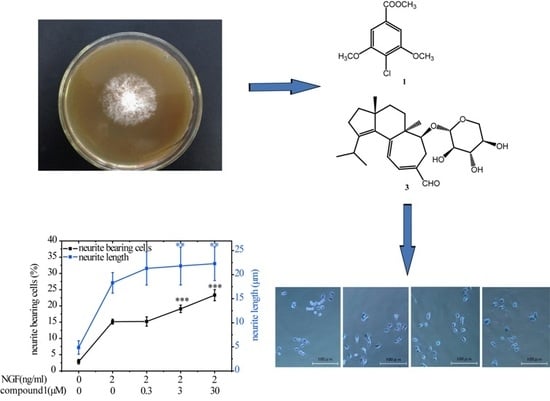
Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway
Abstract
:1. Introduction
2. Results and Discussion
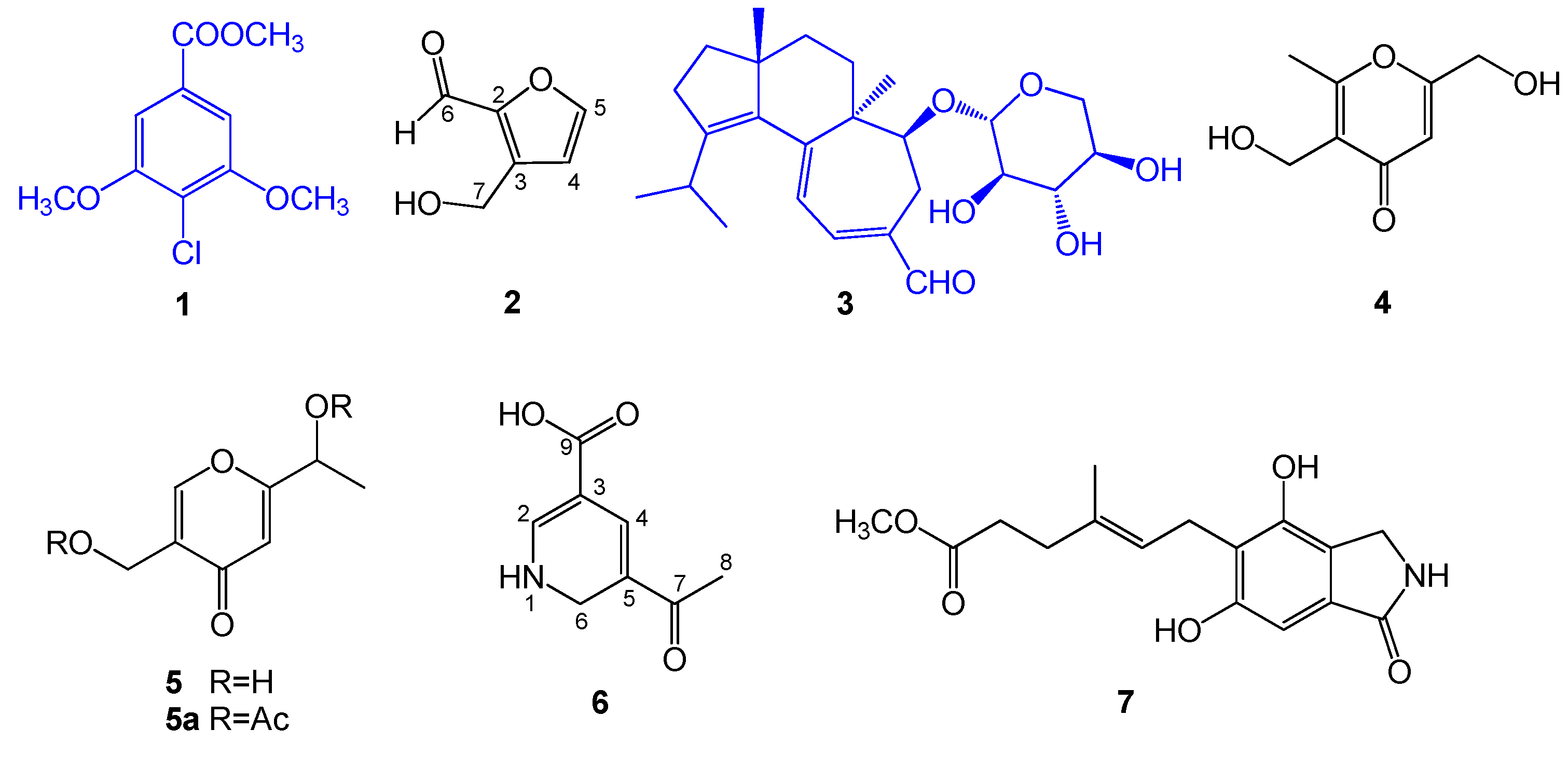
2.1. Structural Elucidation of Compounds 1–7
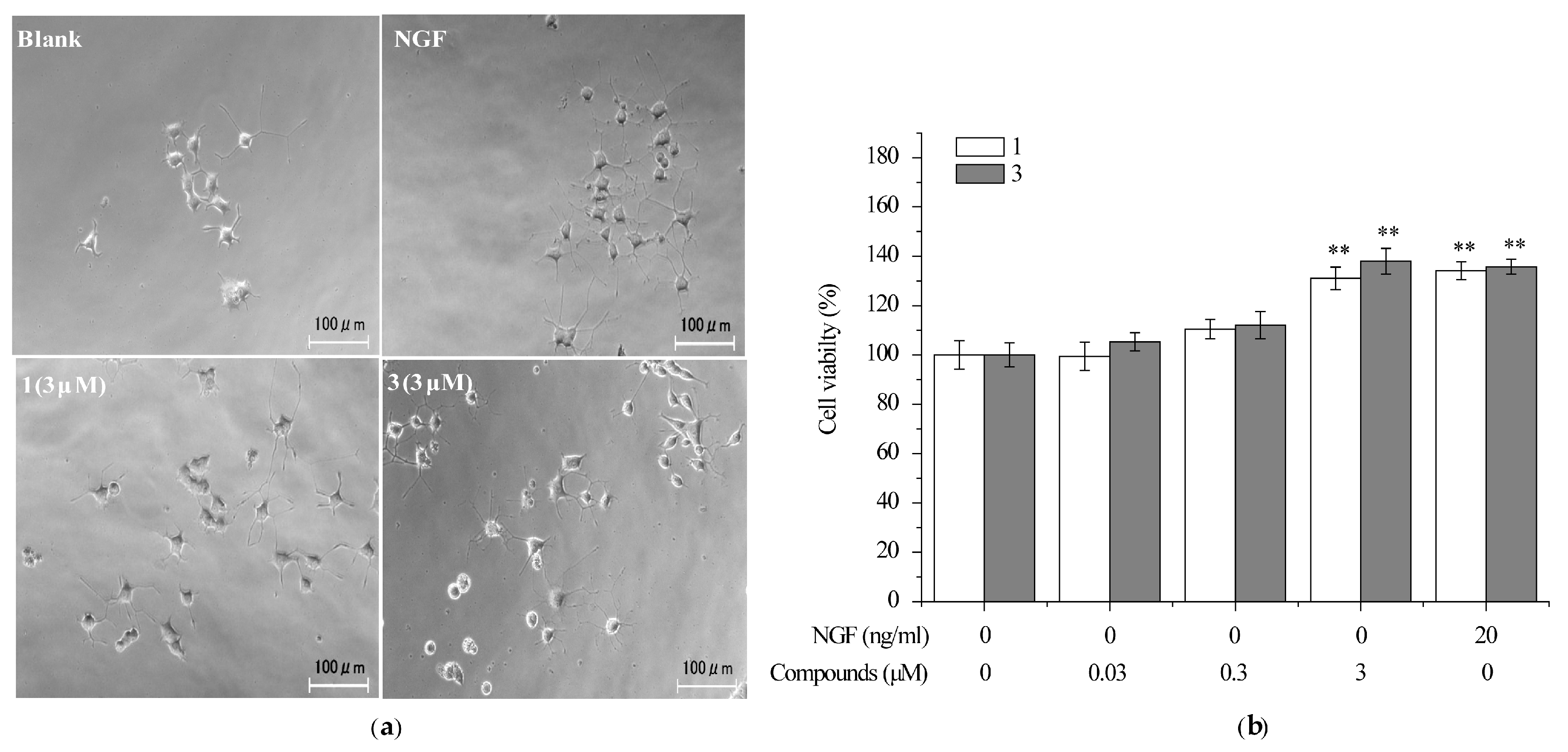
2.2. Compounds 1 and 3 Promoted NGF-Induced Neurite Outgrowth in PC12 Cells
2.3. Compounds 1 and 3 Promoted NGF-Induced Neurite Outgrowth by Activating TrkA/Erk1/2 Pathway in PC12 Cells
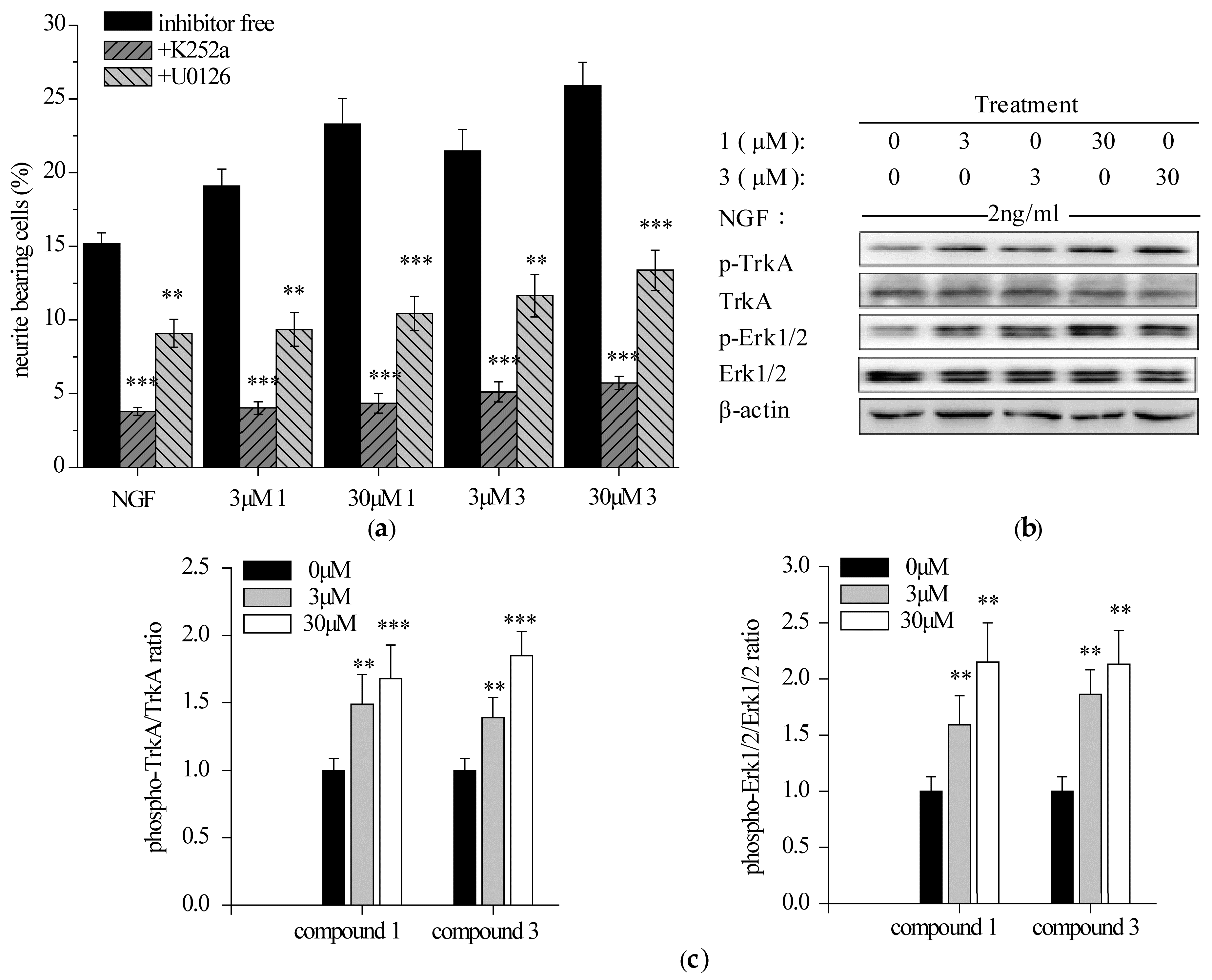
2.3.1. Specific TrkA and Erk1/2 Inhibitors Reduced Compounds 1 and 3-Potentiated Neurite Outgrowth in PC12 Cells
2.3.2. Compounds 1 and 3 Enhanced NGF-Induced TrkA and Erk1/2 Phosphorylation in PC12 Cells
2.4. Compounds 1 and 3 Prevented the Death of Neuronally-Differentiated PC12 Cells against the Removal of NGF
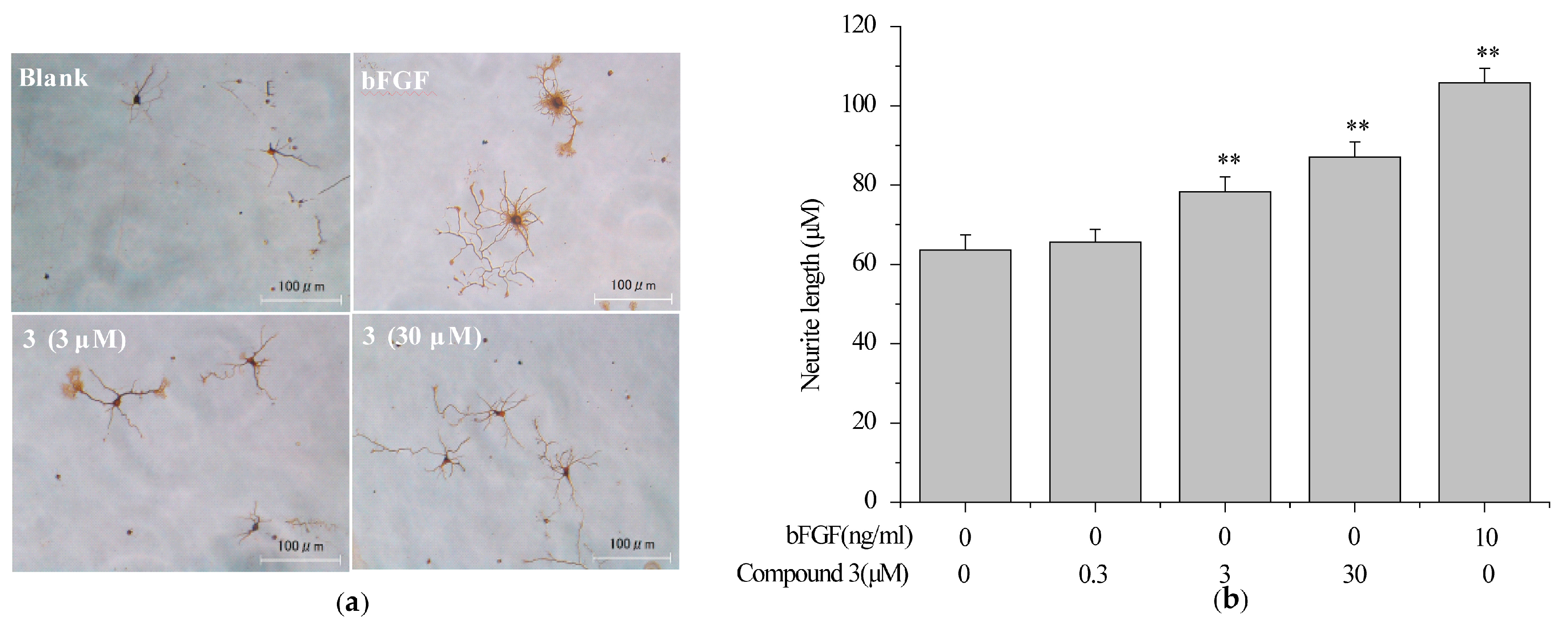
2.5. Compound 3 Induced Neurite Outgrowth in Primary Rat Cortex Neurons
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. MTT Assay for Cell Viability
3.6. Assay of Neurite Outgrowth of PC12 Cells
3.7. Western Blotting Analysis
3.8. Immunocytochemical Staining
