Separate and Combined Response to UV-B Radiation and Jasmonic Acid on Photosynthesis and Growth Characteristics of Scutellaria baicalensis
Abstract
1. Introduction
2. Results
2.1. Light-Response Curves
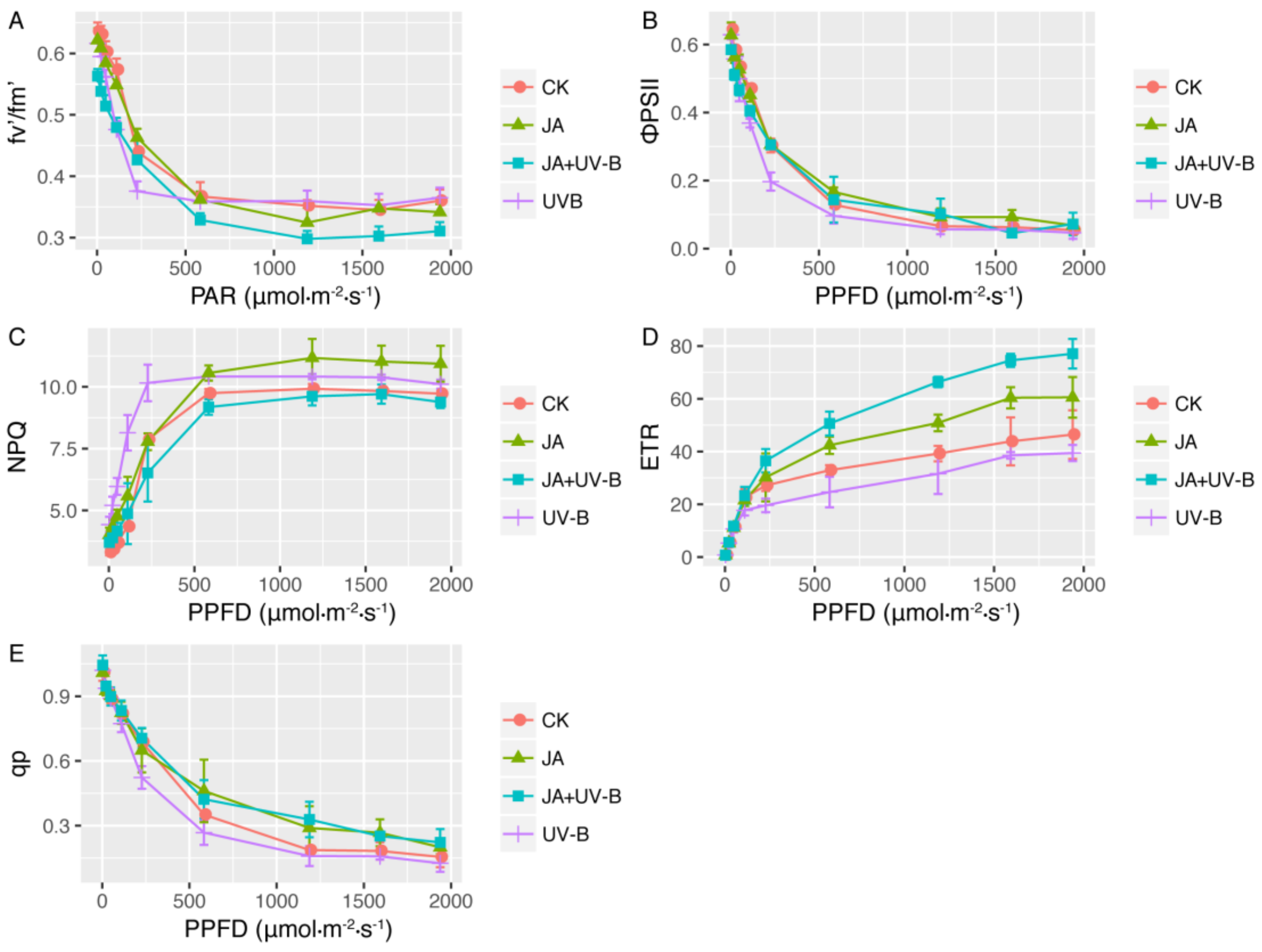
2.2. Chlorophyll Fluorescence
2.3. Chloroplast Pigment Content
2.4. Growth Parameters and Phenotyping Analysis of Root System Architecture
3. Discussion
3.1. The Effects of JA and UV-B on Photosynthesis
3.2. The Effects of JA and UV-B on Growth
4. Materials and Methods
4.1. Plant Material
4.2. JA Treatment
4.3. UV-B Treatment
4.4. Light-Response Curves of Photosynthesis
4.5. Chlorophyll Fluorescence Parameters
4.6. Measurement of Photosynthetic Pigments
4.7. Measurement of Growth Parameters
4.8. Phenotyping Analysis of Root System Architecture
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, W.T.; Fang, M.F.; Liu, X.; Yue, M. Simultaneous Quantitative and Qualitative Analysis of Flavonoids from Ultraviolet-B Radiation in Leaves and Roots of Scutellaria baicalensis Georgi Using LC-UV-ESI-Q/TOF/MS. J. Anal. Methods Chem. 2014, 2014, 643879. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Hou, J.Y.; Jiang, C.; Li, G.; Lu, H.; Meng, F.Y.; Shi, L.C. Deep sequencing of the Scutellaria baicalensis Georgi transcriptome reveals flavonoid biosynthetic profiling and organ-specific gene expression. PLoS ONE 2015, 10, e0136397. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.K.; Agrawal, S.B. Ultraviolet-B induced changes in morphological, physiological and biochemical parameters of two cultivars of pea (Pisum sativum L.). Ecotoxicol. Environ. Saf. 2014, 100, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Q.; Yue, M.; Zhang, X.F.; Zhang, R.C.; Zhang, B.; Wang, M. Nitric oxide is involved in integration of UV-B absorbing compounds among parts of clonal plants under a heterogeneous UV-B environment. Physiol. Plant. 2015, 155, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Jansen, M.A.K.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2012, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defense responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M. Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol. Rep. 2014, 8, 279–293. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Guo, J.L.; Zhu, A.J.; Zhang, L.; Zhang, M.M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Shah, K. Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 2014, 108, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Doganlar, Z.B. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Del Amor, F.M.; Cuadra-Crespo, P. Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: Analysis of growth, gas exchange, and isotope composition. Plant Growth Regul. 2011, 63, 55–62. [Google Scholar] [CrossRef]
