Molecular Modeling Studies on Carbazole Carboxamide Based BTK Inhibitors Using Docking and Structure-Based 3D-QSAR
Abstract
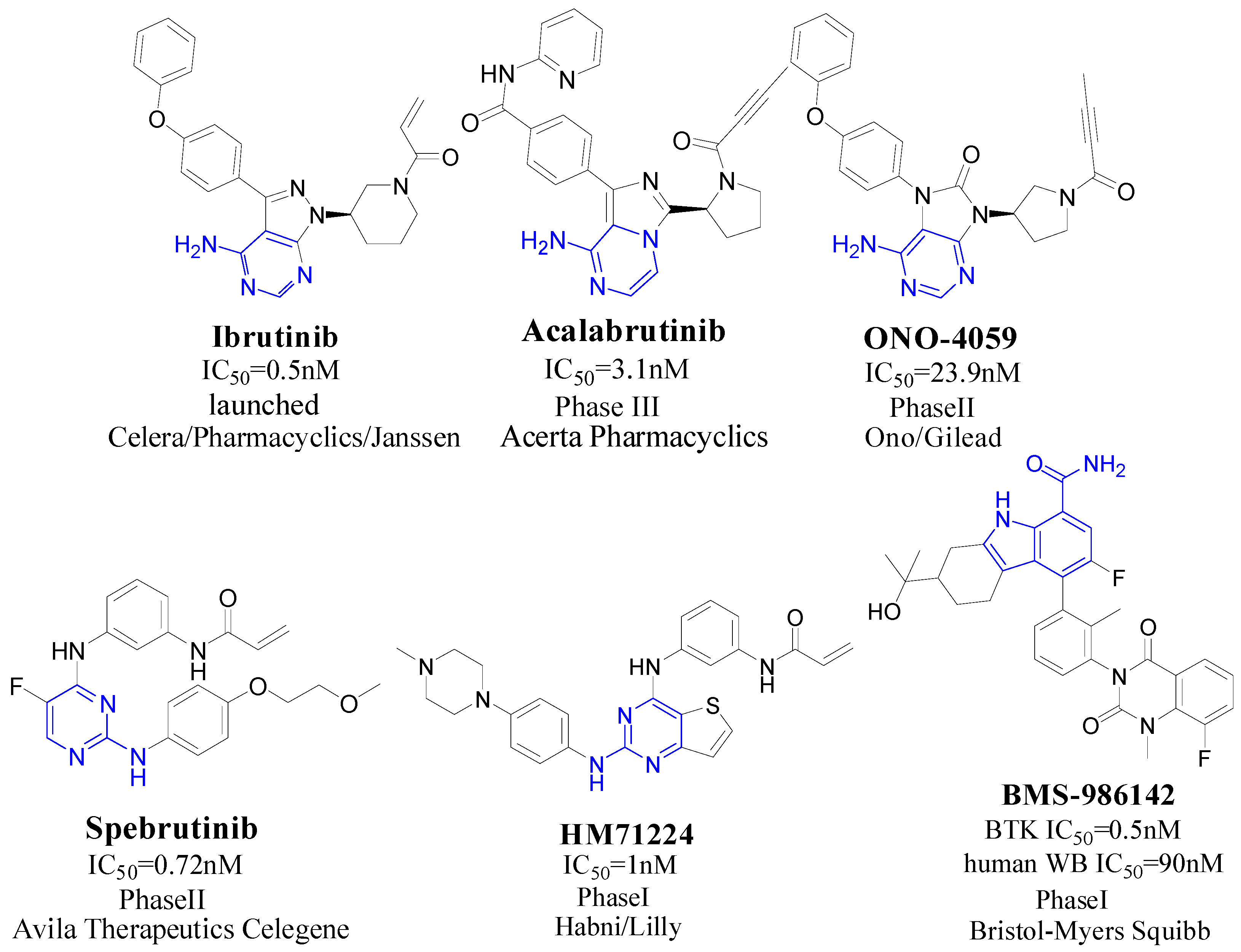
:1. Introduction
2. Results and Discussion
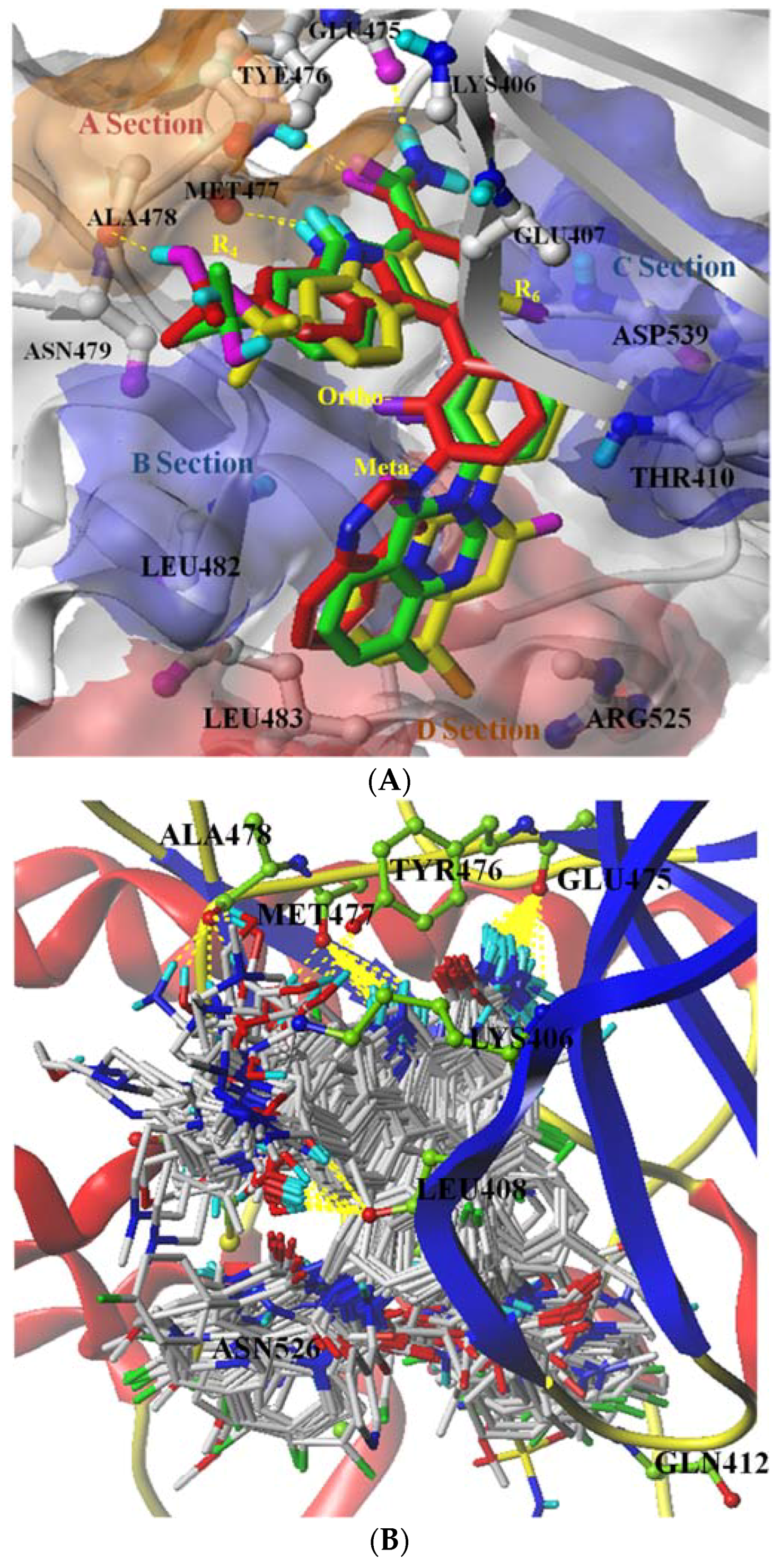
2.1. Molecular Docking
2.2. 3D-QSAR Analysis Studies
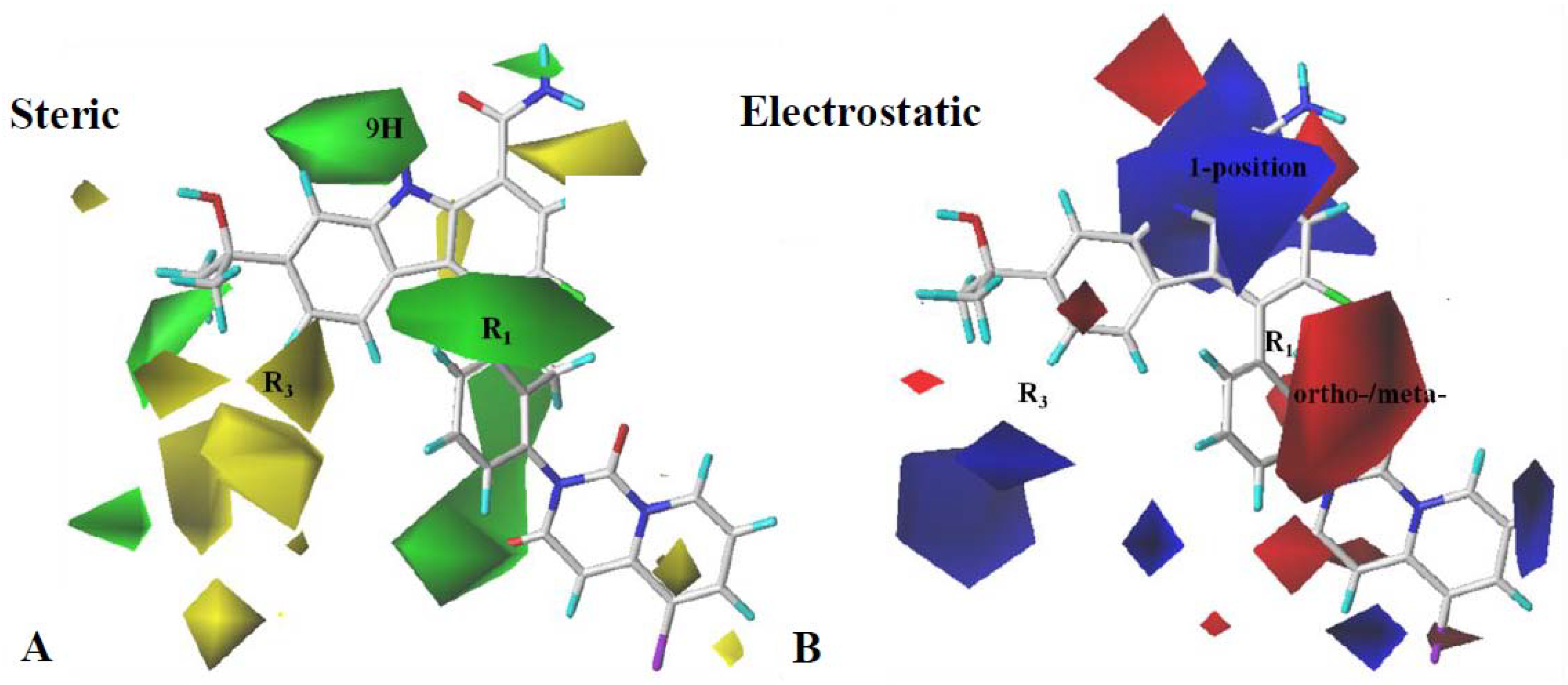
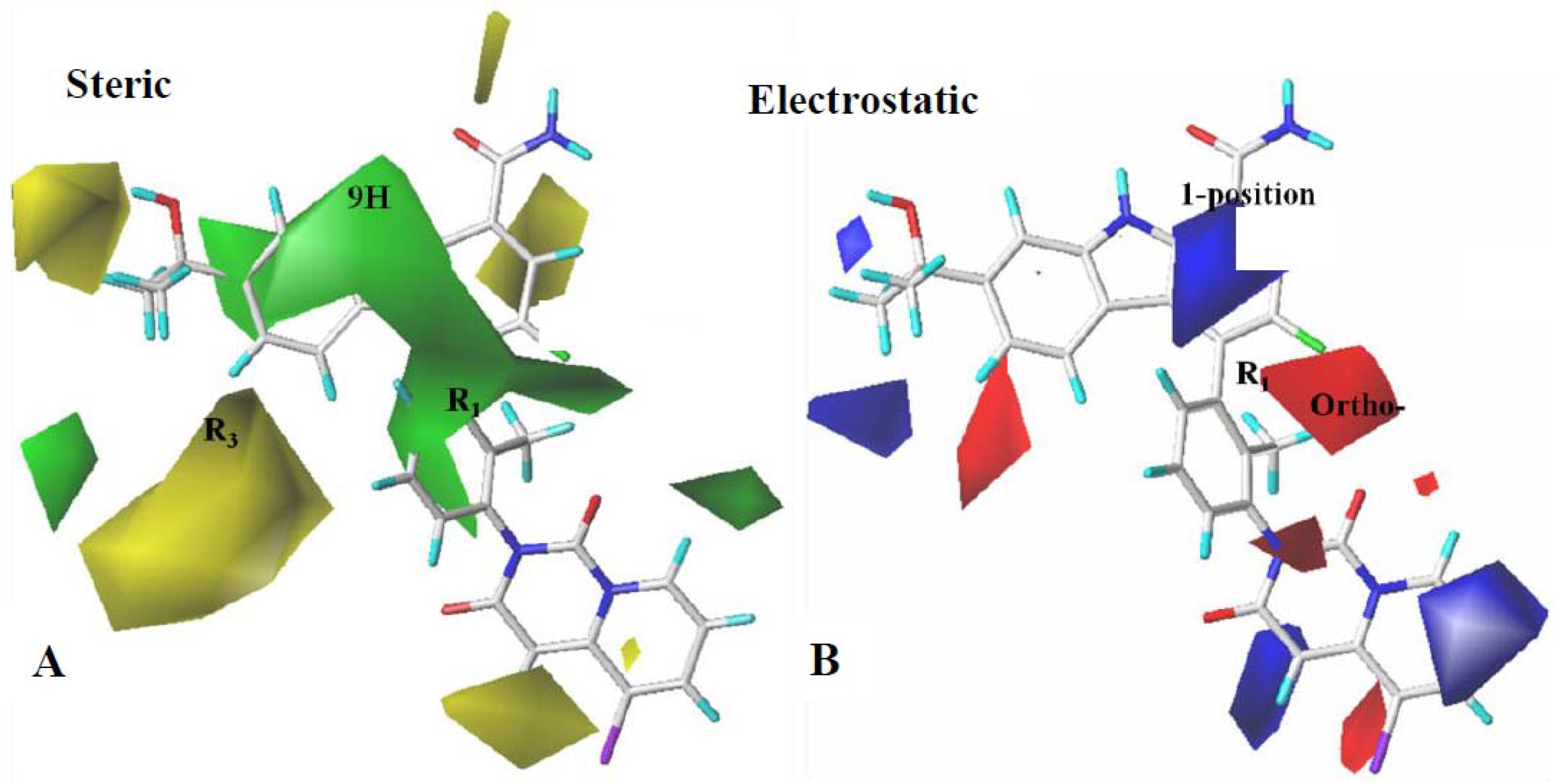
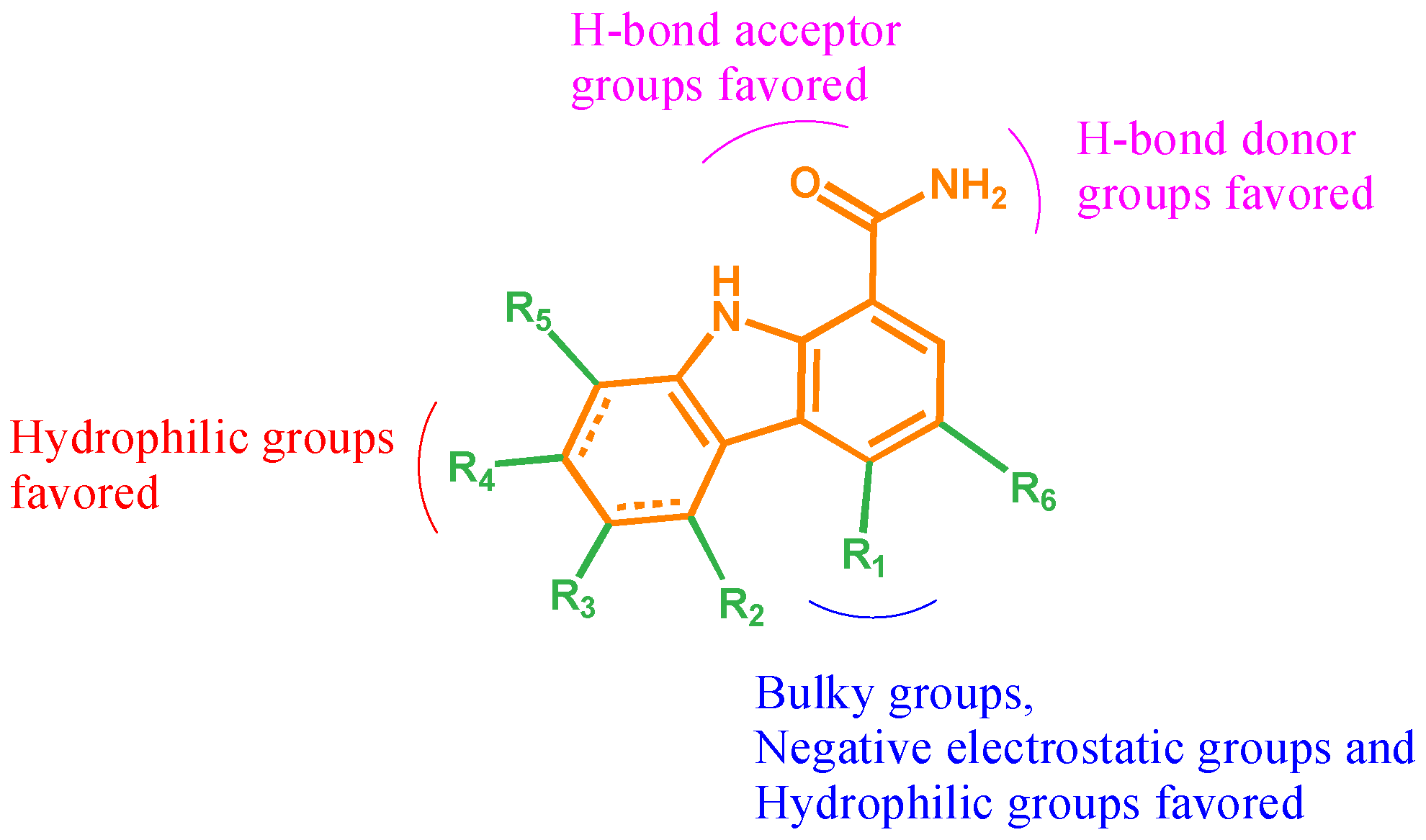
2.3. Contour Map Analysis
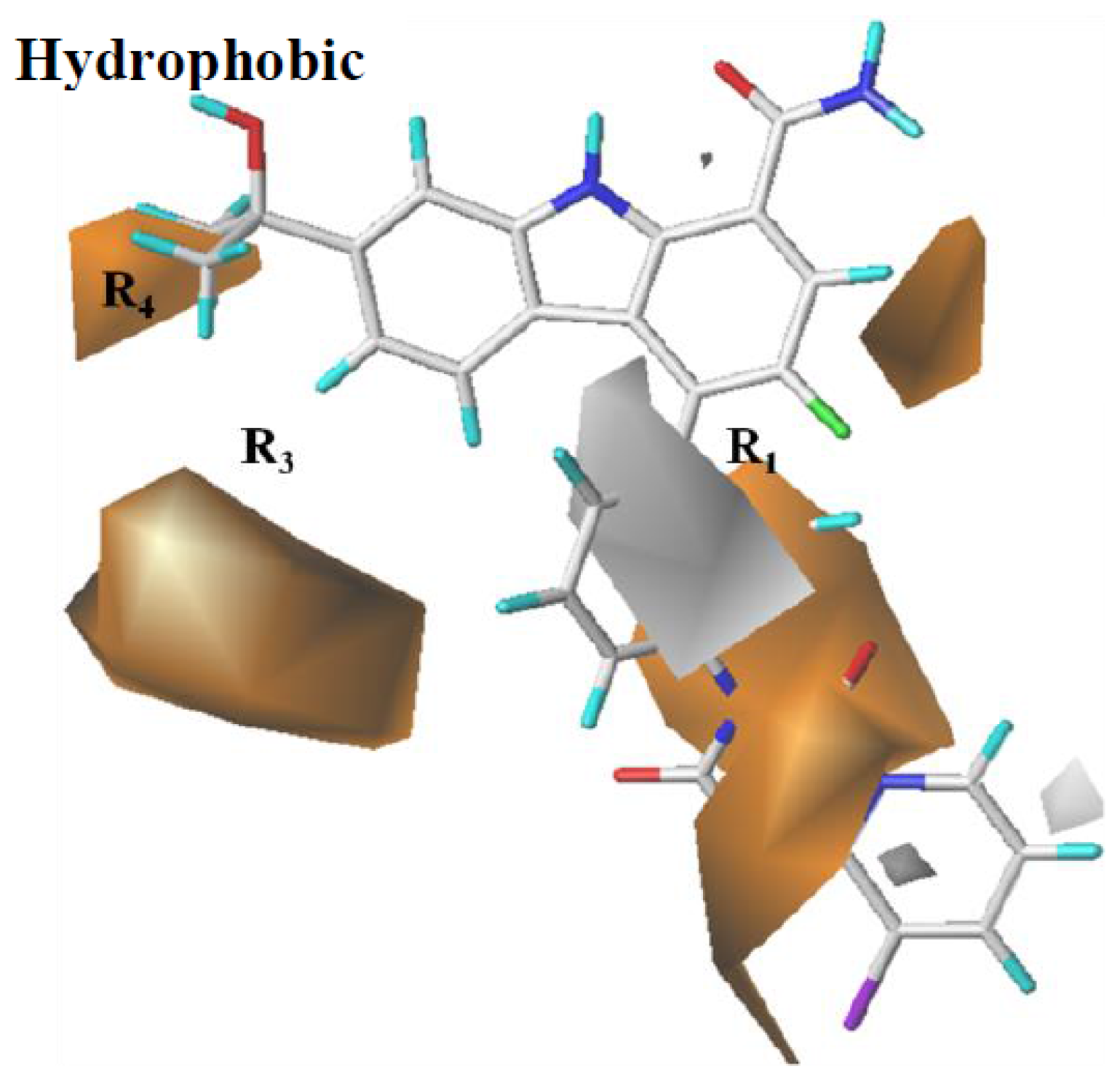
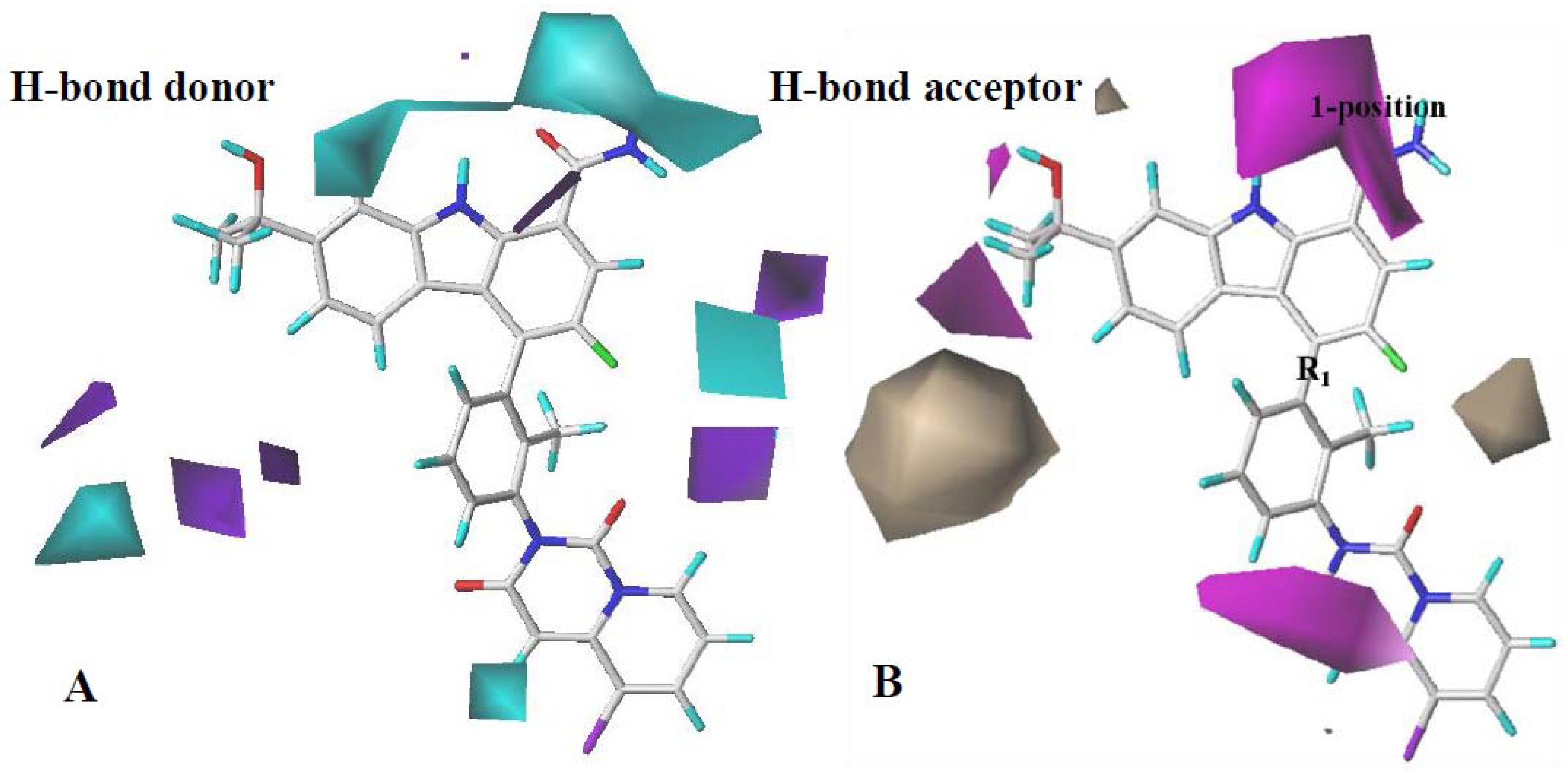
2.3.1. CoMFA Contour Map Analysis
2.3.2. CoMSIA Contour Map Analysis
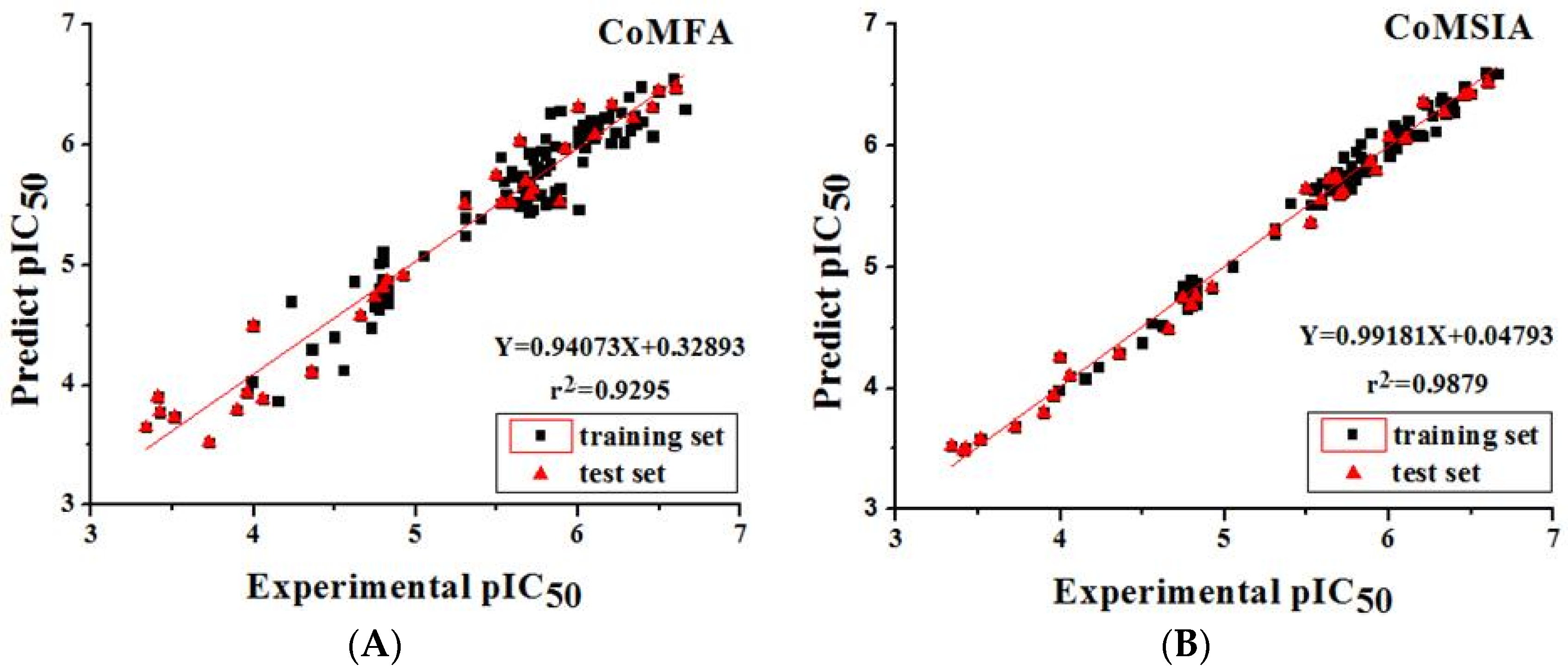
2.4. Model Validation of CoMFA and CoMSIA Models
3. Materials and Methods
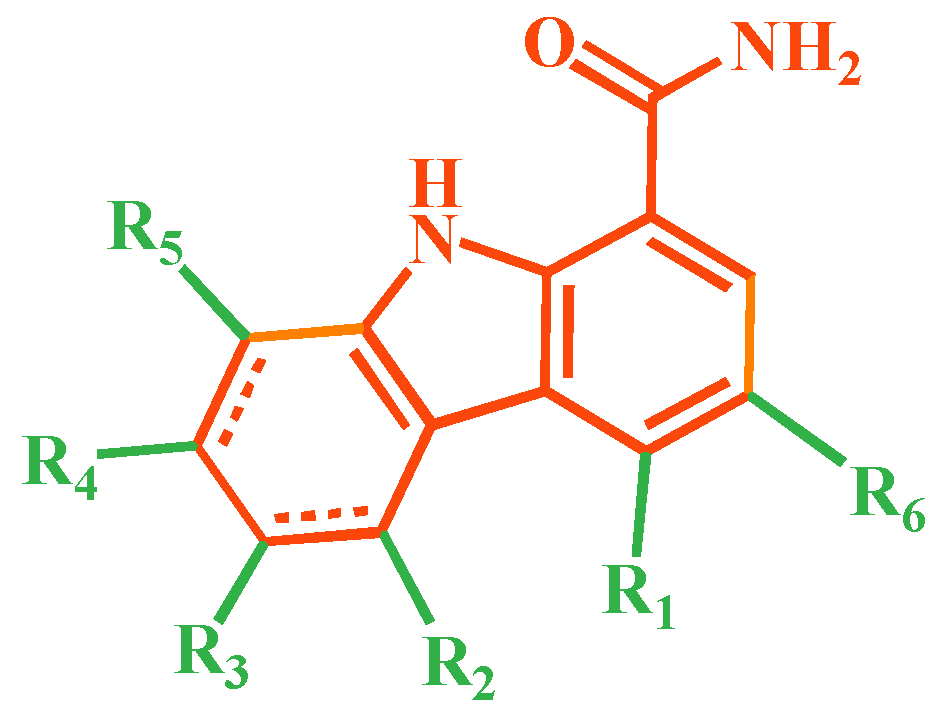
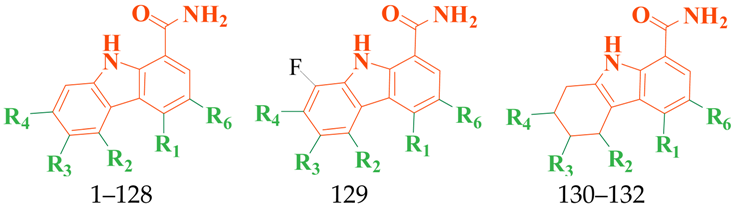
3.1. Collection of the Dataset
3.2. Preparation of Protein
3.3. Molecular Docking and Alignment
3.4. 3D-QSAR Analysis Studies
3.5. Model Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BTK | Broton’s tyrosine kinase |
| RA | Rheumatoid Arthritis |
| BCR | B-cell receptor |
| NSAIDs | non-steroidal anti-inflammatory drug |
| SAARDs | slow acting anti-rheumatic drugs |
| DMARDs | disease-modifying anti rheumatic drugs |
| 3D-QSAR | three-dimensional quantitative structure–activity relationship |
| CoMFA | comparative molecular field analysis |
| CoMSIA | comparative molecular similarity indices analysis |
| PLS | partial least square |
| XLA | X-linked agammaglobulinemia |
| ALL | acute lymphoblastic leukemia |
| CML | chronic myeloid leukemia |
| CLL | chronic lymphocytic leukemia |
References
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid arthritis-associated lung disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brito, Y.; Glassberg, M.K.; Ascherman, D.P. Rheumatoid Arthritis-Associated Interstitial Lung Disease: Current Concepts. Curr. Rheumatol. Rep. 2017, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.; Breedveld, F.C.; Dougados, M.; Emery, P.; Gaujoux-Viala, C.; Gorter, S.; Knevel, R.; Nam, J.; Schoels, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum. Dis. 2010, 69, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.H.; Cope, A.P.; Scott, D.L. Safety of combination therapies in early rheumatoid arthritis: A systematic comparison between antirheumatic drugs and TNF inhibitors with methotrexate. Int. J. Clin. Rheumatol. 2010, 5, 547–554. [Google Scholar] [CrossRef]
- Takata, M.; Kurosaki, T. A Role for Bruton’s Tyrosine Kinase in B Cell Antigen Receptor-mediated Activation of Phospholipase C-gamma 2. J. Exp. Med. 1996, 184, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.J.; Nore, B.F.; Christensson, B.; Smith, C.I. Signalling of Bruton’s Tyrosine Kinase, Btk. Scand. J. Immunol. 1999, 49, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Whang, J.A.; Chang, B.Y. Bruton’s tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. Drug Discov. Today 2014, 19, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Scheerens, H.; Li, S.J.; Schultz, B.E.; Sprengeler, P.A.; Burrill, L.C.; Mendonca, R.V.; Sweeney, M.D.; Scott, K.C.; Grothaus, P.G.; et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2007, 2, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Liu, D. Acalabrutinib (ACP-196): A selective second-generation BTK inhibitor. J. Hematol. Oncol. 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Birkett, J.T.; Kawabata, K. ONO-4059, A Novel Bruton’s Tyrosine Kinase (Btk) Inhibitor: Synergistic Activity in Combination with Chemotherapy in a ABC-DLBCL Cell Line. Blood 2013, 122, 5151. [Google Scholar]