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaplan, D.R.; Stephens, R.M. Neurotrophin signal transduction by the Trk receptor. J. Neurobiol. 1994, 25, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Akagi, M.; Matsui, N.; Akae, H.; Hirashima, N.; Fukuishi, N.; Fukuyama, Y.; Akagi, R. Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer’s disease. J. Pharmacol. Sci. 2015, 127, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Chaturvedi, R.K. Peptide therapeutics in neurodegenerative disorders. Curr. Med. Chem. 2014, 21, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Wang, J.L.; Jin, W.L. The emerging therapeutic role of NGF in Alzheimer’s disease. Neurochem. Res. 2016, 41, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Harzdorf, N.; Khaing, Z.; Kang, D.; Camelio, A.M.; Shaw, T.; Schmidt, C.E.; Siegel, D. Neuronal growth promoting sesquiterpene-neolignans; syntheses and biological studies. Org. Biomol. Chem. 2012, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Liu, L.; Gao, J.M.; Zhang, A.L. Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and their neurite outgrowth-promoting activity. Eur. J. Med. Chem. 2011, 46, 3112–3117. [Google Scholar]
- Gao, J.M. New biologically active metabolites from Chinese higher fungi. Curr. Org. Chem. 2006, 10, 849–871. [Google Scholar]
- Lu, Q.Q.; Tian, J.M.; Wei, J.; Gao, J.M. Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum. Nat. Prod. Res. 2014, 28, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91–113. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion's Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.L.; Naidu, M.; Sabaratnam, V.; Wong, K.H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N.A. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms 2013, 15, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Obara, Y.; Moriya, T.; Inatomi, S.; Nakahata, N. Effects of Hericium erinaceus on amyloid beta(25–35) peptide-induced learning and memory deficits in mice. Biomed. Res. 2011, 32, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Naidu, M.; David, R.P.; Abdulla, M.A.; Abdullah, N.; Kuppusamy, U.R.; Sabaratnam, V. Functional recovery enhancement following injury to rodent peroneal nerve by Lion’s Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2009, 11, 225–236. [Google Scholar] [CrossRef]
- Kawagishi, H.; Zhuang, C. Compounds for dementia from Hericium erinaceum. Drugs Future 2008, 33, 149–155. [Google Scholar] [CrossRef]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617–623. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, Y.; Yew, X.X.; Chen, H.X.; Kim, J.X.; Chang, C.C.; Peng, C.C.; Peng, R.Y. Improvement of erinacine A productivity in Hericium erinaceus mycelia and its neuroprotective bioactivity against the glutamate-insulted apoptosis. LWT Food Sci. Technol. 2016, 65, 1100–1108. [Google Scholar] [CrossRef]
