An Update on Jacalin-Like Lectins and Their Role in Plant Defense
Abstract
:1. Introduction
2. Evolution Drives Formation of Chimeric Proteins
3. Chimeric Proteins with Lectin Domains Are Commonly Involved in Plant Defense
4. Chimeric Proteins with Jacalin-Related Lectin (JRL) Domains Are Widely Distributed among Different Kingdoms
5. Chimeric Dirigent-JRL Proteins Occur Exclusively in Monocotyledons (Liliopsida)
6. Chimeric Dirigent-JRLs and Plant Defense
7. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| JRL | Jacalin Related Lectin |
| BRI1 | Barssinosteroid insensitive gene 1 |
| LRR | Leucin Rich Repeat |
| LecRLK | Lectin Receptor Like Kinase |
| FBA-1 | F-Box Associated-1 |
| PKc | Protein Kinase Catalytic Domain |
| RX-CC | Rx Protein-Coiled-Coil |
| NTPase | Nucleoside Triphosphate Hydrolase |
| L-type | Legum-Lectin Protein-Like Type |
| G-type | Galanthus nivaili Agglutinin-Related Type |
| C-type | Calcium-Dependent Type |
| EUL | Euonymus Lectin |
| ER | Endoplasmatic Reticulum |
| Nictaba | Nicotiana tabacum Agglutinin |
| O-GlcNAc | o-β-n-Acetyl-d-glucosamine |
| SNA-I/SNA-V | Sambucus nigra Agglutinin I/V |
| gJRL | Galactose-Specific Jacalin Related Lectin |
| mJRL | Mannose-Specific Jacalin Related Lectin |
| JAX1 | Jacalin-Type Lectin Required for Potexvirus Resistance1 |
| RNA | Ribonucleic Acid |
| NLR | Nod-Like Receptor |
| EEP | Exonuclease-Endonuclease-Phosphatase |
| metallopep | Metallopeptidase |
| NPP1 | Necrosis-Inducing Phytophthora Protein 1 |
| ORF | Open Reading Frame |
| CC | Coiled-Coil |
| NB | Nucleotide Binding |
| NTP | Nucleoside Triphosphate |
| ATP | Adenosine Triphosphate |
| GTP | Guanosine Triphosphate |
| ETX | Epsilon Toxin |
| MTX | Mosquitocidal Toxin |
| OsJAC1 | Oryza sativa Jacalin-Related Lectin1 |
| TaJA1 | Triticum aestivum Jacalin-Related Lectin1 |
| HcJAC1 | Hordeu vulgare Jacalin-Related Lectin1 |
References
- Chow, B.; McCourt, P. Plant hormone receptors: Perception is everything. Genes Dev. 2006, 20, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.M.; Moffett, P. NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci. 2009, 14, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Labbe, J.; Muchero, W.; Yang, X.H.; Jawdy, S.S.; Kennedy, M.; Johnson, J.; Sreedasyam, A.; Schmutz, J.; Tuskan, G.A.; et al. Genome-wide analysis of lectin receptor-like kinases in Populus. BMC Genom. 2016, 17, 699. [Google Scholar] [CrossRef] [PubMed]
- Weidenbach, D.; Esch, L.; Möller, C.; Hensel, G.; Kumlehn, J.; Höfle, C.; Hückelhoven, R.; Schaffrath, U. Polarized defense against fungal pathogens is mediated by the jacalin-related lectin domain of modular Poaceae-specific proteins. Mol. Plant 2016, 9, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Xu, W.; Xiang, Y.; Jia, H.; Zhang, L.; Ma, Z. Association of jacalin-related lectins with wheat responses to stresses revealed by transcriptional profiling. Plant Mol. Biol. 2014, 84, 95–110. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, L.; Xu, H.; Xi, J.; Cao, X.; Xu, H.; Rong, S.; Dong, Y.; Wang, C.; Chen, R.; et al. OsJRL, a rice jacalin-related mannose-binding lectin gene, enhances Escherichia coli viability under high-salinity stress and improves salinity tolerance of rice. Plant Biol. 2017, 19, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Mahajan, S.K.; Whitham, S.A.; Yamamoto, M.L.; Carrington, J.C. Cloning of the Arabidopsis RTM1 gene, which controls restriction of long-distance movement of tobacco etch virus. Proc. Natl. Acad. Sci. USA 2000, 97, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Van Damme, E.J. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014. [CrossRef] [PubMed]
- Apic, G.; Gough, J.; Teichmann, S.A. Domain combinations in archaeal, eubacterial and eukaryotic proteomes. J. Mol. Biol. 2001, 310, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.A.; Teichmann, S.A. How do proteins gain new domains? Genome Biol. 2010, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Pasek, S.; Risler, J.-L.; Brézellec, P. Gene fusion/fission is a major contributor to evolution of multi-domain bacterial proteins. Bioinformatics 2006, 22, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Bashton, M.; Chothia, C. The generation of new protein functions by the combination of domains. Structure 2007, 15, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta (BBA) Gen. Subj. 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Haudek, K.C.; Spronk, K.J.; Voss, P.G.; Patterson, R.J.; Wang, J.L.; Arnoys, E.J. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; van Damme, E.J.M. Nucleocytoplasmic plant lectins. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Gentry, M.S.; Dixon, J.E. Laforin: A dual specificity phosphatase that dephosphorylates complex carbohydrates. J. Biol. Chem. 2006, 281, 30412–30418. [Google Scholar] [CrossRef] [PubMed]