- Evans, E.K.; Tester, R.; Aslanian, S.; Karp, R.; Sheets, M.; Labenski, M.T.; Witowski, S.R.; Lounsbury, H.; Chaturvedi, P.; Mazdiyasni, H.; et al. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J. Pharmacol. Exp. Ther. 2013, 346, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Byun, J.Y.; Park, J.A.; Kim, Y.Y.; Lee, Y.J.; Oh, J.I.; Jang, S.Y.; Kim, Y.H.; Song, Y.W.; Son, J.; et al. HM71224, a novel Bruton’s tyrosine kinase inhibitor, suppresses B cell and monocyte activation and ameliorates arthritis in a mouse model: A potential drug for rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Watterson, S.H.; De Lucca, G.V.; Shi, Q.; Langevine, C.M.; Liu, Q.; Batt, D.G.; Beaudoin Bertrand, M.; Gong, H.; Dai, J.; Yip, S.; et al. Discovery of 6-Fluoro-5-(R)-(3-(S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8-carboxamide (BMS-986142): A Reversible Inhibitor of Bruton’s Tyrosine Kinase (BTK) Conformationally Constrained by Two Locked Atropisomers. J. Med. Chem. 2016, 59, 9173–9200. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Xing, J.; Catlett, I.M.; Adamczyk, R.; Griffies, A.; Liu, A.; Murthy, B.; Nowak, M. Safety, pharmacokinetics, and pharmacodynamics of BMS-986142, a novel reversible BTK inhibitor, in healthy participants. Eur. J. Clin. Pharmacol. 2017, 73, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, K.M.; Pulicicchio, C.; Pattoli, M.A.; Cheng, L.; Skala, S.; Heimrich, E.M.; McIntyre, K.W.; Taylor, T.L.; Kukral, D.W.; Dudhgaonkar, S.; et al. Bruton’s tyrosine kinase inhibitor BMS-986142 in experimental models of rheumatoid arthritis enhances efficacy of agents representing clinical standard-of-care. PLoS ONE 2017, 12, e0181782. [Google Scholar] [CrossRef] [PubMed]
- Cramer, R.D.; Patterson, D.E.; Bunce, J.D. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 1988, 110, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Klebe, G.; Abraham, U.; Mietzner, T. Molecular Similarity Indices in a Comparative Analysis (CoMSIA) of Drug Molecules to Correlate and Predict Their Biological Activity. J. Med. Chem. 1994, 37, 4130–4146. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zuo, Y.; Tang, G.; Wang, Y.; Zhou, Y.; Wang, X.; Guo, T.; Xia, M.; Ding, N.; Pan, Z. Discovery of a series of 2,5-diaminopyrimidine covalent irreversible inhibitors of Bruton’s tyrosine kinase with in vivo antitumor activity. J. Med. Chem. 2014, 57, 5112–5128. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, G.V.; Shi, Q.; Liu, Q.; Batt, D.G.; Beaudoin Bertrand, M.; Rampulla, R.; Mathur, A.; Discenza, L.; D’Arienzo, C.; Dai, J.; et al. Small Molecule Reversible Inhibitors of Bruton’s Tyrosine Kinase (BTK): Structure-Activity Relationships Leading to the Identification of 7-(2-Hydroxypropan-2-yl)-4-[2-methyl-3-(4-oxo-3,4-dihydroquinazolin-3-yl)phenyl]-9H-carbazole-1-carboxamide (BMS-935177). J. Med. Chem. 2016, 59, 7915–7935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Batt, D.G.; Lippy, J.S.; Surti, N.; Tebben, A.J.; Muckelbauer, J.K.; Chen, L.; An, Y.; Chang, C.; Pokross, M.; et al. Design and synthesis of carbazole carboxamides as promising inhibitors of Bruton’s tyrosine kinase (BTK) and Janus kinase 2 (JAK2). Bioorg. Med. Chem. Lett. 2015, 25, 4265–4269. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Batt, D.G.; DeLucca, G.V.; Shi, Q.; Tebben, A.J. Carbazole Carboxamide Compounds Useful as Kinase Inhibitors. U.S. Patent 8,362,065, 9 January 2013. [Google Scholar]
- Batt, D.G.; Bertrand, M.B.; DeLucca, G.; Galella, M.A.; Ko, S.S.; Langevine, C.M.; Liu, Q.; Shi, Q.; Srivastava, A.S.; Tino, J.A.; et al. Substituted Tetrahydrocarbazole and Carbazole Carboxamide Compounds. U.S. Patent 9,334,290, 10 May 2016. [Google Scholar]
- Clark, M.; Cramer, R.D.; Van Opdenbosch, N. Validation of the General Purpose Tripos 5.2 Force Field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Cramer, R.D., III; Patterson, D.E.; Bunce, J.D. Recent advances in comparative molecular field analysis (CoMFA). Prog. Clin. Biol. Res. 1989, 291, 161–165. [Google Scholar] [PubMed]
