Molecular Structural Changes in Alfalfa Detected by ATR-FTIR Spectroscopy in Response to Silencing of TT8 and HB12 Genes
Abstract
1. Introduction
2. Results and Discussion
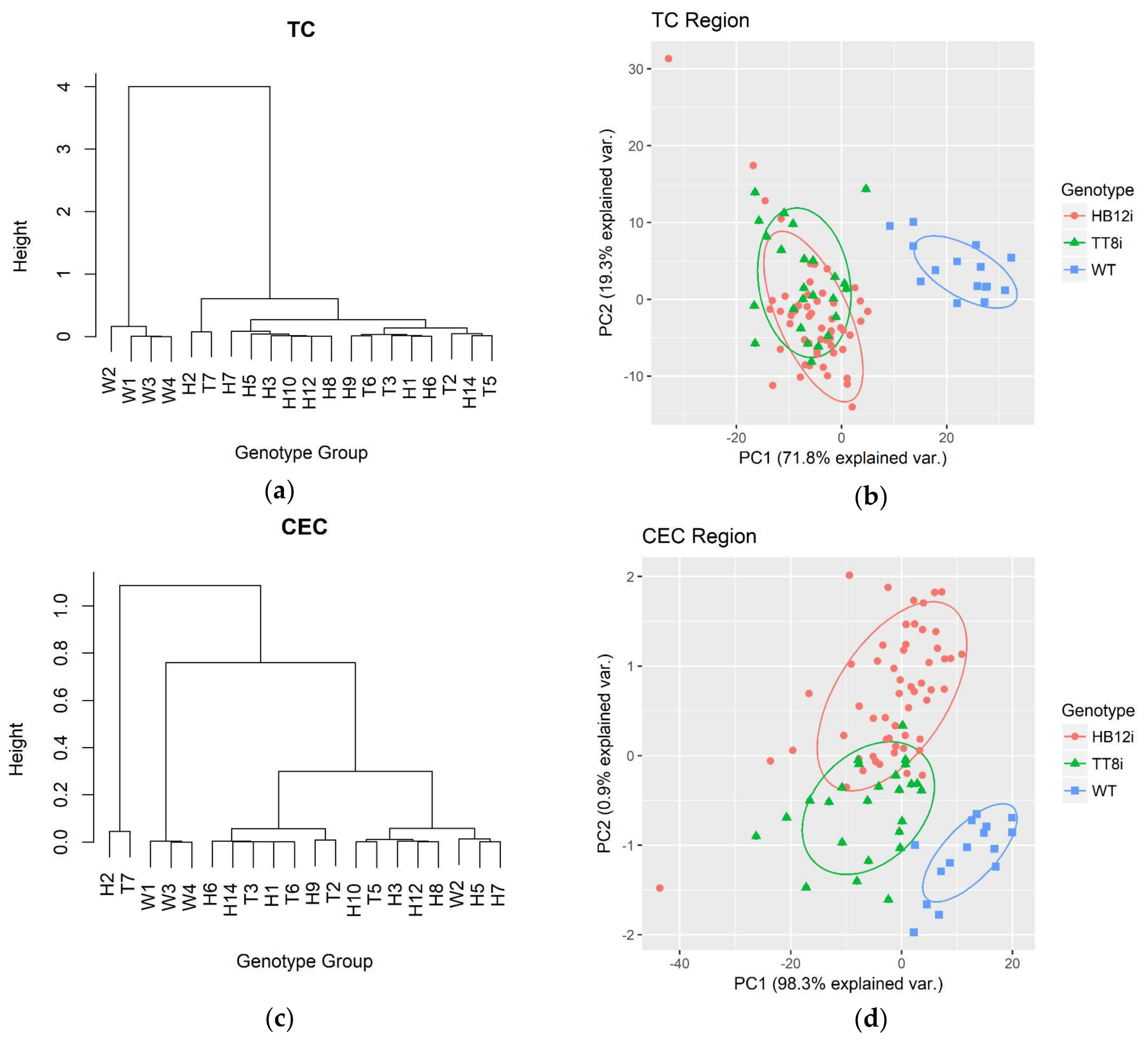
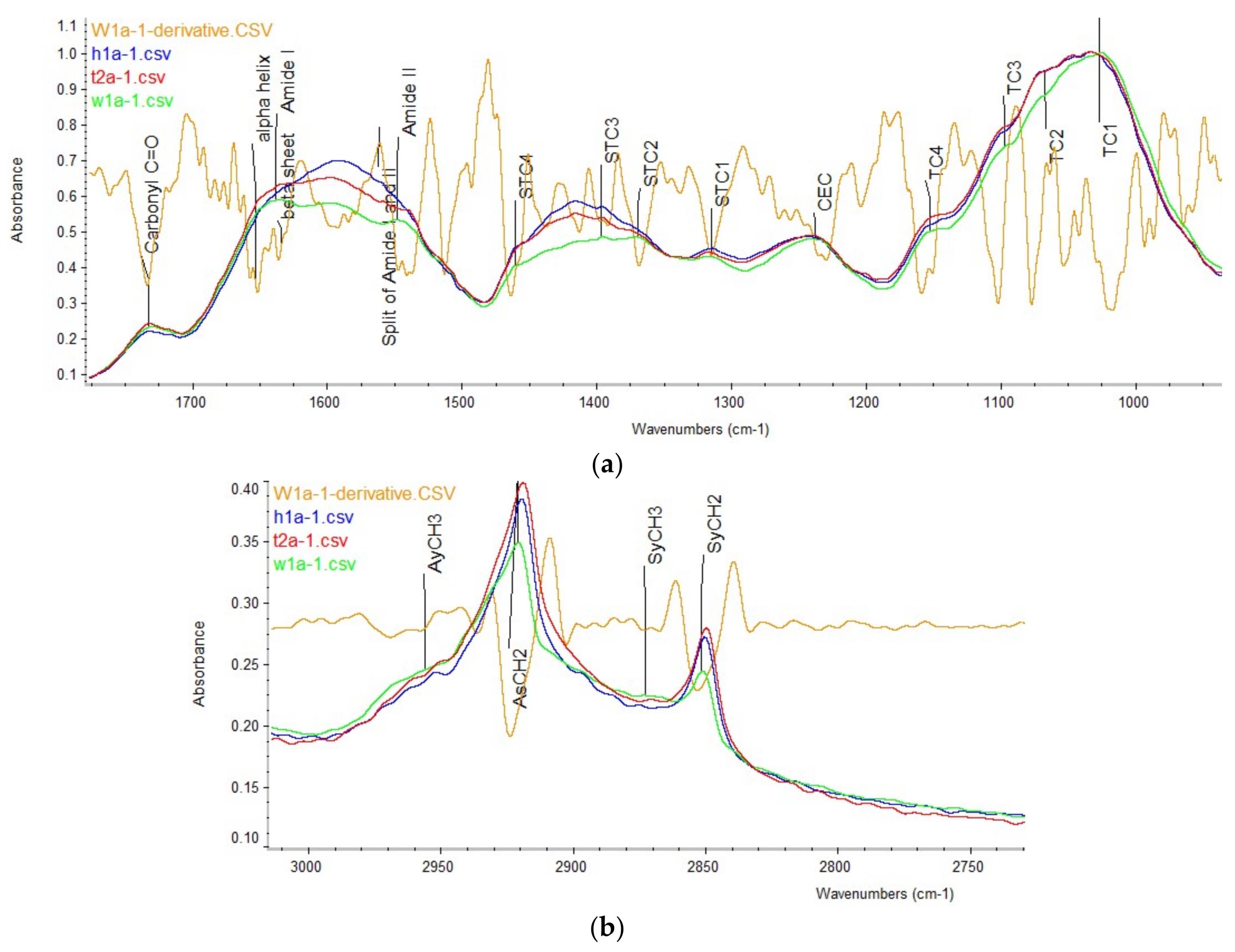
2.1. Carbohydrate Structure-Related Spectral Profiles
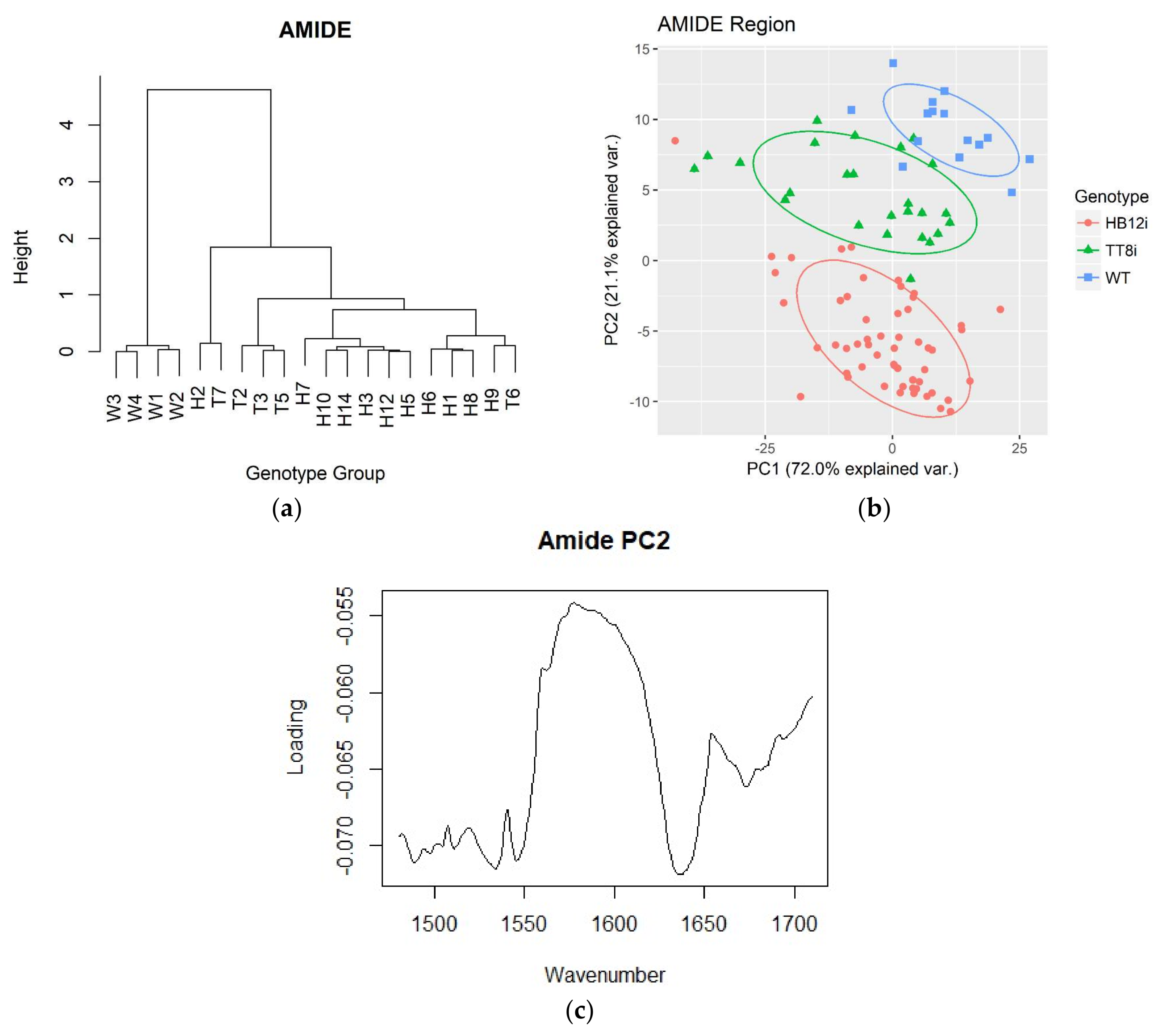
2.2. Amide and Secondary Structure Related Spectral Profiles
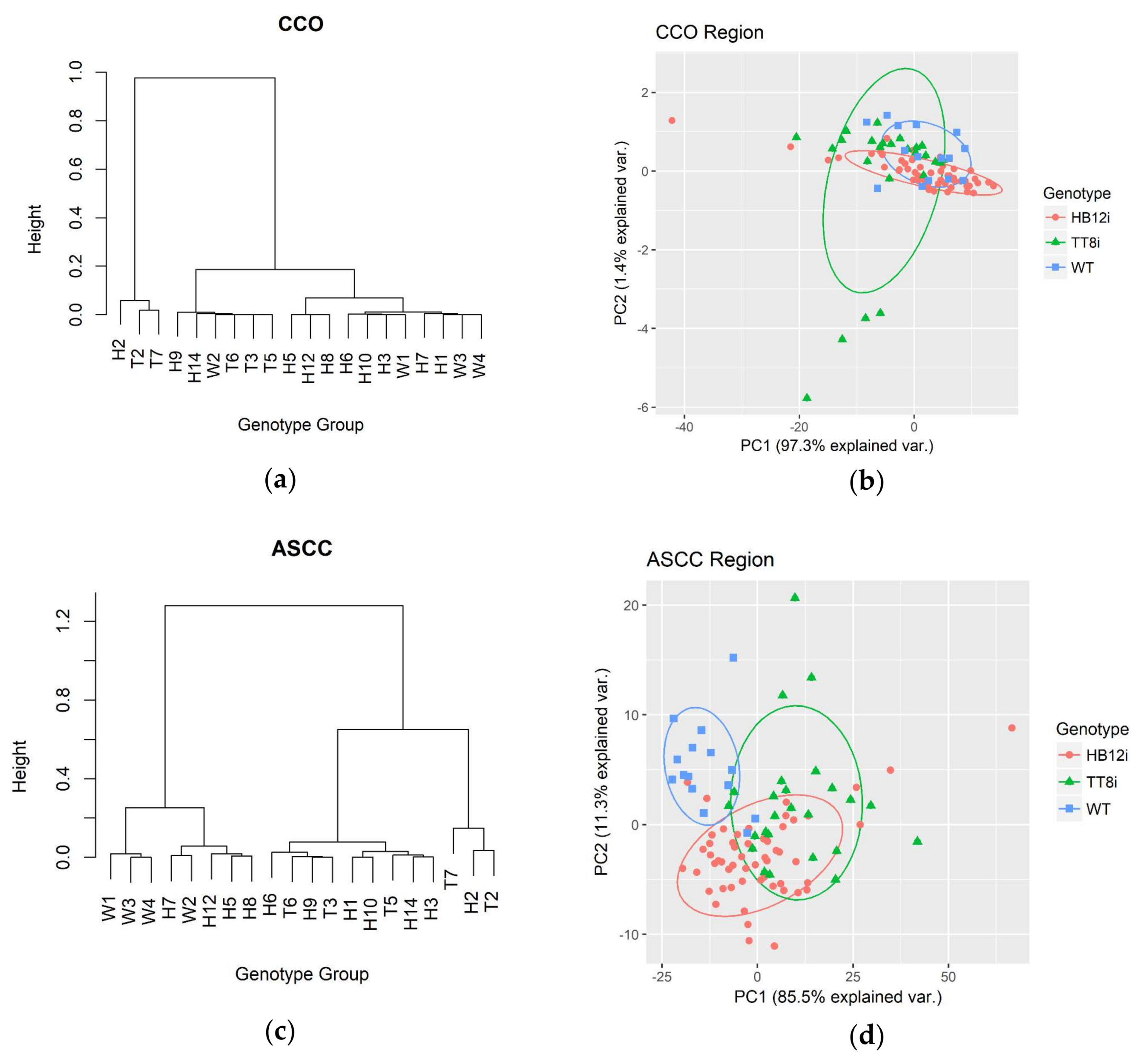
2.3. Lipid-Related Structure Spectral Profiles
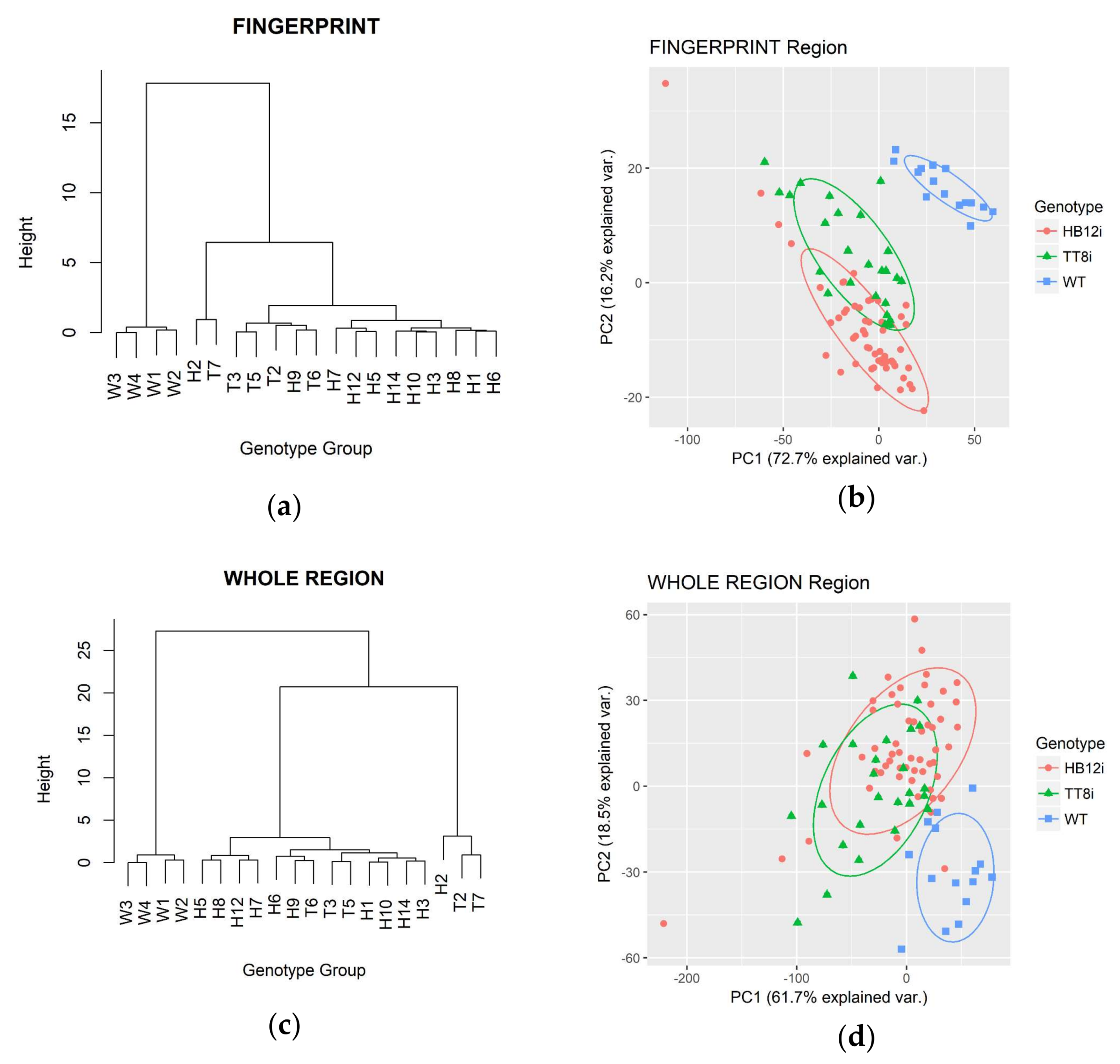
2.4. Multivariate Analysis in Fingerprint and Whole Region
3. Materials and Methods
3.1. RNAi Transformation, Growth Condition and Sampling
3.2. ATR-FTIR Spectroscopy
3.3. Univariate Analysis
3.4. Multivariate Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TT8i | TT8 silenced alfalfa |
| HB12i | HB12 silenced alfalfa |
| WT | Wild type control alfalfa |
| TT8i | Transparent Testa 8-silenced alfalfa |
| HB12i | Homeobox 12-silenced alfalfa |
| TC | Total carbohydrate |
| TCA | TC peak area |
| STC | Structural carbohydrate |
| STCA | STC peak area |
| CP | Crude protein |
| SEM | Standard error of mean |
| CEC | Cellulosic compounds |
| CECA | CEC peak area |
| CHO | Total carbohydrate |
| CCO | Carbonyl C=O |
| CCOA | CCO peak area |
| AA | Whole amide area |
| AIA | Amide I peak area |
| AIIA | Amide II peak area |
| SyCH2 | Symmetric CH2 |
| AsCH2 | Asymmetric CH2 |
| SyCH3 | Symmetric CH3 |
| AsCH3 | Asymmetric CH3 |
| ASCC | Asymmetric and symmetric CH2 and CH3 |
| ASCCA | ASCC peak area |
| HCA | Hierarchical cluster analysis |
| PCA | Principle component analysis |
References