- Lee, K.F.; Chen, J.H.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Lu, C.C.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073–15089. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Lu, C.C.; Shen, C.H.; Tung, S.Y.; Hsieh, M.C.; Lee, K.C.; Lee, L.Y.; Chen, C.C.; Teng, C.C.; Huang, W.S.; et al. Hericium erinaceus mycelium and its isolated erinacine A protection from MPTP-induced neurotoxicity through the ER stress, triggering an apoptosis cascade. J. Transl. Med. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Kodani, S.; Kubo, M.; Masuno, K.; Sekiya, A.; Nagai, K.; Kawagishi, H. Endoplasmic reticulum (ER) stress-suppressive compounds from scrap cultivation beds of the mushroom Hericium erinaceum. Biosci. Biotechnol. Biochem. 2009, 73, 1908–1910. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Qian, F.G.; Xu, G.Y.; Du, S.J.; Li, M.H. Isolation and identification of two new pyrone compounds from the culture of Herictum erinaceus. Yao Xue Xue Bao 1990, 25, 522–525. [Google Scholar] [PubMed]
- Wang, K.; Bao, L.; Qi, Q.; Zhao, F.; Ma, K.; Pei, Y.; Liu, H. Erinacerins C-L, isoindolin-1-ones with α-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J. Nat. Prod. 2015, 78, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schwiderski, M.; Kruse, A. Catalytic effect of aluminium chloride on the example of the conversion of sugar model compounds. J. Mol. Catal. 2015, 402, 64–70. [Google Scholar] [CrossRef]
- Phan, C.W.; Lee, G.S.; Hong, S.L.; Wong, Y.T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A-C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Lange-Carter, C.A.; Johnson, G.L. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science 1994, 265, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Hempstead, B.L. p75 and Trk: A two-receptor system. Trends Neurosci. 1995, 18, 321–326. [Google Scholar] [CrossRef]
- Vaudry, D.; Stork, P.J.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Zhang, R.; Liu, S.; Xia, Z.; Hu, Y. Catalpol ameliorates beta amyloid-induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors. Neuroscience 2009, 163, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Ye, Y.; Xiang, L.; Osada, H.; Qi, J.H. Lindersin B from Lindernia crustacea induces neuritogenesis by activation of tyrosine kinase A/phosphatidylinositol 3 kinase/extracellular signal-regulated