- Balasubramanian, P.K.; Balupuri, A.; Gadhe, C.G.; Cho, S.J. 3D QSAR modeling study on 7-aminofuro [2,3-c] pyridine derivatives as TAK1 inhibitors using CoMFA and COMSIA. Med. Chem. Res. 2014, 24, 2347–2365. [Google Scholar] [CrossRef]
- Cai, B.Q.; Jin, H.X.; Yan, X.J.; Zhu, P.; Hu, G.X. 3D-QSAR and 3D-QSSR studies of thieno[2,3-d]pyrimidin-4-yl hydrazone analogues as CDK4 inhibitors by CoMFA analysis. Acta Pharmacol. Sin. 2014, 35, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Aalizadeh, R.; Pourbasheer, E.; Ganjali, M.R. Analysis of B-Raf V600E inhibitors using 2D and 3D-QSAR, molecular docking and pharmacophore studies. Mol. Divers. 2015, 19, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Wehrens, R.; Putter, H.; Buydens, L.M. The bootstrap: A tutorial. Chemometr. Intell. Lab. Syst. 2000, 54, 35–52. [Google Scholar] [CrossRef]
| CoMFA | NOC | q2 | r2 | SEE | F Value | Field Contributions | ||||
| S | E | H | D | A | ||||||
| S+E | 6 | 0.761 | 0.933 | 0.202 | 291.45 | 0.46 | 0.54 | - | - | - |
| CoMSIA | NOC | q2 | r2 | SEE | F Value | Field Contributions | ||||
| S | E | H | D | A | ||||||
| S+E | 5 | 0.851 | 0.941 | 0.188 | 404.01 | 0.198 | 0.802 | - | - | - |
| S+E+H | 7 | 0.862 | 0.972 | 0.132 | 606.51 | 0.110 | 0.554 | 0.336 | - | - |
| S+E+D | 4 | 0.863 | 0.930 | 0.205 | 420.26 | 0.117 | 0.515 | - | 0.367 | - |
| S+E+A | 7 | 0.863 | 0.974 | 0.127 | 657.51 | 0.122 | 0.535 | - | - | 0.342 |
| S+E+H+D | 9 | 0.875 | 0.985 | 0.095 | 920.97 | 0.069 | 0.424 | 0.235 | 0.272 | - |
| S+E+H+A | 9 | 0.880 | 0.986 | 0.095 | 923.65 | 0.073 | 0.411 | 0.254 | - | 0.262 |
| S+E+D+A | 10 | 0.878 | 0.985 | 0.092 | 1031.44 | 0.078 | 0.400 | - | 0.270 | 0.253 |
| S+E+H+D+A | 9 | 0.891 | 0.988 | 0.088 | 1076.36 | 0.053 | 0.342 | 0.193 | 0.208 | 0.203 |
| Training Set Compounds | pIC50 | CoMFA | CoMSIA | ||
| Predicted | Residuals | Predicted | Residuals | ||
| 1 | 4.3565 | 4.304 | 0.0525 | 4.286 | 0.0705 |
| 3 | 5.6778 | 5.554 | 0.1239 | 5.730 | −0.0526 |
| 4 | 5.8239 | 5.526 | 0.2981 | 5.790 | 0.0344 |
| 5 | 5.6576 | 5.649 | 0.0082 | 5.673 | −0.0157 |
| 6 | 5.7447 | 5.758 | −0.0135 | 5.753 | −0.008 |
| 7 | 5.6576 | 5.524 | 0.1337 | 5.697 | −0.0393 |
| 8 | 5.6576 | 5.751 | −0.0938 | 5.659 | −0.0011 |
| 9 | 5.6576 | 5.735 | −0.0776 | 5.656 | 0.0015 |
| 10 | 5.6383 | 5.493 | 0.1456 | 5.640 | −0.0015 |
| 11 | 5.7959 | 6.058 | −0.2625 | 5.957 | −0.1615 |
| 12 | 6.0969 | 6.060 | 0.0366 | 6.102 | −0.0048 |
| 13 | 5.6990 | 5.631 | 0.0675 | 5.653 | 0.0459 |
| 14 | 6.0000 | 6.042 | −0.0422 | 5.925 | 0.0755 |
| 15 | 6.1551 | 6.234 | −0.0789 | 6.087 | 0.0681 |
| 17 | 5.8861 | 5.641 | 0.2452 | 5.885 | 0.0008 |
| 18 | 5.6990 | 5.583 | 0.1156 | 5.704 | −0.0049 |
| 19 | 5.7447 | 5.821 | −0.0766 | 5.761 | −0.0162 |
| 20 | 5.5376 | 5.698 | −0.16 | 5.638 | −0.1001 |
| 22 | 5.8539 | 5.615 | 0.2385 | 5.855 | −0.0012 |
| 23 | 5.7213 | 5.465 | 0.2565 | 5.751 | −0.0294 |
| 24 | 5.7959 | 5.917 | −0.1211 | 5.731 | 0.0644 |
| 25 | 5.5229 | 5.901 | −0.3785 | 5.516 | 0.0073 |
| 26 | 5.7213 | 5.876 | −0.1547 | 5.908 | −0.1865 |
| 28 | 6.0924 | 6.208 | −0.1156 | 6.099 | 0.0066 |
| 29 | 6.2076 | 6.337 | −0.1291 | 6.36 | −0.1519 |
| 31 | 5.5528 | 5.597 | −0.0445 | 5.654 | −0.1009 |
| 32 | 5.0458 | 5.081 | −0.0354 | 5.010 | 0.0357 |
| 33 | 5.5850 | 5.744 | −0.1586 | 5.518 | 0.0669 |
| 34 | 5.8239 | 6.272 | −0.4485 | 6.017 | −0.1929 |
| 36 | 5.7695 | 5.951 | −0.1814 | 5.642 | 0.1275 |
| 37 | 6.2007 | 6.017 | 0.1837 | 6.080 | 0.1203 |
| 38 | 6.2840 | 6.026 | 0.2581 | 6.119 | 0.165 |
| 39 | 6.0269 | 5.864 | 0.1626 | 6.068 | −0.0413 |
| 40 | 6.0000 | 6.121 | −0.1213 | 5.939 | 0.0609 |
| 42 | 5.7695 | 5.786 | −0.0166 | 5.831 | −0.0613 |
| 43 | 5.7959 | 5.791 | 0.0051 | 5.737 | 0.059 |
| 44 | 5.7959 | 5.513 | 0.2828 | 5.760 | 0.0358 |
| 45 | 6.0315 | 6.176 | −0.1442 | 6.172 | −0.1406 |