- Hristova, V.A.; Popova, L.P. Treatment with methyl jasmonate alleviates the effects of paraquat on photosynthesis in barley plants. Photosynthetica 2002, 40, 567–574. [Google Scholar] [CrossRef]
- Popova, L.P.; Ananieva, E.; Hristova, V.; Christov, K.; Georgieva, K.; Alexieva, V.; Stoinova, Z.H. Salicylic acid-and methyl jasmonate-induced protection on photosynthesis to paraquat oxidative stress. Bulg. J. Plant Physiol. 2003, 133, 152. [Google Scholar]
- Yoon, J.Y.; Hamayun, M.; Lee, S.K.; Lee, I.J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Ervin, E.H. Effects of methyl jasmonate and salicylic acid on UV-B tolerance associated with free radical scavenging capacity in Poa pratensis. Int. Turfgrass Soc. Res. J. 2005, 10, 910–915. [Google Scholar]
- Fedina, I.; Nedeva, D.; Genrgieva, K.; Velitchkova, M. Methyl jasmonate counteract UV-B stress in barley seedlings. J. Agron. Sci. 2009, 195, 204–212. [Google Scholar] [CrossRef]
- Liu, X.; Chi, H.; Yue, M.; Zhang, X.F.; Li, W.J.; Jia, E.P. The regulation of exogenous jasmonic acid on UV-B stress tolerance in wheat. J. Plant Growth Regul. 2012, 31, 436–447. [Google Scholar] [CrossRef]
- Tang, W.T.; Liu, X.; Fang, M.F.; Yue, M. FTIR Analysis of the Effects of Enhanced Ultraviolet-B (UV-B) Radiation on Chemical Composition of Different Parts of Scutellaria baicalensis Georgi. Spectrosc. Spectr. Anal. 2011, 31, 1220–1224, (In Chinese with English abstract). [Google Scholar]
- Surabhi, G.K.; Reddy, K.R.; Singh, S.K. Photosynthesis, fluorescence, shoot biomass and seed weight responses of three cowpea (Vigna unguiculata (L.) Walp.) cultivars with contrasting sensitivity to UV-B radiation. Environ. Exp. Bot. 2009, 66, 160–171. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Rodrigues, G.C.; Jansen, M.A.K.; van den Noort, M.E.; van Rensen, J.J. Evidence for the semireduced primary quinone electron acceptor of photosystem II being a photosensitizer for UV-B damage to the photosynthetic apparatus. Plant Sci. 2006, 170, 283–290. [Google Scholar] [CrossRef]
- Shi, S.B.; Zhu, W.Y.; Li, H.M.; Zhou, D.W.; Han, F.; Zhao, X.Q.; Tang, Y.H. Photosynthesis of Saussurea superba and Gentiana straminea is not reduced after long-term enhancement of UV-B radiation. Environ. Exp. Bot. 2004, 51, 75–83. [Google Scholar] [CrossRef]