- Lei, Y.; Hannoufa, A.; Yu, P. The Use of Gene Modification and Advanced Molecular Structure Analyses towards Improving Alfalfa Forage. Int. J. Mol. Sci. 2017, 18, 298. [Google Scholar] [CrossRef] [PubMed]
- Pumure, I.; Ford, S.; Shannon, J.; Kohen, C.; Mulcahy, A.; Frank, K.; Sisco, S.; Chaukura, N. Analysis of ATR-FTIR Absorption-Reflection Data from 13 Polymeric Fabric Materials Using Chemometrics. Am. J. Anal. Chem. 2015, 6, 305–312. [Google Scholar] [CrossRef]
- Prates, L.L.; Lei, Y.; Refat, B.; Zhang, W.; Yu, P. Effects of heat processing methods on protein subfractions and protein degradation kinetics in dairy cattle in relation to protein molecular structure of barley grain using advanced molecular spectroscopy. J. Cereal Sci. 2018, in press. [Google Scholar] [CrossRef]
- Mueller, D.; Ferrão, M.F.; Marder, L.; da Costa, A.B.; de Cássia de Souza Schneider, R. Fourier Transform Infrared Spectroscopy (FTIR) and Multivariate Analysis for Identification of Different Vegetable Oils Used in Biodiesel Production. Sensors 2013, 13, 4258–4271. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Y.; Sun, B.; Cai, C.; Ma, R.; Jin, Z. Measurement and characterization of external oil in the fried waxy maize starch granules using ATR-FTIR and XRD. Food Chem. 2018, 242, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Mukherjee, R.; Routray, A.; Ghosh, A.K.; Roy, S.; Ghosh, B.P.; Mandal, P.B.; Bose, S.; Chakraborty, C. Serum-based diagnostic prediction of oral submucous fibrosis using FTIR spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Fahey, L.M.; Nieuwoudt, M.K.; Harris, P.J. Predicting the cell-wall compositions of Pinus radiata (radiata pine) wood using ATR and transmission FTIR spectroscopies. Cellulose 2017, 24, 5275–5293. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-01113-3. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Heendeniya, R.G.; Yu, P. Gene-Transformation-Induced Changes in Chemical Functional Group Features and Molecular Structure Conformation in Alfalfa Plants Co-Expressing Lc-bHLH and C1-MYB Transcriptive Flavanoid Regulatory Genes: Effects of Single-Gene and Two-Gene Insertion. Int. J. Mol. Sci. 2017, 18, 664. [Google Scholar] [CrossRef] [PubMed]
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- McCaslin, M.; Weakley, D.; Temple, S.; Reisen, P. New technologies in alfalfa. Adv. Dairy Technol. West. Can. Dairy Semin. 2015, 27, 215–222. [Google Scholar]
- Pu, Y.; Chen, F.; Ziebell, A.; Davison, B.H.; Ragauskas, A.J. NMR Characterization of C3H and HCT Down-Regulated Alfalfa Lignin. BioEnergy Res. 2009, 2, 198. [Google Scholar] [CrossRef]
- Tong, Z.; Li, H.; Zhang, R.; Ma, L.; Dong, J.; Wang, T. Co-downregulation of the hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase and coumarate 3-hydroxylase significantly increases cellulose content in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2015, 239, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Hannoufa, A.; Yu, P. Transformation with TT8 and HB12 RNAi Constructs in Model Forage (Medicago sativa, Alfalfa) Affects Carbohydrate Structure and Metabolic Characteristics in Ruminant Livestock Systems. J. Agric. Food Chem. 2015, 63, 9590–9600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hannoufa, A.; Zhang, Y.; Yu, P. Gene-Silencing-Induced Changes in Carbohydrate Conformation in Relation to Bioenergy Value and Carbohydrate Subfractions in Modeled Plant (Medicago sativa) with Down-Regulation of HB12 and TT8 Transcription Factors. Int. J. Mol. Sci. 2016, 17, 720. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D.; Yu, P. Structural Makeup, Biopolymer Conformation, and Biodegradation Characteristics of a Newly Developed Super Genotype of Oats (CDC SO-I versus Conventional Varieties): A Novel Approach. J. Agric. Food Chem. 2010, 58, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; McKinnon, J.J.; Christensen, D.A.; Beattie, A.D.; Yu, P. Characterizing the molecular structure features of newly developed hulless barley cultivars with altered carbohydrate traits (Hordeum vulgare L.) by globar-sourced infrared spectroscopy in relation to nutrient utilization and availability. J. Cereal Sci. 2014, 60, 48–59. [Google Scholar] [CrossRef]
- Ban, Y.; Prates, L.; Yu, P. Investigating Molecular Structures of Bio-Fuel and Bio-Oil Seeds as Predictors to Estimate Protein Bioavailability for Ruminants by Advanced Nondestructive Vibrational Molecular Spectroscopy. J. Agric. Food Chem. 2017, 65, 9147–9157. [Google Scholar] [CrossRef] [PubMed]
- Refat, B.; Prates, L.L.; Khan, N.A.; Lei, Y.; Christensen, D.A.; McKinnon, J.J.; Yu, P. Physiochemical Characteristics and Molecular Structures for Digestible Carbohydrates of Silages. J. Agric. Food Chem. 2017, 65, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Ami, D.; Mereghetti, P.; Maria, S. Multivariate Analysis for Fourier Transform Infrared Spectra of Complex Biological Systems and Processes. In Multivariate Analysis in Management, Engineering and the Sciences; Freitas, L., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-0921-1. [Google Scholar]