| 46 | 5.6383 | 6.035 | −0.397 | 5.728 | −0.0898 |
| 47 | 5.8239 | 5.844 | −0.02 | 5.909 | −0.0852 |
| 48 | 5.5850 | 5.787 | −0.2015 | 5.695 | −0.1097 |
| 49 | 5.3010 | 5.244 | 0.0572 | 5.325 | −0.0241 |
| 50 | 5.3010 | 5.393 | −0.0917 | 5.275 | 0.026 |
| 52 | 4.7959 | 5.112 | −0.3163 | 4.728 | 0.0682 |
| 54 | 4.7447 | 4.654 | 0.0905 | 4.849 | −0.1045 |
| 55 | 4.7695 | 4.684 | 0.0851 | 4.826 | −0.0568 |
| 56 | 4.8239 | 4.779 | 0.045 | 4.703 | 0.1207 |
| 57 | 4.7959 | 4.792 | 0.0037 | 4.902 | −0.1061 |
| 59 | 4.7695 | 4.634 | 0.1357 | 4.657 | 0.1121 |
| 60 | 5.6990 | 5.694 | 0.0045 | 5.628 | 0.0712 |
| 61 | 5.6990 | 5.440 | 0.2595 | 5.636 | 0.0632 |
| 62 | 4.7959 | 4.760 | 0.0359 | 4.871 | −0.0747 |
| 63 | 4.7695 | 4.812 | −0.0421 | 4.753 | 0.0165 |
| 64 | 5.3010 | 5.582 | −0.2807 | 5.326 | −0.0249 |
| 65 | 4.8239 | 4.686 | 0.1384 | 4.849 | −0.0248 |
| 66 | 5.7213 | 5.880 | −0.159 | 5.762 | −0.0407 |
| 67 | 5.6990 | 5.454 | 0.2446 | 5.714 | −0.0154 |
| 68 | 5.6778 | 5.651 | 0.0267 | 5.790 | −0.1123 |
| 69 | 5.8539 | 5.991 | −0.1368 | 5.792 | 0.0624 |
| 70 | 5.7695 | 5.587 | 0.183 | 5.727 | 0.0424 |
| 71 | 5.7959 | 5.955 | −0.1589 | 5.760 | 0.0362 |
| 72 | 6.1805 | 6.234 | −0.0532 | 6.087 | 0.0931 |
| 73 | 6.3872 | 6.487 | −0.1002 | 6.355 | 0.0321 |
| 76 | 5.3979 | 5.391 | 0.007 | 5.528 | −0.1299 |
| 77 | 6.3468 | 6.220 | 0.1271 | 6.272 | 0.0749 |
| 79 | 6.6576 | 6.305 | 0.3527 | 6.596 | 0.0612 |
| 80 | 6.1135 | 6.136 | −0.0222 | 6.213 | −0.0992 |
| 81 | 6.3188 | 6.131 | 0.188 | 6.402 | −0.0837 |
| 83 | 6.2291 | 6.107 | 0.1225 | 6.339 | −0.1095 |
| 85 | 6.0000 | 6.025 | −0.0254 | 6.029 | −0.0285 |
| 86 | 6.3098 | 6.404 | −0.0941 | 6.365 | −0.0557 |
| 89 | 5.8861 | 6.291 | −0.4053 | 6.112 | −0.2261 |
| 90 | 6.0915 | 6.111 | −0.0196 | 6.131 | −0.0396 |
| 92 | 6.3566 | 6.171 | 0.186 | 6.281 | 0.0751 |
| 93 | 6.2602 | 6.274 | −0.0138 | 6.251 | −0.0092 |
| 94 | 6.0706 | 6.208 | −0.1374 | 6.099 | −0.0288 |
| 95 | 6.0410 | 5.983 | 0.0577 | 5.981 | 0.0595 |
| 96 | 6.0000 | 5.468 | 0.5325 | 5.981 | 0.0187 |
| 97 | 6.0458 | 6.086 | −0.0406 | 6.038 | 0.0077 |
| 98 | 5.6990 | 5.934 | −0.2348 | 5.638 | 0.0608 |
| 99 | 6.3468 | 6.242 | 0.1046 | 6.366 | −0.0187 |
| 100 | 6.4559 | 6.074 | 0.3816 | 6.496 | −0.0397 |
| 105 | 4.5528 | 4.125 | 0.4278 | 4.538 | 0.015 |
| 108 | 4.8239 | 4.736 | 0.0878 | 4.826 | −0.0023 |
| 109 | 4.4949 | 4.403 | 0.0923 | 4.379 | 0.1156 |
| 110 | 4.6198 | 4.870 | −0.2505 | 4.523 | 0.0971 |
| 111 | 4.1487 | 3.873 | 0.2756 | 4.082 | 0.0663 |
| 112 | 4.2291 | 4.703 | −0.4742 | 4.182 | 0.0468 |
| 113 | 3.9872 | 4.031 | −0.0437 | 3.984 | 0.0036 |
| 117 | 4.7212 | 4.481 | 0.2403 | 4.758 | −0.0366 |
| 119 | 4.7959 | 4.747 | 0.0485 | 4.809 | −0.0131 |
| 121 | 4.7959 | 4.881 | −0.0849 | 4.889 | −0.0936 |
| 122 | 4.8239 | 4.695 | 0.1286 | 4.871 | −0.0473 |
| 123 | 4.7959 | 4.888 | −0.0918 | 4.820 | −0.0243 |
| 124 | 4.7695 | 5.019 | −0.2491 | 4.821 | −0.0511 |
| 125 | 4.7959 | 5.032 | −0.2358 | 4.751 | 0.0454 |
| 129 | 6.3979 | 6.202 | 0.1955 | 6.281 | 0.1174 |
| 130 | 6.0458 | 6.086 | −0.0406 | 6.038 | 0.0077 |
| 131 | 6.0000 | 5.468 | 0.5325 | 5.981 | 0.0187 |
| 132 | 6.5850 | 6.559 | 0.0255 | 6.608 | −0.0229 |
| Test Set Compounds | pIC50 | CoMFA | CoMSIA | ||
| Predicted | Residuals | Predicted | Residuals | ||
| 2 * | 5.3010 | 5.505 | −0.2035 | 5.296 | 0.0055 |
| 16 * | 5.8861 | 5.526 | 0.3598 | 5.871 | 0.0147 |
| 21 * | 5.4948 | 5.751 | −0.2562 | 5.648 | −0.1528 |
| 27 * | 5.7213 | 5.631 | 0.0898 | 5.618 | 0.1034 |
| 30 * | 5.585 | 5.522 | 0.0629 | 5.554 | 0.0312 |
| 35 * | 5.6383 | 6.035 | −0.397 | 5.728 | −0.0898 |
| 41 * | 5.6778 | 5.696 | −0.0185 | 5.738 | −0.0601 |
| 51 * | 5.6990 | 5.581 | 0.1182 | 5.596 | 0.103 |
| 53 * | 4.7447 | 4.732 | 0.0132 | 4.745 | −0.0001 |
| 58 * | 5.5229 | 5.515 | 0.0076 | 5.364 | 0.1585 |
| 74 * | 6.1024 | 6.085 | 0.0172 | 6.059 | 0.043 |