- Xu, C.; Sullivan, J.H. Reviewing the technical designs for experiments with ultraviolet-B radiation and impact on photosynthesis, DNA and secondary metabolism. J. Integr. Plant Biol. 2010, 52, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Li, W.; Yuan, Z.Y.; Cui, H.Y.; Lv, C.G.; Gao, Z.P.; Han, B.; Gong, Y.Z.; Chen, G.X. The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica 2013, 51, 33–44. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarfordoei, A.; Samson, R.; Damme, P.V. Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 2011, 49, 107–111. [Google Scholar] [CrossRef]
- Láposi, R.; Veres, S.; Mile, O.; Mészáros, I. Effects of supplemental UV-B radiation on the photosynthesis physiological properties and flavonoid content of beech seedlings (Fagus sylvatica L.) in outdoor conditions. Acta Biol. Szeged. 2005, 49, 151–153. [Google Scholar]
- Szôllôsi, E.; Veres, S.; Kanalas, P.; Oláh, V.; Solti, Á.; Sárvári, É.; Mészáros, I. Effects of UV-B radiation and water stress on chlorophyll fluorescence parameters and activity of xanthophyll cycle in leaves of sessile oak (Quercus petraea) seedlings. Acta Biol. Szeged. 2008, 52, 241–242. [Google Scholar]
- Bukhov, N.G.; Wiese, U.H.; Shuvalov, V.A. Energy dissipation in photosynthesis: Does the quenching of chlorophyll fluorescence originate from antenna complexes of photosystem II or form the reaction center? Planta 2001, 212, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.A.; Adams, W.W.; Adams, B.D. Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Funct. Plant Biol. 2007, 34, 853–859. [Google Scholar] [CrossRef]
- Weidhase, R.; Lehmann, J.; Kramell, H.M.; Sembdner, G.; Parthier, B. Degradation of ribulose-1,5-bisphosphate carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methylester, and counteraction by cytokinins. Physiol. Plant. 1987, 69, 161–166. [Google Scholar] [CrossRef]
- Rossato, L.; MacDuff, J.H.; Laine, P.; Le Deunff, E.; Ourry, A. Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: Effects of methyl jasmonate on nitrate uptake, senescence, growth, and VSP accumulation. J. Exp. Bot. 2002, 53, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Xie, X.Y.; Farooq, M.; Wang, L.C.; Xue, L.L.; Shahbaz, M.; Salhab, J. Effect of exogenous methyl jasmonate on growth, gas exchange and chlorophyll contents of soybean subjected to drought. Afr. J. Biotechnol. 2011, 10, 9640–9646. [Google Scholar]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [PubMed]
- Raghavendra, A.S.; Reddy, K.B. Action of proline on stomata differs from that of abscisic acid, G-Substances, or methyl jasmonate. Plant Physiol. 1987, 83, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhao, G.X.; Li, M.; Zhang, M.T.; Zhang, L.F.; Zhang, X.F.; An, L.Z.; Xu, S.J. C:N:P stoichiometry and leaf traits of halophytes in an arid saline environment, northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Paul, N.D.; Whittaker, J.B.; Taylor, J.E. Exogenous jasmonic acid mimics hervibore-induced systemic increase in cell wall bound peroxidase activity and reduction in leaf expansion. Funct. Ecol. 2003, 17, 549–554. [Google Scholar] [CrossRef]