- Yu, P.; Jonker, A.; Gruber, M. Molecular basis of protein structure in proanthocyanidin and anthocyanin-enhanced Lc-transgenic alfalfa in relation to nutritive value using synchrotron-radiation FTIR microspectroscopy: A novel approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yari, M.; Valizadeh, R.; Nnaserian, A.A.; Jonker, A.; Yu, P. Carbohydrate and lipid spectroscopic molecular structures of different alfalfa hay and their relationship with nutrient availability in ruminants. Asian-Australas. J. Anim. Sci. 2017, 30, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Yu, P. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: a new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Khan, N.A.; Zhang, F.; Yang, L.; Yu, P. Microwave Irradiation Induced Changes in Protein Molecular Structures of Barley Grains: Relationship to Changes in Protein Chemical Profile, Protein Subfractions, and Digestion in Dairy Cows. J. Agric. Food Chem. 2014, 62, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Relationship of feeds protein structural makeup in common Prairie feeds with protein solubility, in situ ruminal degradation and intestinal digestibility. Anim. Feed Sci. Technol. 2014, 194, 58–70. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Yu, P. Impact of ethanol bioprocessing on association of protein structures at a molecular level to protein nutrient utilization and availability of different co-products from cereal grains as energy feedstocks. Biomass Bioenergy 2014, 69, 47–57. [Google Scholar] [CrossRef]