| 75 * | 5.9208 | 5.971 | −0.0502 | 5.797 | 0.124 |
| 78 * | 6.3372 | 6.220 | 0.1176 | 6.272 | 0.0654 |
| 82 * | 6.4559 | 6.312 | 0.1437 | 6.417 | 0.0393 |
| 84 * | 6.4948 | 6.451 | 0.0443 | 6.432 | 0.0631 |
| 87 * | 6.6021 | 6.472 | 0.1298 | 6.523 | 0.079 |
| 88 * | 6.0000 | 6.316 | −0.3157 | 6.074 | −0.0739 |
| 91 * | 6.2076 | 6.337 | −0.1291 | 6.360 | −0.1519 |
| 101 * | 3.7235 | 3.525 | 0.1989 | 3.684 | 0.0392 |
| 102 * | 3.5114 | 3.734 | −0.2221 | 3.580 | −0.0684 |
| 103 * | 3.3363 | 3.648 | −0.3118 | 3.523 | −0.1868 |
| 104 * | 3.9586 | 3.937 | 0.0218 | 3.940 | 0.0186 |
| 106 * | 4.0555 | 3.886 | 0.1694 | 4.104 | −0.048 |
| 107 * | 3.4214 | 3.776 | −0.3549 | 3.506 | −0.0851 |
| 114 * | 4.3565 | 4.110 | 0.2468 | 4.286 | 0.0708 |
| 115 * | 3.8962 | 3.795 | 0.1014 | 3.799 | 0.0972 |
| 116 * | 3.9957 | 4.494 | −0.4982 | 4.254 | −0.2585 |
| 118 * | 4.6576 | 4.579 | 0.0789 | 4.488 | 0.1701 |
| 120 * | 4.9208 | 4.915 | 0.0063 | 4.831 | 0.0897 |
| 126 * | 4.7959 | 4.816 | −0.0204 | 4.685 | 0.1113 |
| 127 * | 3.4089 | 3.904 | −0.4953 | 3.483 | −0.0741 |
| 128 * | 4.8239 | 4.873 | −0.0489 | 4.758 | 0.0663 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Mol. | R1 | R2 | R3 | R4 | R6 | IC50 (nM) | pIC50 |
| 1 |  | H | H |  | H | 44 | 4.357 |
| 2 * |  | H | H | 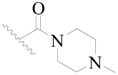 | H | 5.0 | 5.301 |
| 3 | 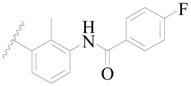 | H | H |  | H | 2.1 | 5.678 |
| 4 | 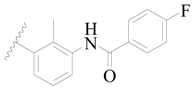 | H | H | 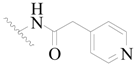 | H | 15 | 5.824 |
| 5 | 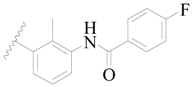 | H | H |  | H | 2.2 | 5.658 |
| 6 |  | H | H | 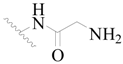 | H | 1.8 | 5.745 |
| 7 | 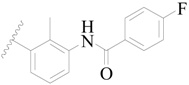 | H | H | 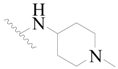 | H | 2.2 | 5.658 |
| 8 |  | H | H |  | H | 2.2 | 5.658 |
| 9 |  | H | H | 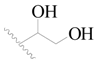 | H | 2.2 | 5.658 |
| 10 | 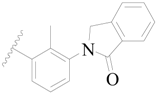 | H | H | 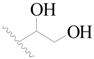 | H | 2.3 | 5.638 |
| 11 | 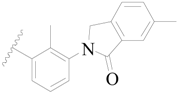 | H | H | 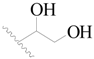 | H | 1.6 | 5.796 |
| 12 | 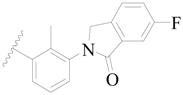 | H | H | 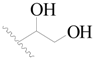 | H | 0.8 | 6.097 |
| 13 |  | H | H | 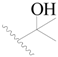 | H | 2.0 | 5.699 |
| 14 | 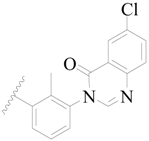 | H | H |  | H | 1.0 | 6.000 |
| 15 | 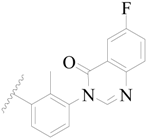 | H | H |  | H | 0.7 | 6.155 |
| 16 * | 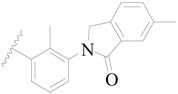 | H | H | 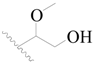 | H | 1.3 | 5.886 |
| 17 | 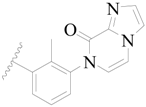 | H | H | 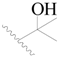 | H | 1.3 | 5.886 |
| 18 | 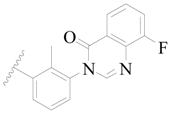 | H | H |  | H | 2.0 | 5.699 |
| 19 |  | H | H | 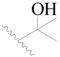 | H | 1.8 | 5.745 |
| 20 | 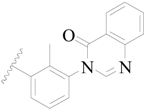 | H | H | 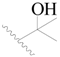 | H | 2.9 | 5.538 |
| 21 * | 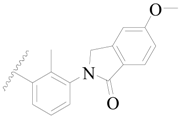 | H | H | 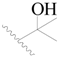 | H | 3.2 | 5.495 |
| 22 | 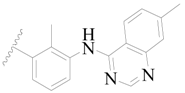 | H | H |  | H | 1.4 | 5.854 |
| 23 | 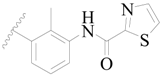 | H | H | 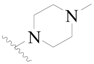 | H | 1.9 | 5.721 |
| 24 | 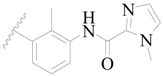 | H | H | 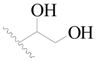 | H | 1.6 | 5.796 |
| 25 | 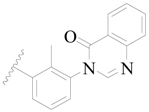 | H | H | CH2OH | H | 3.0 | 5.523 |
| 26 | 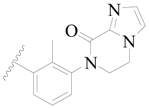 | H | H | 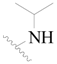 | H | 1.9 | 5.721 |
| 27 * |  | H | H | 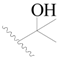 | H | 1.9 | 5.721 |
| 28 | 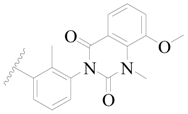 | H | H | 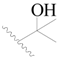 | H | 0.81 | 6.092 |
