Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells
Abstract
1. Introduction
2. Results
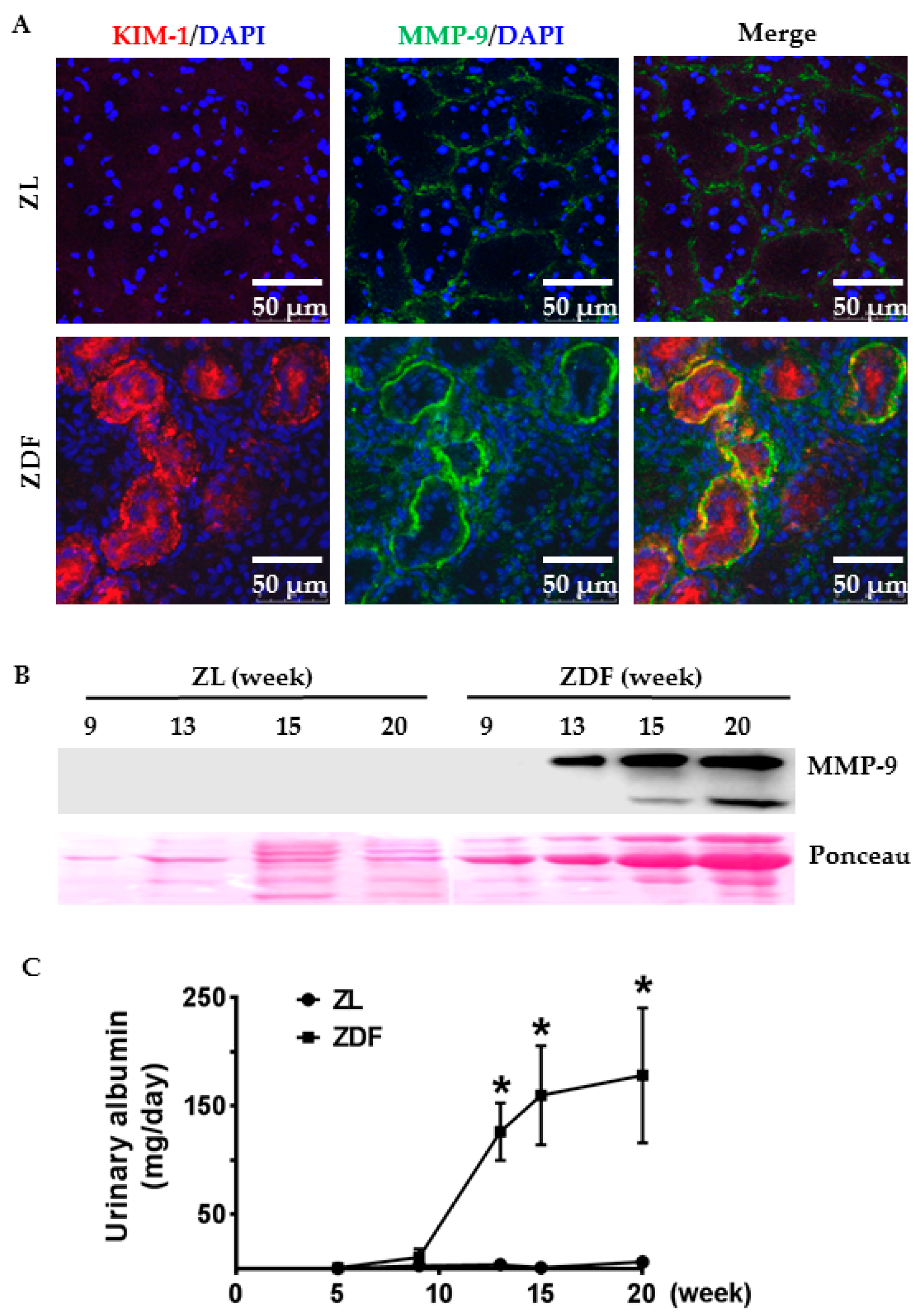
2.1. Tubular Expression and Urinary Excretion of MMP-9 Protein Were Increased in Association with Albumin Overload in the Diabetic Kidneys
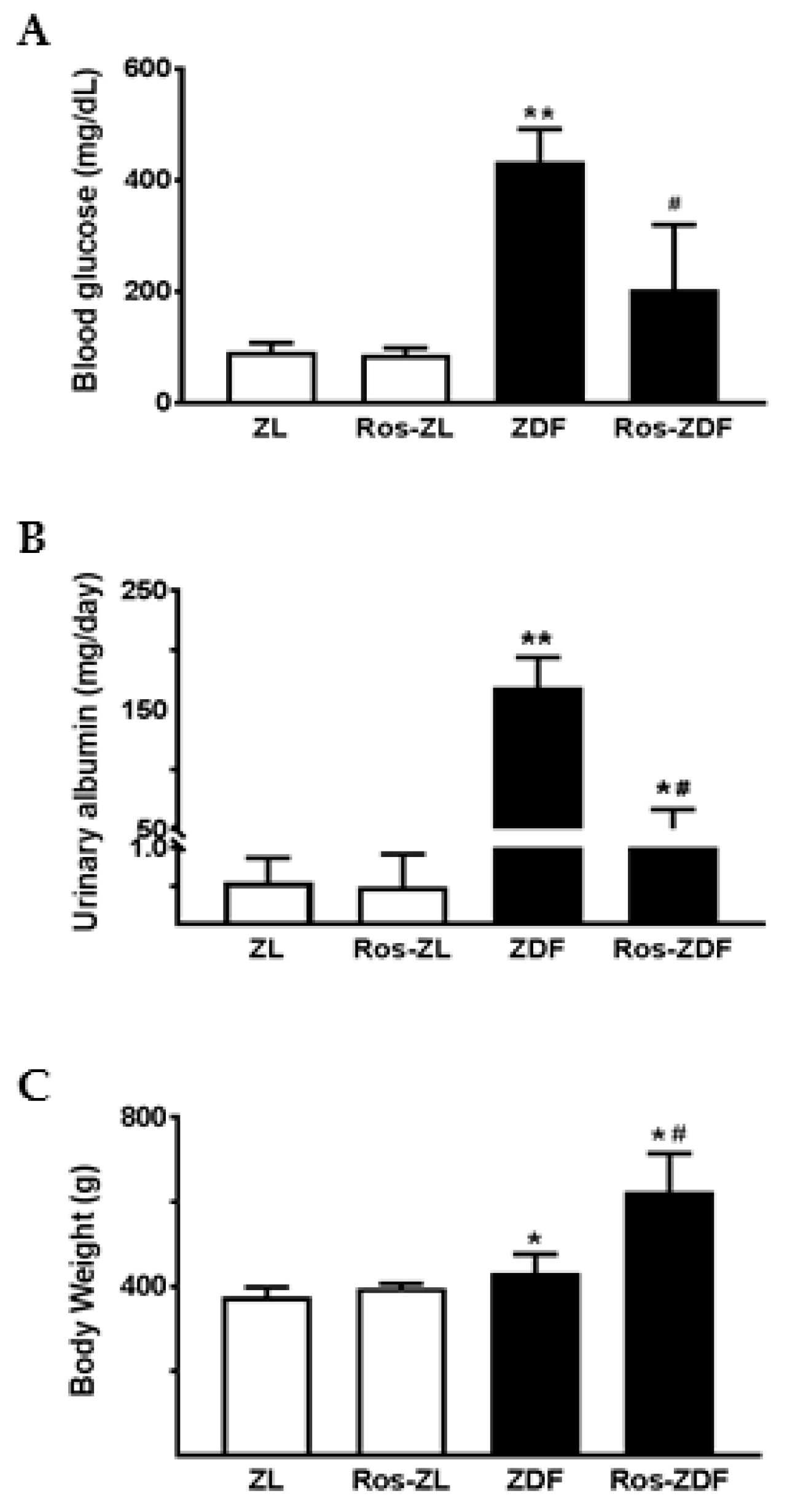
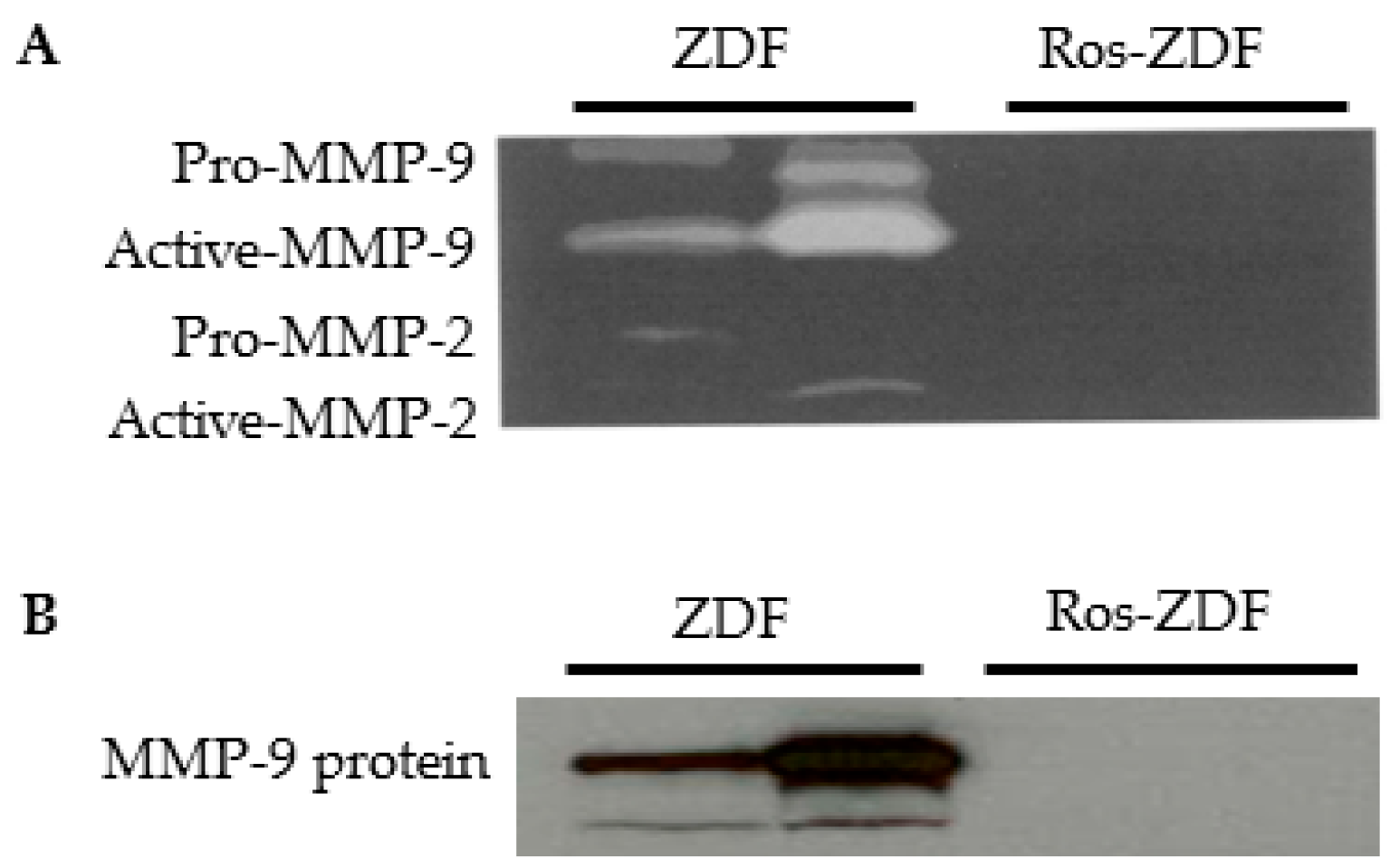
2.2. Rosiglitazone Reduced Blood Glucose and Urinary Excretion of Albumin and MMP-9 in ZDF Rats
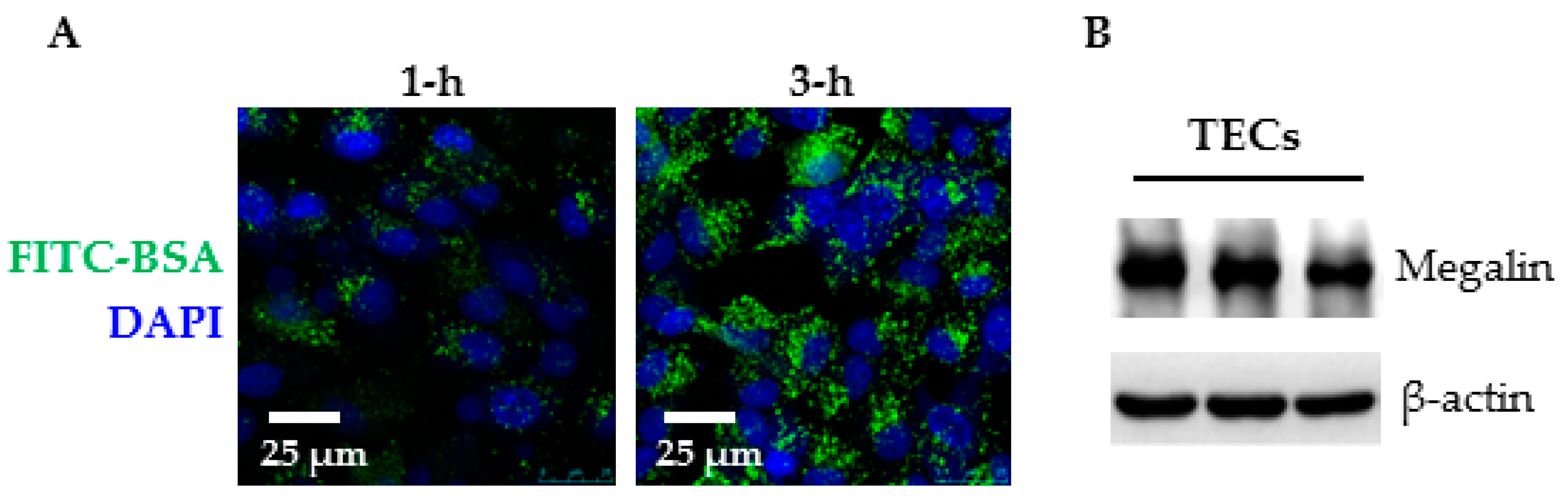
2.3. Primary Cell Culture and Albumin Endocytosis
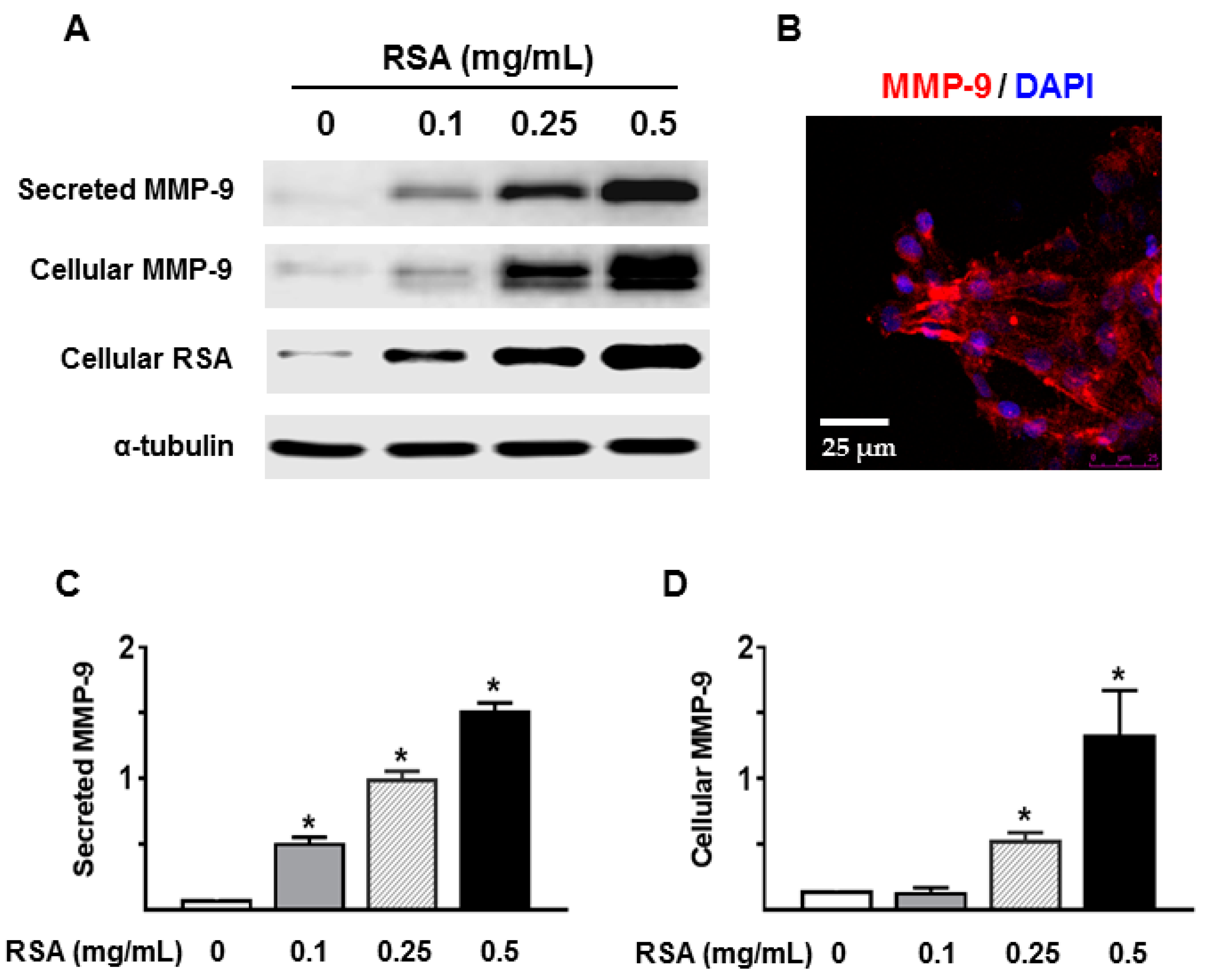
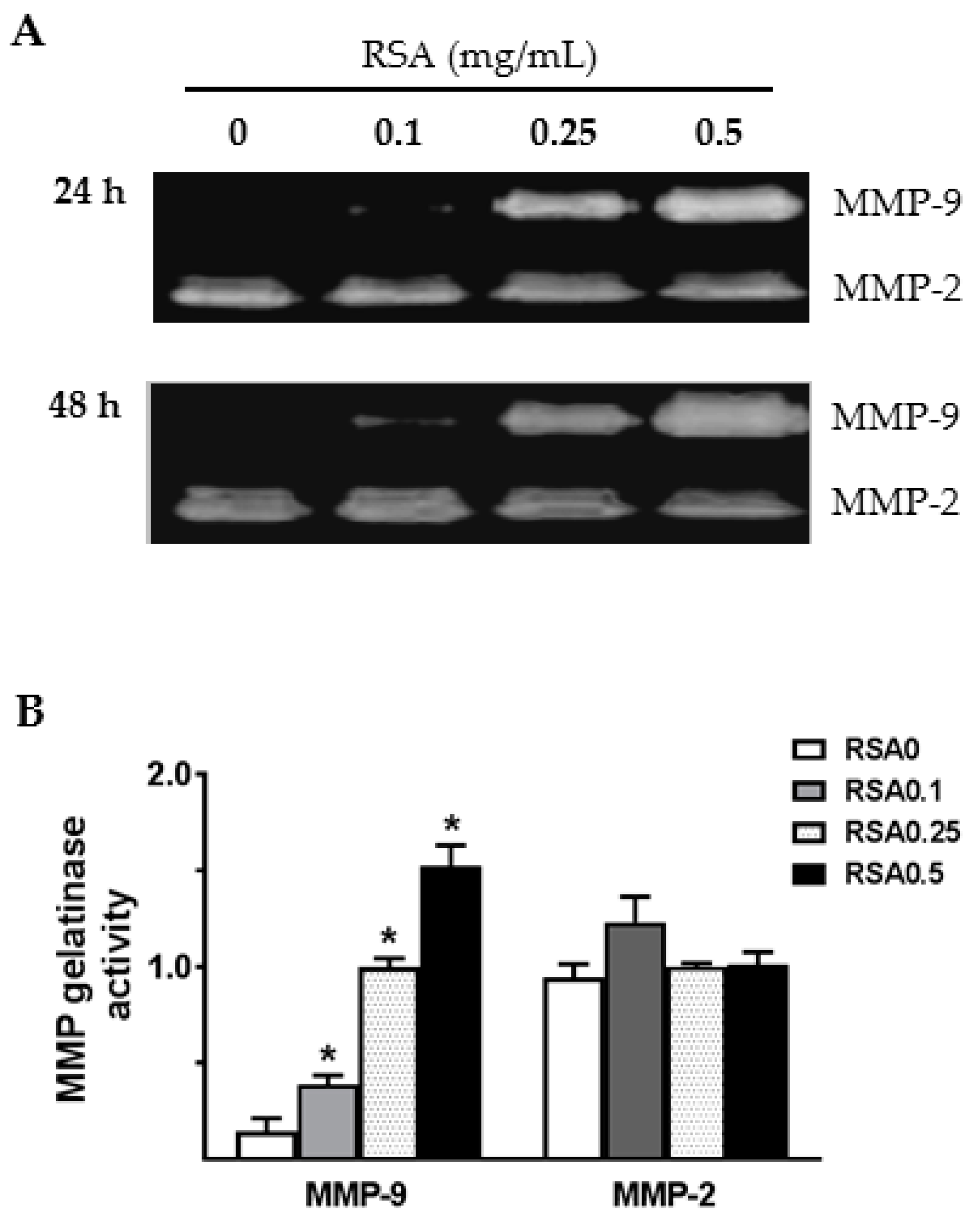
2.4. Albumin Exposure Induced MMP-9 Expression and Secretion by Primary Rat TECs
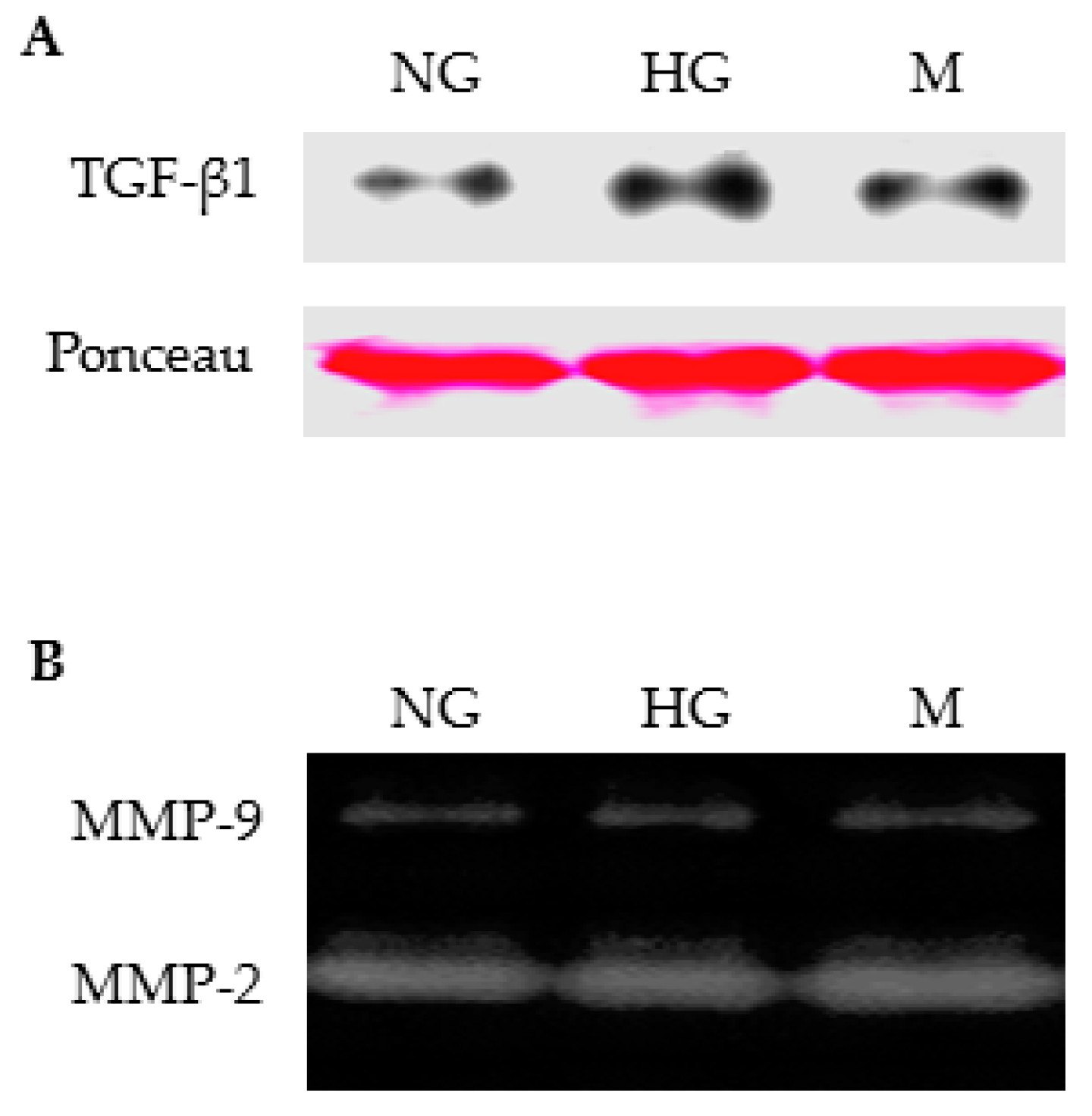
2.5. High Glucose Alone Did not Induce MMP-9 Secretion by Primary Rat TECs
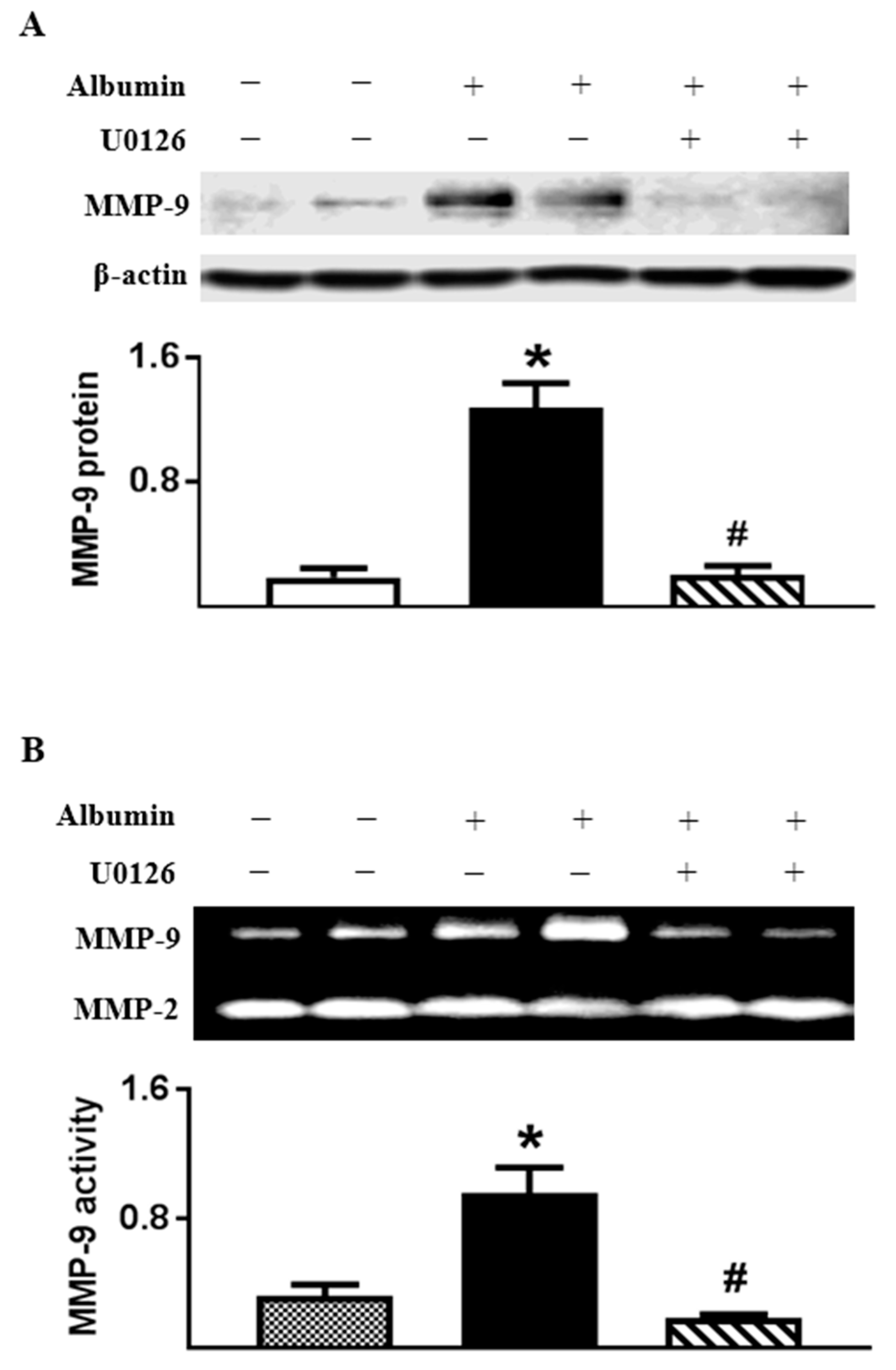
2.6. Albumin-Induced MMP-9 Was Dependent on ERK1/2 Activation in Cultured TECs
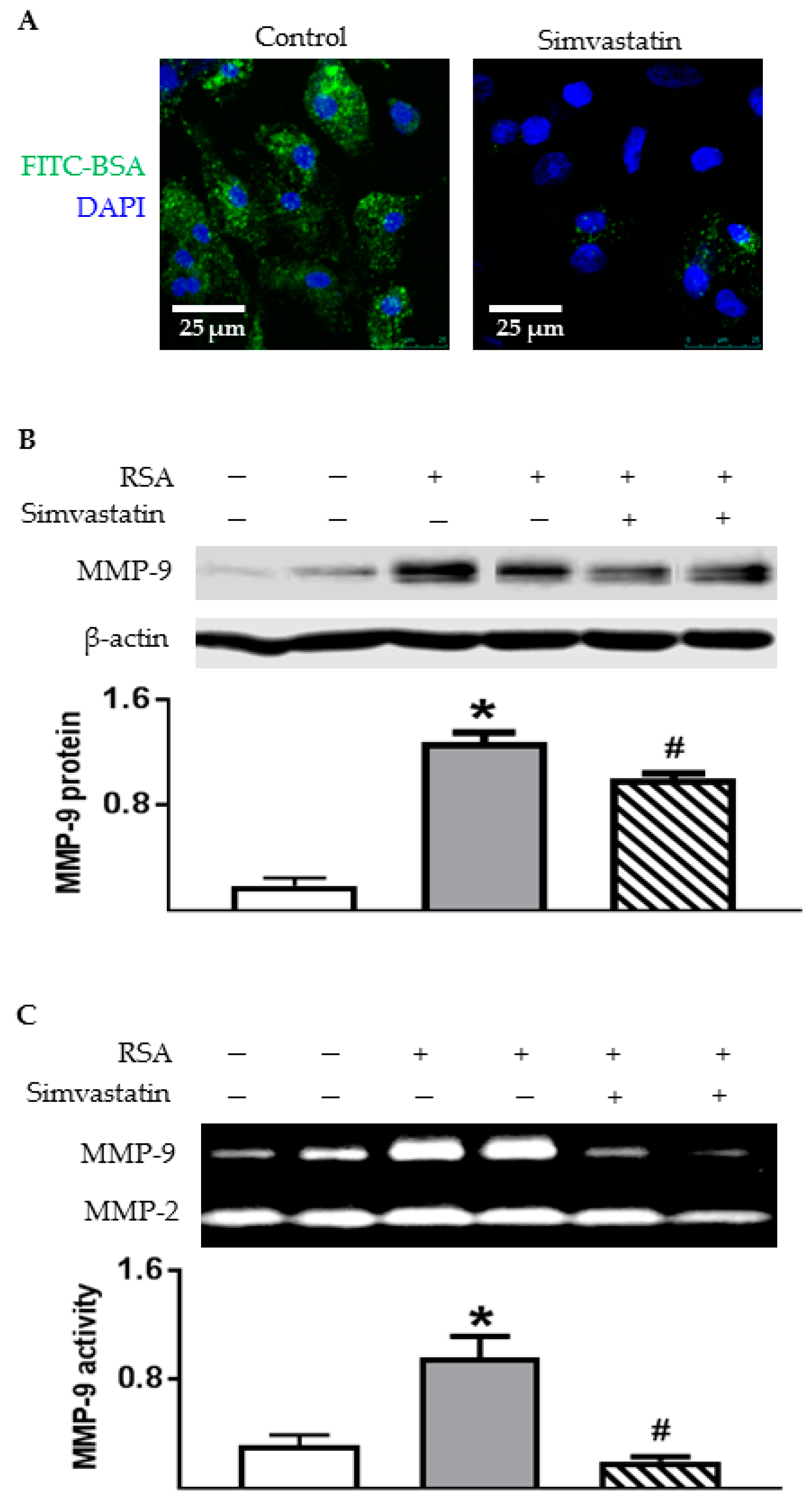
2.7. Inhibiting Endocytosis Attenuated Albumin-Induced MMP-9 Secretion by TECs
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Measurement of Urinary Albumin, Protein, and Creatinine
4.3. Culture of Primary Tubular Epithelial Cells
4.4. Effect of Inhibitors on MMP-9 Release
4.5. Albumin Endocytosis
4.6. Immunofluorescence Staining
4.7. Gelatin Zymography
4.8. Immunoblot Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ERK | Extracellular signal-regulated kinase |
