, F.; Gerrits, N.; Kortstee, A.; van Kampen, M.; Borrias, M.; Weisbeek, P.; Smeekens, S. Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J. 1998, 15, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.B. Sugar as a key component of the shoot branching regulation network. Plant Cell Environ. 2015, 38, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N. Determination of sucrose. In Plant Physiology Lab Guide; Zhang, Z.L., Qu, W.J., Eds.; Higher Education Press: Beijing, China, 2003; pp. 128–129. (In Chinese) [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Ahmad, S.; Cheng, T.; Wang, J.; Pan, H.; Zhao, L.; Zhang, Q. Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Int. J. Mol. Sci. 2018, 19, 1590. https://doi.org/10.3390/ijms19061590
Yuan C, Ahmad S, Cheng T, Wang J, Pan H, Zhao L, Zhang Q. Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’). International Journal of Molecular Sciences. 2018; 19(6):1590. https://doi.org/10.3390/ijms19061590
Chicago/Turabian StyleYuan, Cunquan, Sagheer Ahmad, Tangren Cheng, Jia Wang, Huitang Pan, Liangjun Zhao, and Qixiang Zhang. 2018. "Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’)" International Journal of Molecular Sciences 19, no. 6: 1590. https://doi.org/10.3390/ijms19061590
APA StyleYuan, C., Ahmad, S., Cheng, T., Wang, J., Pan, H., Zhao, L., & Zhang, Q. (2018). Red to Far-Red Light Ratio Modulates Hormonal and Genetic Control of Axillary bud Outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’). International Journal of Molecular Sciences, 19(6), 1590. https://doi.org/10.3390/ijms19061590