| 29 | 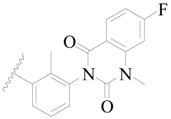 | H | H |  | H | 0.62 | 6.208 |
| 30 * |  | H | H | 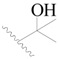 | H | 2.6 | 5.585 |
| 31 | 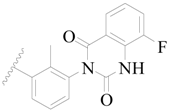 | H | H | 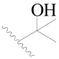 | H | 2.8 | 5.553 |
| 32 | 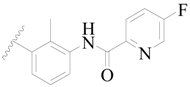 | H | H | 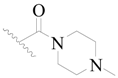 | H | 9.0 | 5.046 |
| 33 | 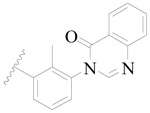 | H | H | 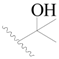 | H | 2.6 | 5.585 |
| 34 | 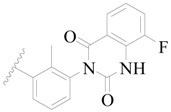 | H | H | 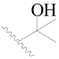 | H | 1.5 | 5.824 |
| 35 * | 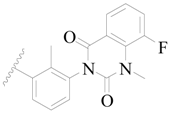 | H | H | 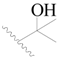 | H | 2.3 | 5.638 |
| 36 | 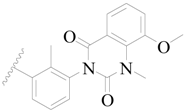 | H | H |  | H | 1.7 | 5.770 |
| 37 |  | H | H | 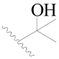 | H | 0.63 | 6.201 |
| 38 |  | H | H |  | H | 0.52 | 6.284 |
| 39 |  | H | H | 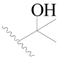 | H | 0.94 | 6.027 |
| 40 | 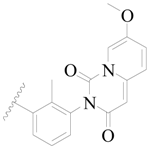 | H | H | 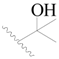 | H | 1.0 | 6.000 |
| 41 * | 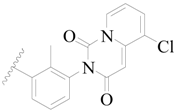 | H | H |  | H | 2.1 | 5.678 |
| 42 | 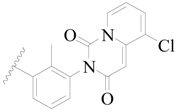 | H | H |  | H | 1.7 | 5.770 |
| 43 | 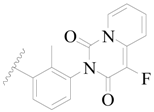 | H | H | 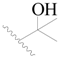 | H | 1.6 | 5.796 |
| 44 | 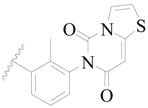 | H | H |  | H | 1.6 | 5.796 |
| 45 |  | H | H | 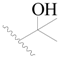 | H | 0.93 | 6.032 |
| 46 | 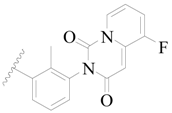 | H | H |  | H | 2.3 | 5.638 |
| 47 | 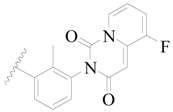 | H | H |  | H | 1.5 | 5.824 |
| 48 | 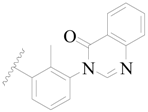 | H | H | 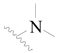 | H | 2.6 | 5.585 |
| 49 | 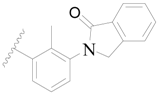 | H | H | 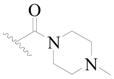 | H | 5.0 | 5.301 |
| 50 | 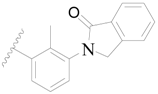 | H | H | 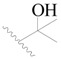 | H | 5.0 | 5.301 |
| 51 * | 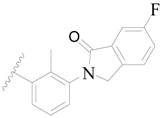 | H | H | 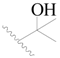 | H | 2.0 | 5.699 |
| 52 | 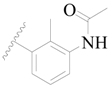 | H | H | 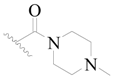 | H | 16 | 4.796 |
| 53 * |  | H | H | 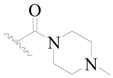 | H | 18 | 4.745 |
| 54 | 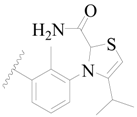 | H | H | 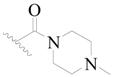 | H | 18 | 4.745 |
| 55 | 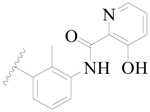 | H | H |  | H | 17 | 4.770 |
| 56 | 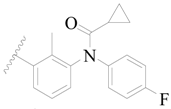 | H | H | 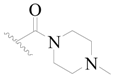 | H | 15 | 4.824 |
| 57 | 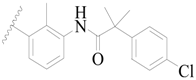 | H | H |  | H | 16 | 4.796 |
| 58 * | 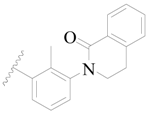 | H | H |  | H | 3.0 | 5.523 |
| 59 | 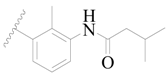 | H | H |  | H | 17 | 4.770 |
| 60 |  | H | H |  | H | 2.0 | 5.699 |
| 61 | 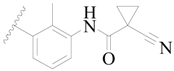 | H | H | 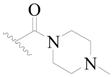 | H | 18 | 5.699 |
| 62 | 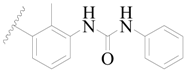 | H | H | 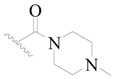 | H | 16 | 4.796 |
| 63 |  | H | H | 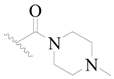 | H | 17 | 4.770 |
| 64 | 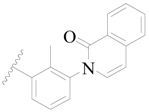 | H | H | 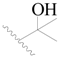 | H | 5.0 | 5.301 |
| 65 | 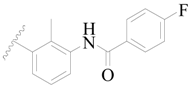 | H | H | 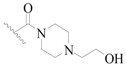 | H | 15 | 4.824 |
| 66 | 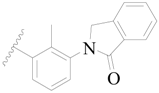 | H | H | 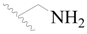 | H | 1.9 | 5.721 |
| 67 | 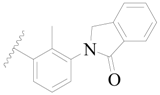 | H | H | 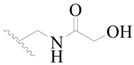 | H | 2.0 | 5.699 |