kinase signaling pathway. Phytomedicine 2017, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheng, Y.Y.; Fan, W.; Yang, C.B.; Ye, S.F.; Cui, W.; Wei, W.; Lao, L.X.; Cai, J.; Han, Y.F.; et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci. Ther. 2015, 21, 61–70. [Google Scholar] [PubMed]
- Jesky, R.; Chen, H. The neuritogenic and neuroprotective potential of senegenin against Aβ-induced neurotoxicity in PC 12 cells. BMC Complement. Altern. Med. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Shen, J.; Zhang, A.L.; Zhu, W.; Zhang, X.; Liu, J.K. Chemical constituents of the fungus Leccinum extremiorientale. Chin. J. Org. Chem. 2003, 23, 853–857. [Google Scholar]
- Kubo, M.; Ishii, R.; Ishino, Y.; Harada, K.; Matsui, N.; Akagi, M.; Kato, E.; Hosoda, S.; Fukuyama, Y. Evaluation of constituents of Piper retrofractum fruits on neurotrophic activity. J. Nat. Prod. 2013, 76, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, C.C.; Yin, X.; Wei, j.; Gao, J.M. Cyathane diterpenoids with neurotrophic activity from cultures of the fungus. J. Nat. Prod. 2015, 78, 783–788. [Google Scholar]
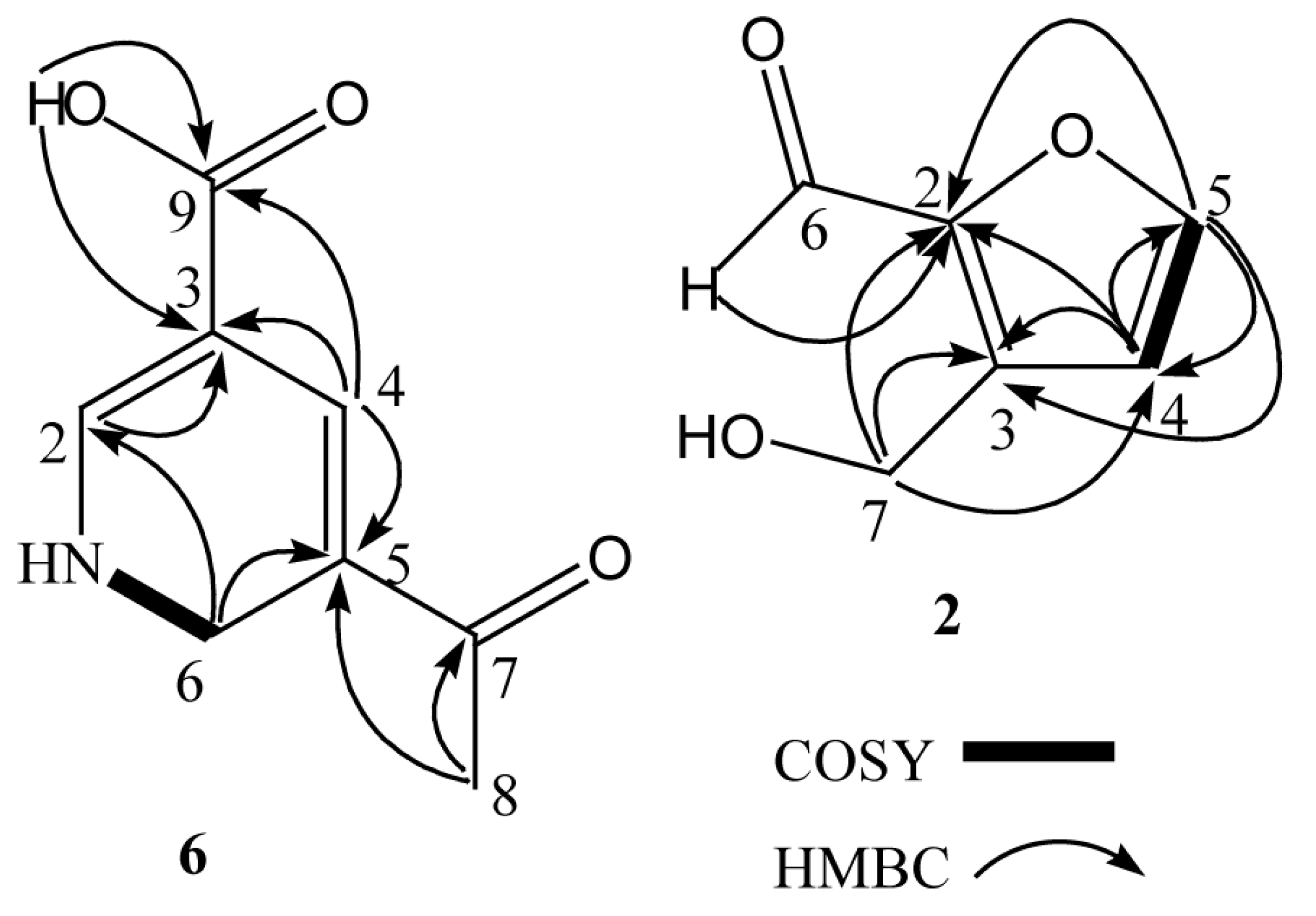

| Position | δH | δC | HMBC |
|---|---|---|---|
| 2 | 6.88 (1H, d, J = 0.5 Hz) | 109.3 | C-3 |
| 3 | 116.6 | ||