| MMP | Matrix metalloproteinase |
| TECs | Tubule epithelial cells |
| ZDF | Zucker diabetic fatty |
| ZL | Zucker lean |
| RSA | Rat serum albumin |
| BSA | Bovine serum albumin |
| DN | Diabetic nephropathy |
| RANTES | Regulated on activation, normal T cell expressed and secreted |
| MCP-1 | Monocyte chemoattractant protein-1 |
| TGF-β | Transforming growth factor-β |
| ECM | Extracellular matrix |
| EMT | Epithelial-mesenchymal transition |
| KIM-1 | Kidney injury molecule-1 |
References
- Nangaku, M. Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern. Med. 2004, 43, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zoja, C.; Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 2006, 17, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Donadelli, R.; Colleoni, S.; Figliuzzi, M.; Bonazzola, S.; Morigi, M.; Remuzzi, G. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-κB activation. Kidney Int. 1998, 53, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Koya, D.; Isono, M.; Sugimoto, T.; Sugaya, T.; Kashiwagi, A.; Haneda, M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2003, 284, F1037–F1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Chen, L.; Tay, Y.C.; Rangan, G.K.; Harris, D.C. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J. Am. Soc. Nephrol. 1997, 8, 1537–1545. [Google Scholar] [PubMed]
- Diwakar, R.; Pearson, A.L.; Colville-Nash, P.; Brunskill, N.J.; Dockrell, M.E. The role played by endocytosis in albumin-induced secretion of TGF-β1 by proximal tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 2007, 292, F1464–F1470. [Google Scholar] [CrossRef] [PubMed]