| 68 |  | H | H | 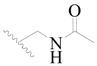 | H | 2.1 | 5.678 |
| 69 | 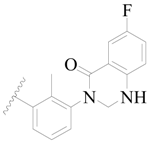 | H | H | 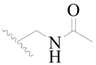 | H | 1.4 | 5.854 |
| 70 | 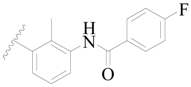 | H | H |  | H | 1.7 | 5.770 |
| 71 |  | H | H | OH | H | 1.6 | 5.796 |
| 72 | 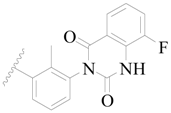 | H | H | 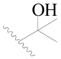 | CH3 | 0.66 | 6.180 |
| 73 | 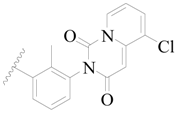 | H | H | 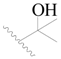 | CH3 | 0.41 | 6.387 |
| 74 * | 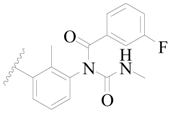 | CH3 | H | 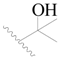 | H | 0.79 | 6.102 |
| 75 * | 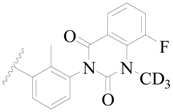 | CH3 | H | 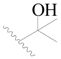 | H | 1.2 | 5.921 |
| 76 | 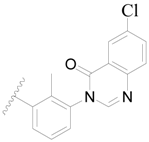 | H | H | 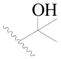 | H | 4.0 | 5.398 |
| 77 | 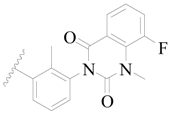 | H | H | 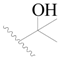 | F | 0.45 | 6.347 |
| 78 * | 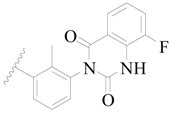 | H | H | 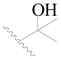 | F | 0.46 | 6.337 |
| 79 | 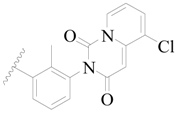 | H | H | 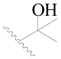 | F | 0.22 | 6.658 |
| 80 | 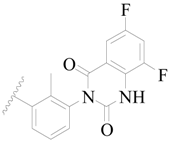 | H | H |  | F | 0.77 | 6.114 |
| 81 | 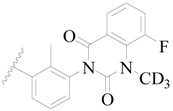 | H | H | 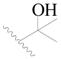 | F | 0.48 | 6.319 |
| 82 * | 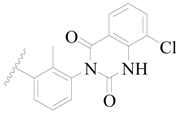 | H | H |  | F | 0.35 | 6.456 |
| 83 | 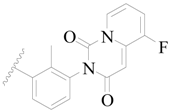 | H | H | 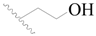 | F | 0.59 | 6.229 |
| 84 * | 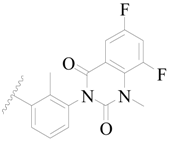 | H | H | 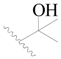 | F | 0.32 | 6.495 |
| 85 |  | H | H | 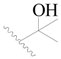 | CN | 1.0 | 6.000 |
| 86 | 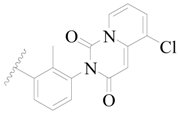 | H | H |  | CN | 0.49 | 6.310 |
| 87 * | 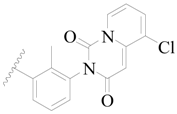 | H | H | 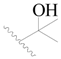 | Cl | 0.25 | 6.602 |
| 88 * |  | H | H | 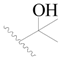 | Cl | 1.0 | 6.000 |
| 89 | 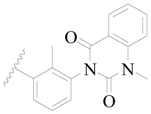 | H | H | 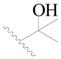 | Cl | 1.3 | 5.886 |
| 90 | 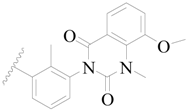 | H | H |  | Cl | 0.81 | 6.092 |
| 91 * | 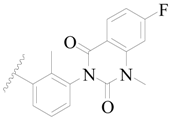 | H | H | 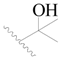 | Cl | 0.62 | 6.208 |
| 92 |  | H | H | 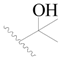 | Cl | 0.44 | 6.357 |
| 93 | 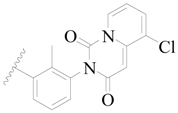 | H | H | 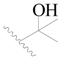 | Cl | 0.55 | 6.260 |
| 94 | 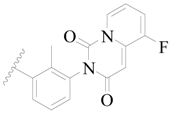 | H | H | 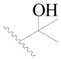 | Cl | 0.85 | 6.071 |
| 95 | 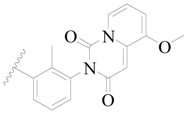 | H | H |  | Cl | 0.91 | 6.041 |
| 96 | 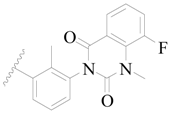 | H | H |  | Cl | 1.0 | 6.000 |
| 97 | 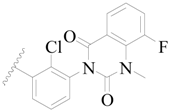 | H | H | 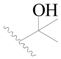 | H | 0.9 | 6.046 |
| 98 | 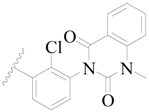 | H | H | 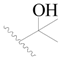 | Cl | 2.0 | 5.699 |
| 99 | 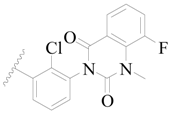 | H | H | 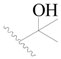 | Cl | 0.45 | 6.347 |
| 100 | 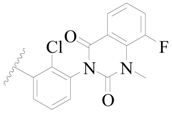 | H | H | 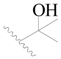 | F | 0.35 | 6.456 |
| 101 * | H | H | 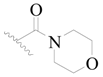 | H |  | 189 | 3.724 |
| 102 * | H | H | 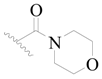 | H | 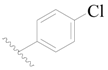 | 308 | 3.511 |
| 103 * | H | H |  | H | 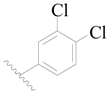 | 461 | 3.336 |
| 104 * | H | H |  | H | 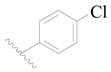 | 110 | 3.959 |