- Heijari, J.; Nerg, A.M.; Kainulainen, P.; Viiri, H.; Vuorinen, M.; Holopainen, J.K. Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomol. Exp. Appl. 2005, 115, 117–124. [Google Scholar] [CrossRef]
- Noir, S.; Bömer, M.; Takahashi, N.; Ishida, T.; Tsui, T.L.; Balbi, V.; Shanahan, H.; Sugimoto, K.; Devoto, A. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-By mode. Plant Physiol. 2013, 161, 1930–1951. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A Review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Photosynthesis, Respiration, and Long-Distance Transport. In Plant Physiological Ecology; Lambers, H., Ed.; Springer: New York, NY, USA, 2006; pp. 10–153. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars (Olea europaea L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Academic Press Inc.: San Diego, CA, USA, 1987; pp. 350–382. [Google Scholar]

| Treatments | Rd [μmol−2·s−1] | Ф [μmolCO2·μmol−1photon] | Pmax [μmol−2·s−1] | θ | LCP [μmol−2·s−1] | LSP [μmol−2·s−1] | Fo | Fm | Fv/Fm | ETRmax |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 1.02 ± 0.09 c | 0.05 ± 0.003 b | 14.16 ± 0.12 a | 0.91 ± 0.04 a | 17.56 ± 0.89 b | 364.65 ± 23.29 c | 164.50 ± 18.54 ab | 790.19 ± 52.22 ab | 0.79 ± 0.01 a | 41.76 ± 4.87 c |
| UV-B | 1.66 ± 0.08 b | 0.08 ± 0.007 a | 9.28 ± 0.09 c | 0.74 ± 0.03 c | 16.93 ± 0.91 b | 425.34 ± 19.89 c | 203.22 ± 25.26 a | 953.93 ± 111.51 a | 0.79 ± 0.00 a | 37.86 ± 3.34 c |
| JA | 2.00 ± 0.11 a | 0.05 ± 0.000 b | 8.74 ± 0.90 c | 0.83 ± 0.03 b | 42.66 ± 1.21 a | 601.48 ± 25.28 b | 119.13 ±18.08 c | 551.60 ± 123.55 b | 0.78 ± 0.02 a | 58.22 ± 3.21 b |
| JA+UV-B | 0.89 ± 0.05 c | 0.02 ± 0.003 c | 10.76 ± 0.96 b | 0.82 ± 0.06 b | 44.79 ± 1.90 a | 1042.69 ± 59.68 a | 124.86 ± 28.09 b c | 475.76 ± 23.46 b | 0.78 ± 0.01 a | 75.12 ± 5.12 a |
| Photosynthesis Pigments | CK | JA | UV-B | JA+UV-B |
|---|---|---|---|---|
| Chl a [mg/g] | 10.06 ± 1.15 a | 6.56 ± 0.75 c | 8.19 ± 0.89 b | 8.98 ± 0.68 a,b |
| Chl b [mg/g] | 3.88 ± 0.24 a | 3.79 ± 0.34 a | 2.83 ± 0.22 b | 4.04 ± 0.16 a |
| Chl (a + b) [mg/g] | 13.72 ± 0.27 a | 11.99 ± 1.77 b | 11.41 ± 1.66 b | 12.03 ± 2.31 a |
| Car [mg/g] | 4.30 ± 0.49 a | 4.06 ± 0.56 a | 3.72 ± 0.22 a | 3.93 ± 0.17 a |
| Chl a/b [mg/g] | 2.69 ± 0.65 a | 2.21 ± 0.24 b | 2.72 ± 0.09 a | 2.26 ± 0.19 b |
| Characters | CK | JA | UV-B | JA+UV-B |
|---|---|---|---|---|