- Yard, B.A.; Chorianopoulos, E.; Herr, D.; Van der Woude, F.J. Regulation of endothelin-1 and transforming growth factor-β1 production in cultured proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol. Dial. Transplant. 2001, 16, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y.; Zhang, Y.; Harris, D.C.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Strutz, F.; Zeisberg, M.; Ziyadeh, F.N.; Yang, C.Q.; Kalluri, R.; Muller, G.A.; Neilson, E.G. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002, 61, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shultz, R.W.; Mars, W.M.; Wegner, R.E.; Li, Y.; Dai, C.; Nejak, K.; Liu, Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Investig. 2002, 110, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Lauhio, A.; Sorsa, T.; Srinivas, R.; Stenman, M.; Tervahartiala, T.; Stenman, U.H.; Gronhagen-Riska, C.; Honkanen, E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann. Med. 2008, 40, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Huang, P.H.; Yang, A.H.; Tarng, D.C.; Yang, W.C.; Lin, C.C.; Chen, J.W.; Schmid-Schonbein, G.; Lin, S.J. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int. 2014, 86, 358–369. [Google Scholar] [CrossRef] [PubMed]
- McKittrick, I.B.; Bogaert, Y.; Nadeau, K.; Snell-Bergeon, J.; Hull, A.; Jiang, T.; Wang, X.; Levi, M.; Moulton, K.S. Urinary matrix metalloproteinase activities: Biomarkers for plaque angiogenesis and nephropathy in diabetes. Am. J. Physiol. Ren. Physiol. 2011, 301, F1326–F1333. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Moreau, C.S.; Cockrell, G.E.; Jo, C.H.; Bunn, R.C.; Morales-Pozzo, A.E.; Lumpkin, C.K.; Fowlkes, J.L. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine 2010, 37, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Van der Zijl, N.J.; Hanemaaijer, R.; Tushuizen, M.E.; Schindhelm, R.K.; Boerop, J.; Rustemeijer, C.; Bilo, H.J.; Verheijen, J.H.; Diamant, M. Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin. Biochem. 2010, 43, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; George, J.; Li, Y.; Olufade, R.; Zhao, X. Matrix metalloproteinase-9 expression is enhanced in renal parietal epithelial cells of zucker diabetic fatty rats and is induced by albumin in in vitro primary parietal cell culture. PLoS ONE 2015, 10, e0123276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lv, L.L.; Cao, Y.H.; Zhang, J.D.; Wu, M.; Ma, K.L.; Phillips, A.O.; Liu, B.C. Urinary mRNA markers of epithelial-mesenchymal transition correlate with progression of diabetic nephropathy. Clin. Endocrinol. 2012, 76, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, S.; Gburek, J.; Hamard, G.; Nielsen, R.; Willnow, T.E.; Devuyst, O.; Nexo, E.; Verroust, P.J.; Christensen, E.I.; Kozyraki, R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J. Am. Soc. Nephrol. 