- Vaid, N.; Macovei, A.; Tuteja, N. Knights in action: Lectin receptor-like kinases in plant development and stress responses. Mol. Plant 2013, 6, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Lannoo, N.; Peumans, W.J. Plant lectins. Adv. Bot. Res. 2008, 48, 107–209. [Google Scholar]
- Wu, J.; Luo, X.; Guo, H.; Xiao, J.; Tian, Y. Transgenic cotton, expressing Amaranthus caudatus agglutinin, confers enhanced resistance to aphids. Plant Breed. 2006, 125, 390–394. [Google Scholar] [CrossRef]
- Williams, D.B. Beyond lectins: The calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 2006, 119, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peumans, W.J.; Hause, B.; Bras, J.; Kumar, M.; Proost, P.; Barre, A.; Rougé, P.; van Damme, E.J.M. Jasmonate methyl ester induces the synthesis of a cytoplasmic/nuclear chitooligosaccharide-binding lectin in tobacco leaves. FASEB J. 2002, 16, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peumans, W.J.; van Damme, E.J.M. The Sambucus nigra type-2 ribosome-inactivating protein SNA-I’ exhibits in planta antiviral activity in transgenic tobacco. FEBS Lett. 2002, 516, 27–30. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Desmyter, S.; Ciani, M.; Proost, P.; Peumans, W.J.; van Damme, E.J.M. Analysis of the in planta antiviral activity of elderberry ribosome-inactivating proteins. Eur. J. Biochem. 2004, 271, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-Q.; Liu, R.-S.; Wang, Q.; Liu, W.-Y. Toxicity of two type II ribosome-inactivating proteins (cinnamomin and ricin) to domestic silkworm larvae. Arch. Insect Biochem. Physiol. 2004, 57, 160–165. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Moreira, R.; Ainouz, I. Lectins from seeds of jack fruit (Artocarpus integrifolia L.): Isolation and purification of two isolectins from the albumin fraction. Biol. Plant 1981, 23, 186–192. [Google Scholar] [CrossRef]
- Rabijns, A.; Barre, A.; van Damme, E.J.M.; Peumans, W.J.; de Ranter, C.J.; Rougé, P. Structural analysis of the jacalin-related lectin MornigaM from the black mulberry (Morus nigra) in complex with mannose. FEBS J. 2005, 272, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peumans, W.J.; Barre, A.; Houles Astoul, C.; Rovira, P.; Rougé, P.; Proost, P.; Truffa-Bachi, P.; Jalali, A.A.H.; van Damme, E.J.M. Isolation and characterization of a jacalin-related mannose-binding lectin from salt-stressed rice (Oryza sativa) plants. Planta 2000, 210, 970–978. [Google Scholar] [PubMed]
- Yamaji, Y.; Maejima, K.; Komatsu, K.; Shiraishi, T.; Okano, Y.; Himeno, M.; Sugawara, K.; Neriya, Y.; Minato, N.; Miura, C.; et al. Lectin-mediated resistance impairs plant virus infection at the cellular level. Plant Cell 2012, 24, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Chisholm, S.T.; Whitham, S.A.; Carrington, J.C. Identification and characterization of a locus (RTM1) that restricts long-distance movement of tobacco etch virus in Arabidopsis thaliana. Plant J. 1998, 14, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Song, M.; Wei, Z.; Tong, J.; Zhang, L.; Xiao, L.; Ma, Z.; Wang, Y. A jacalin-related lectin-like gene in wheat is a component of the plant defence system. J. Exp. Bot. 2011, 62, 5471–5483. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Keller, B. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 2016, 212, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G. Integrated decoys and effector traps: How to catch a plant pathogen. BMC Biol. 2016, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kroj, T.; Chanclud, E.; Michel-Romiti, C.; Grand, X.; Morel, J.-B. Integration of decoy domains derived from protein targets of pathogen effectors into plant immune receptors is widespread. New Phytol. 2016, 210, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCB’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed]
- Lenart, A.; Dudkiewicz, M.; Grynberg, M.; Pawłowski, K. CLCAs—A family of metalloproteases of intriguing phylogenetic distribution and with cases of substituted catalytic sites. PLoS ONE 2013, 8, e62272. [Google Scholar] [CrossRef] [PubMed]
- Gijzen, M.; Nürnberger, T. Nep1-like proteins from plant pathogens: Recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 2006, 67, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Cooley, L. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 1993, 72, 681–693. [Google Scholar] [CrossRef]