- Abeysekara, S.; Damiran, D.; Yu, P. Univariate and multivariate molecular spectral analyses of lipid related molecular structural components in relation to nutrient profile in feed and food mixtures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Yu, P. Detect changes in lipid-related structure of brown-and yellow-seeded Brassica Carinata seed during rumen fermentation in relation to basic chemical profile using ATR-FT/IR molecular spectroscopy with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Khan, N.A.; Falk, K.C.; Yu, P. Mid-Infrared Spectral Characteristics of Lipid Molecular Structures in Brassica carinata Seeds: Relationship to Oil Content, Fatty Acid and Glucosinolate Profiles, Polyphenols, and Condensed Tannins. J. Agric. Food Chem. 2014, 62, 7977–7988. [Google Scholar] [CrossRef] [PubMed]
- Aung, B.; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Vu, V. ggbiplot: A Biplot Based on ggplot2. Available online: https://github.com/vqv/ggbiplot (accessed on 31 March 2018).


| Items | WT | Transgenic Alfalfa | SEM 1 | p | Contrast 2 W vs. G | |
|---|---|---|---|---|---|---|
| HB12i | TT8i | |||||
| Total carbohydrate related spectral profiles 3 | ||||||
| TC1 | 0.646a | 0.612b | 0.606b | 0.007 | 0.003 | 0.001 |
| TC2 | 0.498b | 0.55a | 0.533a | 0.0083 | 0.001 | 0.001 |
| TC3 | 0.338b | 0.381a | 0.373a | 0.0054 | <0.001 | <0.001 |
| TC4 | 0.159 | 0.152 | 0.149 | 0.0028 | 0.088 | 0.035 |
| TCA | 76.091 | 78.571 | 77.296 | 0.9286 | 0.162 | 0.157 |
| Cellulosic compounds related structural profiles 4 | ||||||
| CEC | 0.078 | 0.072 | 0.077 | 0.0029 | 0.175 | 0.33 |
| CECA | 2.786 | 2.504 | 2.712 | 0.1841 | 0.467 | 0.48 |