| 4 | 8.90 (1H, s) | 152.1 | C-3, C-5, C-9 |
| 5 | 166.1 | ||
| 6 | 4.73 (2H, d, J = 4.9 Hz) | 64.2 | C-2, C-5, C-8 |
| 7 | 203.8 | ||
| 8 | 2.69 (3H, s) | 26.4 | C-5, C-7 |
| 9 | 168.3 | ||
| –NH | 3.33 (1H, m) | ||
| –COOH | 12.56 (1H, s) | C-3, C-9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-C.; Cao, C.-Y.; Kubo, M.; Harada, K.; Yan, X.-T.; Fukuyama, Y.; Gao, J.-M. Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. Int. J. Mol. Sci. 2017, 18, 1659. https://doi.org/10.3390/ijms18081659
Zhang C-C, Cao C-Y, Kubo M, Harada K, Yan X-T, Fukuyama Y, Gao J-M. Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. International Journal of Molecular Sciences. 2017; 18(8):1659. https://doi.org/10.3390/ijms18081659
Chicago/Turabian StyleZhang, Cheng-Chen, Chen-Yu Cao, Miwa Kubo, Kenichi Harada, Xi-Tao Yan, Yoshiyasu Fukuyama, and Jin-Ming Gao. 2017. "Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway" International Journal of Molecular Sciences 18, no. 8: 1659. https://doi.org/10.3390/ijms18081659
APA StyleZhang, C.-C., Cao, C.-Y., Kubo, M., Harada, K., Yan, X.-T., Fukuyama, Y., & Gao, J.-M. (2017). Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. International Journal of Molecular Sciences, 18(8), 1659. https://doi.org/10.3390/ijms18081659