2010, 21, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Sun, Y.; Yang, G.; Zhang, A.; Huang, S.; Heiney, K.M.; Zhang, Y. New Insights into the PPAR gamma Agonists for the treatment of diabetic nephropathy. PPAR Res. 2014, 2014, 818530. [Google Scholar] [CrossRef] [PubMed]
- Miceli, I.; Burt, D.; Tarabra, E.; Camussi, G.; Perin, P.C.; Gruden, G. Stretch reduces nephrin expression via an angiotensin II-AT(1)-dependent mechanism in human podocytes: Effect of rosiglitazone. Am. J. Physiol. Ren. Physiol. 2010, 298, F381–F390. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Stafylas, P.C.; Georgianos, P.I.; Saratzis, A.N.; Lasaridis, A.N. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: A meta-analysis. Am. J. Kidney Dis. 2010, 55, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Ma, L.J.; Ma, J.; Fogo, A.B. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 2006, 69, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, J.E.; Davidson, R.G.; McTaggart, F.; Orton, T.C.; Scott, R.C.; Smith, G.J.; Brunskill, N.J. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J. Am. Soc. Nephrol. 2004, 15, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, A.; D’Haese, P.C.; De Broe, M.E. Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J. Am. Soc. Nephrol. 2004, 15, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- McLennan, S.V.; Kelly, D.J.; Cox, A.J.; Cao, Z.; Lyons, J.G.; Yue, D.K.; Gilbert, R.E. Decreased matrix degradation in diabetic nephropathy: Effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia 2002, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.L.; Bilkan, C.M.; Sandberg, K.; Myers, A.K.; Mulroney, S.E. Growth hormone exacerbates diabetic renal damage in male but not female rats. Biol. Sex Differ. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.M.; Sandoval, R.M.; Campos, S.B.; Molitoris, B.A.; Comper, W.D.; Brown, D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Tojo, A.; Onozato, M.L.; Ha, H.; Kurihara, H.; Sakai, T.; Goto, A.; Fujita, T.; Endou, H. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem. Cell Biol. 2001, 116, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.M.; Long, Y.; Song, Y.; Yang, L.; Wei, H.; Coventry, S.; Zheng, S.; Epstein, P.N. Diabetic albuminuria is due to a small fraction of nephrons distinguished by albumin-stained tubules and glomerular adhesions. Am. J. Pathol. 2009, 175, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.P.; Yokoi, H.; Kasahara, M.; Imamaki, H.; Ishii, A.; Kuwabara, T.; Koga, K.; Kato, Y.; Toda, N.; Ohno, S.; et al. Increase of total nephron albumin filtration and reabsorption in diabetic nephropathy. J. Am. Soc. Nephrol. 2017, 28, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.S.; Zheng, S.; Kralik, P.M.; Benz, F.W.; Epstein, P.N. Impaired albumin uptake and processing promote albuminuria in OVE26 diabetic mice. J. Diabetes Res. 2016, 2016, 8749417. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.K.; Zheng, G.; Hsu, T.T.; Lee, S.R.; Zhang, J.; Zhao, Y.; Tian, X.; Wang, Y.; Wang, Y.M.; Cao, Q.; et al. Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab. Investig. 2013, 93, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.; Tritchler, D.; Herzenberg, A.M.; Kassiri, Z.; Zhou, X.; Gao, W.; Scholey, J.W. Albumin activates ERK via EGF receptor in human renal epithelial cells. J. Am. Soc. Nephrol. 2005, 16, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, E.; Kurihara, H.; Sakai, T.; Ohshiro, K.; Yamamoto, T. Phenotypic modulation of parietal epithelial cells of Bowman’s capsule in culture. Cell Tissue Res. 2001, 304, 339–349. [Google Scholar] [PubMed]
- Ralay, R.H.; Zunich, S.M.; Choi, N.; Hodge, J.N.; Wainwright, M.S. Mild stretch-induced injury increases susceptibility to interleukin-1β-induced release of matrix metalloproteinase-9 from astrocytes. J. Neurotrauma 2011, 28, 1757–1766. [Google Scholar] [CrossRef]
- Ralay, R.H.; Hodge, J.N.; Choi, N.; Wainwright, M.S. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J. Neuroinflamm. 2012, 9, 68. [Google Scholar] [CrossRef]

| Header | ZDF (n = 8) | Ros-ZDF (n = 7) | p Value |
|---|---|---|---|
| Food intake (g) | 29 ± 2 | 33 ± 1 | 0.1529 |
| Water intake (mL) | 57 ± 7 | 42 ± 3 | 0.0635 |
| Urine volume (mL/day) | 34 ± 5 | 15 ± 1 | 0.0017 |
| Urinary protein (mg/day) | 326 ± 34 | 149 ± 20 | 0.0005 |
| Protein/creatinine | 36 ± 3 | 17 ± 2 | 0.0002 |