| Structural carbohydrate related structural profiles 5 | ||||||
| STC1 | 0.093b | 0.106a | 0.1ab | 0.0026 | 0.007 | 0.014 |
| STC2 | 0.151b | 0.162a | 0.152b | 0.0028 | 0.006 | 0.122 |
| STC3 | 0.164c | 0.23a | 0.187b | 0.0048 | <0.001 | <0.001 |
| STC4 | 0.131b | 0.168a | 0.143b | 0.0043 | <0.001 | <0.001 |
| STCA | 28.035c | 35.027a | 30.747b | 0.4989 | <0.001 | <0.001 |
| Items | WT | Transgenic Alfalfa | SEM 1 | P | Contrast 2 W vs. G | |
|---|---|---|---|---|---|---|
| HB12i | TT8i | |||||
| Amide heights and spectral ratio profiles | ||||||
| Amide I | 0.356 | 0.341 | 0.348 | 0.0098 | 0.508 | 0.379 |
| Amide II | 0.272 | 0.269 | 0.241 | 0.0085 | 0.042 | 0.15 |
| Amide I/Amide II | 1.311b | 1.258b | 1.436a | 0.0216 | <0.001 | 0.232 |
| Secondary structures heights and ratio profiles | ||||||
| α-helix | 0.346 | 0.333 | 0.333 | 0.0083 | 0.481 | 0.24 |
| β-sheet | 0.335b | 0.386a | 0.369a | 0.0071 | <0.001 | <0.001 |
| α-helix/β-sheet | 1.041a | 0.863c | 0.907b | 0.0106 | <0.001 | <0.001 |
| Amide area and ratio profiles | ||||||
| Amide area (AA) | 49.183b | 54.941a | 50.052b | 1.078 | 0.001 | 0.035 |
| Amide I area (AIA) | 33.168b | 37.906a | 35.545ab | 0.7236 | <0.001 | 0.002 |
| Amide II area (AIIA) | 16.016ab | 16.991a | 14.659b | 0.4144 | 0.001 | 0.735 |
| AIA/AIIA | 2.072c | 2.237b | 2.42a | 0.0351 | <0.001 | <0.001 |
| AIA/AA | 0.674c | 0.691b | 0.707a | 0.0032 | <0.001 | <0.001 |
| Items | WT | Transgenic Alfalfa | SEM 1 | P | Contrast 2 W vs. G | |
|---|---|---|---|---|---|---|
| HB12i | TT8i | |||||
| Carbonyl C=O ester height and area profiles 3 | ||||||
| CCO | 0.063 | 0.059 | 0.056 | 0.0024 | 0.232 | 0.109 |