| 105 | H | H | 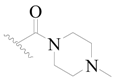 | H | 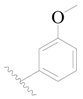 | 28 | 4.553 |
| 106 * | H | H |  | H | 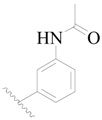 | 88 | 4.056 |
| 107 * | H | H | H | 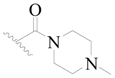 | 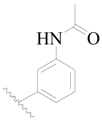 | 379 | 3.421 |
| 108 | H | H | H | 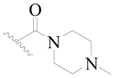 |  | 15 | 4.824 |
| 109 | H | H | H | 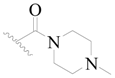 | 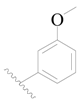 | 32 | 4.495 |
| 110 | H | H | H | 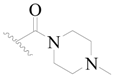 | 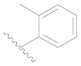 | 24 | 4.620 |
| 111 | H | H | H |  | 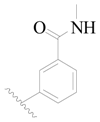 | 71 | 4.149 |
| 112 | H | H | H | 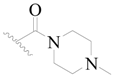 | 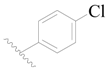 | 59 | 4.229 |
| 113 | H | H | H | 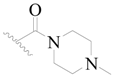 |  | 103 | 3.987 |
| 114 * | H | H | H | 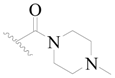 |  | 44 | 4.357 |
| 115 * | H | H | H | 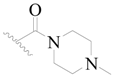 | 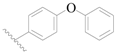 | 127 | 3.896 |
| 116 * | H | H | H | 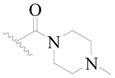 |  | 101 | 3.996 |
| 117 | H | H | H | 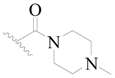 | 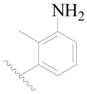 | 19 | 4.721 |
| 118 * | H | H | H | 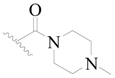 | 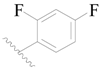 | 22 | 4.658 |
| 119 | H | H | H |  | 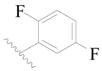 | 16 | 4.796 |
| 120 * | H | H | H | 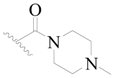 |  | 12 | 4.921 |
| 121 | 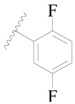 | H | H | 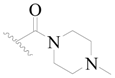 | H | 16 | 4.796 |
| 122 |  | H | H |  | H | 15 | 4.824 |
| 123 | 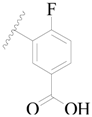 | H | H | 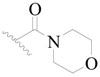 | H | 16 | 4.796 |
| 124 | 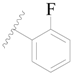 | H | H | 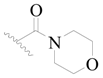 | H | 17 | 4.770 |
| 125 |  | H | H |  | H | 16 | 4.796 |
| 126 * | 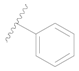 | H | H | 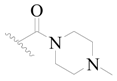 | H | 16 | 4.796 |
| 127 * | 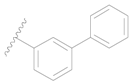 | H | H | 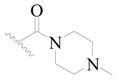 | H | 390 | 3.409 |
| 128 * | 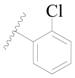 | H | H |  | H | 15 | 4.824 |
| 129 | 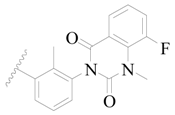 | H | H | 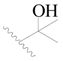 | H | 0.4 | 6.398 |
| 130 | 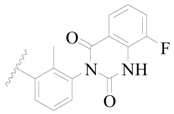 | H | H |  | Cl | 0.90 | 6.046 |
| 131 |  | H | H | 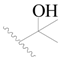 | Cl | 1.0 | 6.000 |
| 132 | 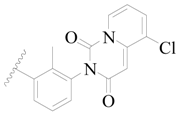 | H | H |  | F | 0.34 | 6.585 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Du, Y.; Gao, Z.; Shen, J. Molecular Modeling Studies on Carbazole Carboxamide Based BTK Inhibitors Using Docking and Structure-Based 3D-QSAR. Int. J. Mol. Sci. 2018, 19, 1244. https://doi.org/10.3390/ijms19041244
Li R, Du Y, Gao Z, Shen J. Molecular Modeling Studies on Carbazole Carboxamide Based BTK Inhibitors Using Docking and Structure-Based 3D-QSAR. International Journal of Molecular Sciences. 2018; 19(4):1244. https://doi.org/10.3390/ijms19041244
Chicago/Turabian StyleLi, Rui, Yongli Du, Zhipei Gao, and Jingkang Shen. 2018. "Molecular Modeling Studies on Carbazole Carboxamide Based BTK Inhibitors Using Docking and Structure-Based 3D-QSAR" International Journal of Molecular Sciences 19, no. 4: 1244. https://doi.org/10.3390/ijms19041244
APA StyleLi, R., Du, Y., Gao, Z., & Shen, J. (2018). Molecular Modeling Studies on Carbazole Carboxamide Based BTK Inhibitors Using Docking and Structure-Based 3D-QSAR. International Journal of Molecular Sciences, 19(4), 1244. https://doi.org/10.3390/ijms19041244