| Cholesterol (mg/dL) | 168 ± 11 | 159 ± 15 | 0.6566 |
| Triglyceride (mg/dL) | 462 ± 80 | 30 ± 6 | 0.0002 |
| Body weight (g) | 407 ± 22 | 654 ± 16 | 0.0001 |
| Pancreas weight (g) | 0.69 ± 0.04 | 0.87 ± 0.05 | 0.0153 |
| Pancreas/body weight (‰) | 1.77 ± 0.14 | 1.28 ± 0.09 | 0.0154 |
| Kidney weight (g) | 1.58 ± 0.07 | 1.46 ± 0.03 | 0.1408 |
| Kidney/body weight (‰) | 3.94 ± 0.26 | 2.15 ± 0.04 | 0.0001 |
| Heart weight (g) | 1.07 ± 0.04 | 1.60 ± 0.04 | 0.0001 |
| Heart/body weight (‰) | 2.66 ± 0.09 | 2.35 ± 0.09 | 0.034 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cobbs, A.; George, J.; Chima, A.; Tuyishime, F.; Zhao, X. Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 1758. https://doi.org/10.3390/ijms18081758
Chen X, Cobbs A, George J, Chima A, Tuyishime F, Zhao X. Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells. International Journal of Molecular Sciences. 2017; 18(8):1758. https://doi.org/10.3390/ijms18081758
Chicago/Turabian StyleChen, Xiaoming, Alyssa Cobbs, Jasmine George, Ashmeer Chima, Fidele Tuyishime, and Xueying Zhao. 2017. "Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells" International Journal of Molecular Sciences 18, no. 8: 1758. https://doi.org/10.3390/ijms18081758
APA StyleChen, X., Cobbs, A., George, J., Chima, A., Tuyishime, F., & Zhao, X. (2017). Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells. International Journal of Molecular Sciences, 18(8), 1758. https://doi.org/10.3390/ijms18081758