| CCOA | 1.827 | 1.69 | 1.657 | 0.0915 | 0.471 | 0.228 |
| Symmetric and asymmetric of CH2 and CH3 profiles 4 | ||||||
| SyCH2 | 0.101b | 0.133a | 0.116b | 0.0051 | 0.001 | 0.004 |
| SyCH3 | 0.059 | 0.062 | 0.06 | 0.0014 | 0.189 | 0.214 |
| AsCH2 | 0.184b | 0.237a | 0.206b | 0.0084 | 0.001 | 0.004 |
| AsCH3 | 0.061 | 0.066 | 0.062 | 0.0016 | 0.063 | 0.214 |
| ASCCA | 11.719b | 13.387a | 12.387ab | 0.3509 | 0.007 | 0.024 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Hannoufa, A.; Christensen, D.; Shi, H.; Prates, L.L.; Yu, P. Molecular Structural Changes in Alfalfa Detected by ATR-FTIR Spectroscopy in Response to Silencing of TT8 and HB12 Genes. Int. J. Mol. Sci. 2018, 19, 1046. https://doi.org/10.3390/ijms19041046
Lei Y, Hannoufa A, Christensen D, Shi H, Prates LL, Yu P. Molecular Structural Changes in Alfalfa Detected by ATR-FTIR Spectroscopy in Response to Silencing of TT8 and HB12 Genes. International Journal of Molecular Sciences. 2018; 19(4):1046. https://doi.org/10.3390/ijms19041046
Chicago/Turabian StyleLei, Yaogeng, Abdelali Hannoufa, David Christensen, Haitao Shi, Luciana L. Prates, and Peiqiang Yu. 2018. "Molecular Structural Changes in Alfalfa Detected by ATR-FTIR Spectroscopy in Response to Silencing of TT8 and HB12 Genes" International Journal of Molecular Sciences 19, no. 4: 1046. https://doi.org/10.3390/ijms19041046
APA StyleLei, Y., Hannoufa, A., Christensen, D., Shi, H., Prates, L. L., & Yu, P. (2018). Molecular Structural Changes in Alfalfa Detected by ATR-FTIR Spectroscopy in Response to Silencing of TT8 and HB12 Genes. International Journal of Molecular Sciences, 19(4), 1046. https://doi.org/10.3390/ijms19041046