- Bork, P.; Doolittle, R.F. Drosophila kelch motif is derived from a common enzyme fold. J. Mol. Biol. 1994, 236, 1277–1282. [Google Scholar] [CrossRef]
- Adams, J.; Kelso, R.; Cooley, L. The kelch repeat superfamily of proteins: Propellers of cell function. Trends Cell Biol. 2000, 10, 17–24. [Google Scholar] [CrossRef]
- Flick, K.; Kaiser, P. Set them free: F-box protein exchange by Cand1. Cell Res. 2013, 23, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Collier, S.M.; Moffett, P.; Chai, J. Structural basis for the interaction between the potato virus X resistance protein (Rx) and its cofactor Ran GTPase-activating Protein 2 (RanGAP2). J. Biol. Chem. 2013, 288, 35868–35876. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Sibbald, P.R.; Wittinghofer, A. The P-loop—A common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 1990, 15, 430–434. [Google Scholar] [CrossRef]
- Cheung, M.-Y.; Li, X.; Miao, R.; Fong, Y.-H.; Li, K.-P.; Yung, Y.-L.; Yu, M.-H.; Wong, K.-B.; Chen, Z.; Lam, H.-M. ATP binding by the P-loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc. Natl. Acad. Sci. USA 2016, 113, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Björklund, Å.K.; Ekman, D.; Elofsson, A. Expansion of protein domain repeats. PLoS Comput. Biol. 2006, 2, e114. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Maier, E.; Gibert, M.; Popoff, M.R.; Benz, R. Clostridium perfringens epsilon toxin induces a rapid change of cell membrane permeability to ions and forms channels in artificial lipid bilayers. J. Biol. Chem. 2001, 276, 15736–15740. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Chaw, S.-M.; Chang, C.-C.; Chen, H.-L.; Li, W.-H. Dating the monocot–dicot divergence and the origin of core eudicots using whole chloroplast genomes. J. Mol. Evol. 2004, 58, 424–441. [Google Scholar] [PubMed]
- Ma, Q.H. Monocot chimeric jacalins: A novel subfamily of plant lectins. Crit. Rev. Biotechnol. 2014, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budinska, E.; et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017. [CrossRef] [PubMed]
- Davin, L.B.; Wang, H.-B.; Crowell, A.L.; Bedgar, D.L.; Martin, D.M.; Sarkanen, S.; Lewis, N.G. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 1997, 275, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Maruyama, M.; Yamauchi, S.; Nakashima, Y.; Nakato, T.; Tago, R.; Sugahara, T.; Kishida, T.; Koba, Y. Antimicrobiological activity of lignan: Effect of benzylic oxygen and stereochemistry of 2,3-dibenzyl-4-butanolide and 3,4-dibenzyltetrahydrofuran lignans on activity. Biosci. Biotechnol. Biochem. 2007, 71, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, I.; Harport, M.; Pfannstiel, J.; Klaiber, I.; Schaller, A. Expression in Pichia pastoris and characterization of two novel dirigent proteins for atropselective formation of gossypol. Appl. Microbiol. Biotechnol. 2016, 101, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Date, S.V. The rosetta stone method. In Bioinformatics: Structure, Function and Applications; Keith, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 169–180. [Google Scholar]

| Protein Domain Composition 1 | No. of Proteins | Taxonomic Groups 2 |
|---|---|---|
 | 1363 | B, P, A&O, F, Mn, Mv, M, Fr, oS, E, L |
 | 281 | B, A&O, F, Mn, Mv, oS, E, L |
 | 264 | B, A&O, M, oS, E, L |
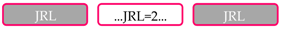 | 110 | A&O, F, M, E, L |
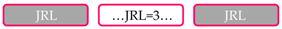 | 18 | A&O, E |
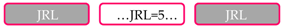 | 3 | A&O, E |
 | 234 | B, A&O, F |
 | 7 | B, F |
 | 22 | A&O |
 | 8 | B, F, Mn, Mv |
 | 93 | F, Mn |
 | 2 | F, E |
 | 29 | E |
 | 8 | E, L |
 | 2 | E, L |
 | 21 | E, L |
 | 14 | E, L |
 | 22 | L |
 | 172 | L |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esch, L.; Schaffrath, U. An Update on Jacalin-Like Lectins and Their Role in Plant Defense. Int. J. Mol. Sci. 2017, 18, 1592. https://doi.org/10.3390/ijms18071592
Esch L, Schaffrath U. An Update on Jacalin-Like Lectins and Their Role in Plant Defense. International Journal of Molecular Sciences. 2017; 18(7):1592. https://doi.org/10.3390/ijms18071592
Chicago/Turabian StyleEsch, Lara, and Ulrich Schaffrath. 2017. "An Update on Jacalin-Like Lectins and Their Role in Plant Defense" International Journal of Molecular Sciences 18, no. 7: 1592. https://doi.org/10.3390/ijms18071592
APA StyleEsch, L., & Schaffrath, U. (2017). An Update on Jacalin-Like Lectins and Their Role in Plant Defense. International Journal of Molecular Sciences, 18(7), 1592. https://doi.org/10.3390/ijms18071592