| Seedling height [cm] | 18.88 ± 1.88 a | 16.13 ± 2.36 b | 19.89 ± 2.78 a | 15.84 ± 2.12 b |
| Total biomass [g] | 0.18 ± 0.02 a | 0.12 ± 0.02 b | 0.14 ± 0.03 b | 0.13 ± 0.02 b |
| Roots biomass [g] | 0.10 ± 0.03 a | 0.07 ± 0.007 a | 0.08 ± 0.007 a | 0.06 ± 0.006 b |
| Stem biomass [g] | 0.02 ± 0.005 a | 0.01 ± 0.002 b | 0.01 ± 0.003 b | 0.02 ± 0.003 b |
| Leaf biomass/each plant [g] | 0.06 ± 0.008 a | 0.03 ± 0.008 b | 0.03 ± 0.008 b | 0.04 ± 0.007 ab |
| Biomass/each leaf [g] | 0.005 ± 0.0006 b | 0.004 ± 0.0009 b | 0.006 ± 0.0008 a | 0.005 ± 0.0006 b |
| Dry mass of above-ground [g] | 0.08 ± 0.01 a | 0.05 ± 0.001 b | 0.036 ± 0.004 b | 0.05 ± 0.006 b |
| Leaf area [cm2] | 1. 30 ± 0.15 b | 1.19 ± 0.20 b | 1.59 ± 0.18 a | 1.25 ± 0.19 b |
| Specific leaf area (SLA) [cm2/g] | 236.16 ± 21.26 a | 225.75 ± 38.62 a | 205.55 ± 6.95 b | 234.83 ± 20.97 a |
| Total root length [cm] | 318.51 ± 30.51 ab | 241.93 ± 85.38 ab | 326.29 ± 37.75 a | 185.14 ± 20.47 b |
| Root surface area [cm2] | 27.22 ± 8.48 b | 25.83 ± 9.56 b | 46.72 ± 2.74 a | 22.46 ± 3.89 b |
| Average root diameter [mm] | 0.37 ± 0.05 b | 0.34 ± 0.02 b | 0.47 ± 0.02 a | 0.37 ± 0.02 b |
| Treatments | Interpretation |
|---|---|
| CK | Control |
| UV-B | Seedlings were treated with 15 days of UV-B radiation |
| JA | Seedlings were sprayed with 1 mM JA |
| JA+UV-B | Seedlings were sprayed with 1 mM JA before additional 15 days of UV-B |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, J.; Song, S.; Abdulrashid, K.; Chai, Y.; Yue, M.; Liu, X. Separate and Combined Response to UV-B Radiation and Jasmonic Acid on Photosynthesis and Growth Characteristics of Scutellaria baicalensis. Int. J. Mol. Sci. 2018, 19, 1194. https://doi.org/10.3390/ijms19041194
Quan J, Song S, Abdulrashid K, Chai Y, Yue M, Liu X. Separate and Combined Response to UV-B Radiation and Jasmonic Acid on Photosynthesis and Growth Characteristics of Scutellaria baicalensis. International Journal of Molecular Sciences. 2018; 19(4):1194. https://doi.org/10.3390/ijms19041194
Chicago/Turabian StyleQuan, Jiaxin, Shanshan Song, Kadir Abdulrashid, Yongfu Chai, Ming Yue, and Xiao Liu. 2018. "Separate and Combined Response to UV-B Radiation and Jasmonic Acid on Photosynthesis and Growth Characteristics of Scutellaria baicalensis" International Journal of Molecular Sciences 19, no. 4: 1194. https://doi.org/10.3390/ijms19041194
APA StyleQuan, J., Song, S., Abdulrashid, K., Chai, Y., Yue, M., & Liu, X. (2018). Separate and Combined Response to UV-B Radiation and Jasmonic Acid on Photosynthesis and Growth Characteristics of Scutellaria baicalensis. International Journal of Molecular Sciences, 19(4), 1194. https://doi.org/10.3390/ijms19041194




