A Membrane-Fusion Model That Exploits a β-to-α Transition in the Hydrophobic Domains of Syntaxin 1A and Synaptobrevin 2
Abstract
:1. Introduction
2. Description of the BAT Models
2.1. The BAT Models: Assumptions
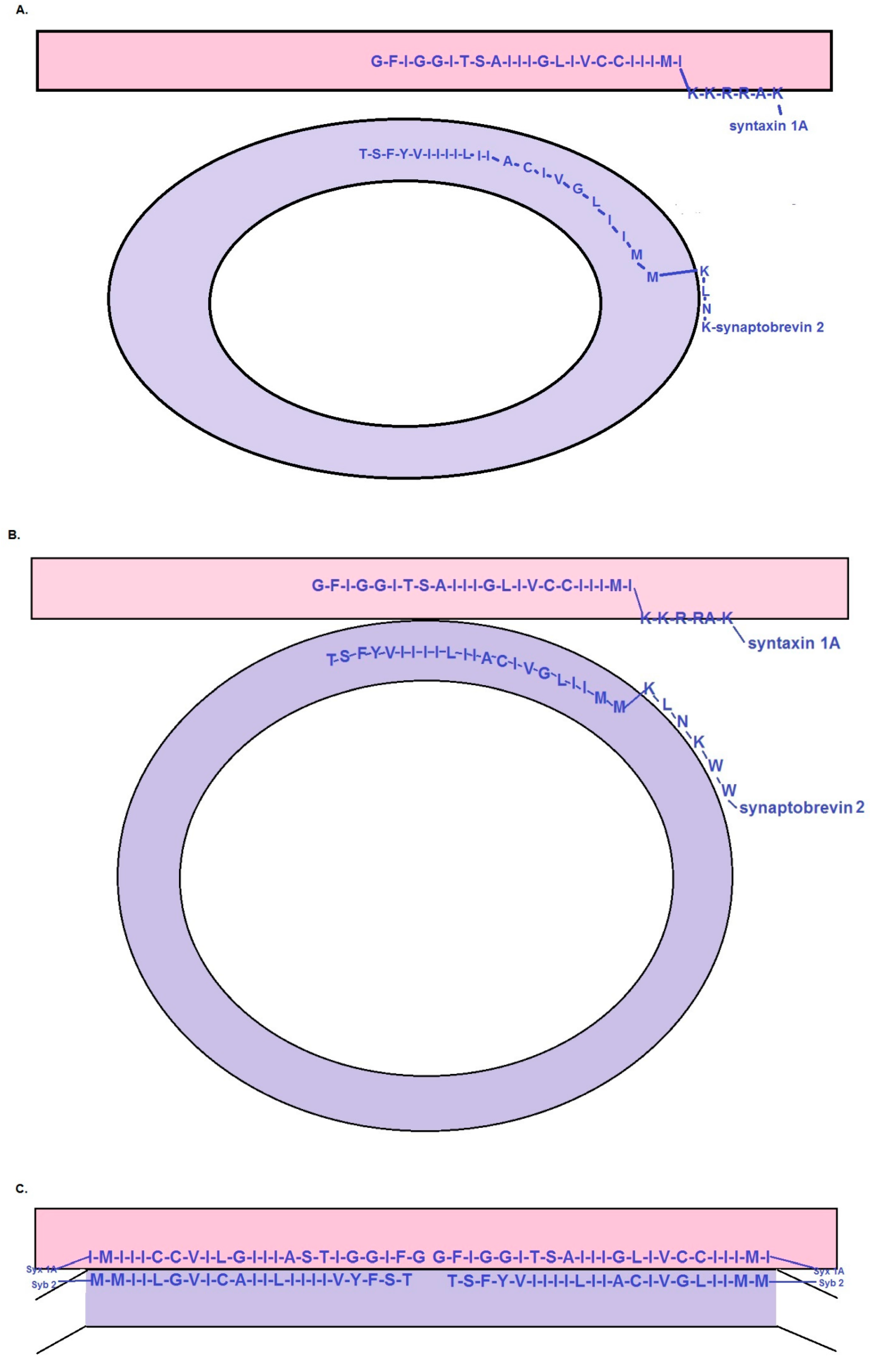
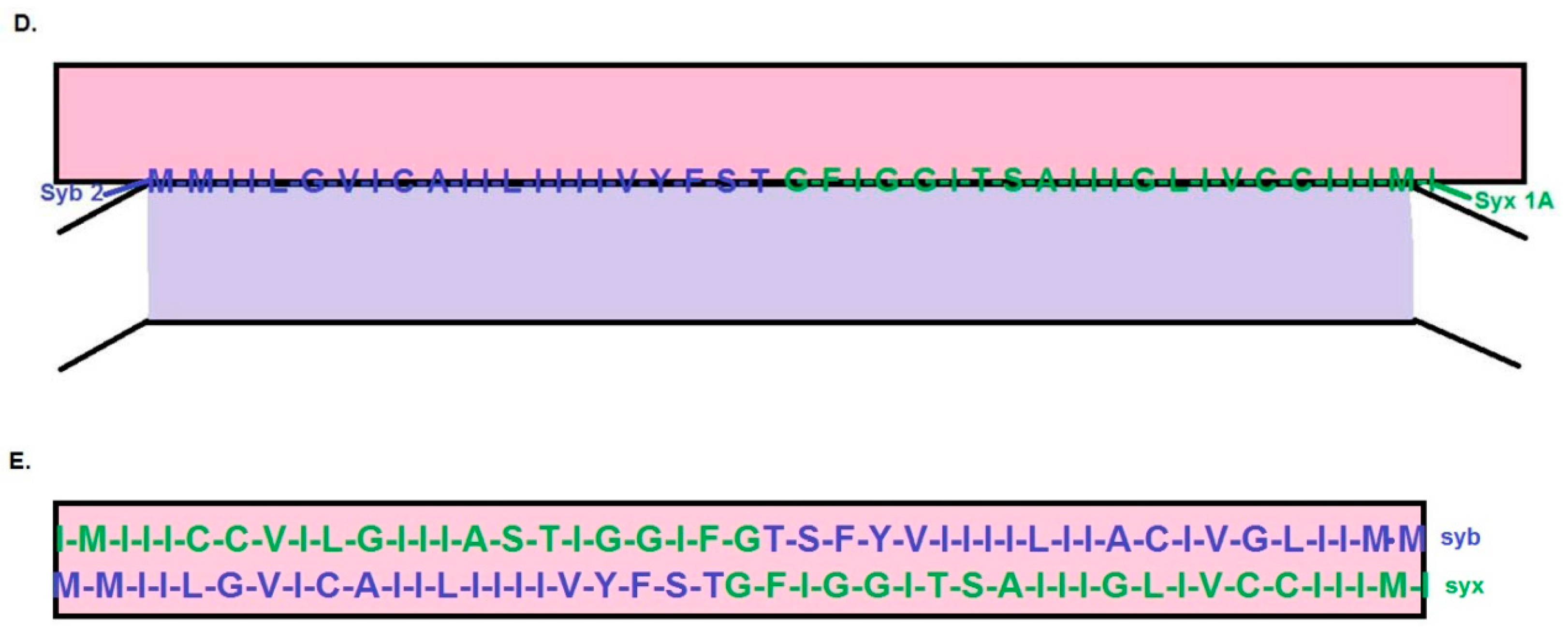
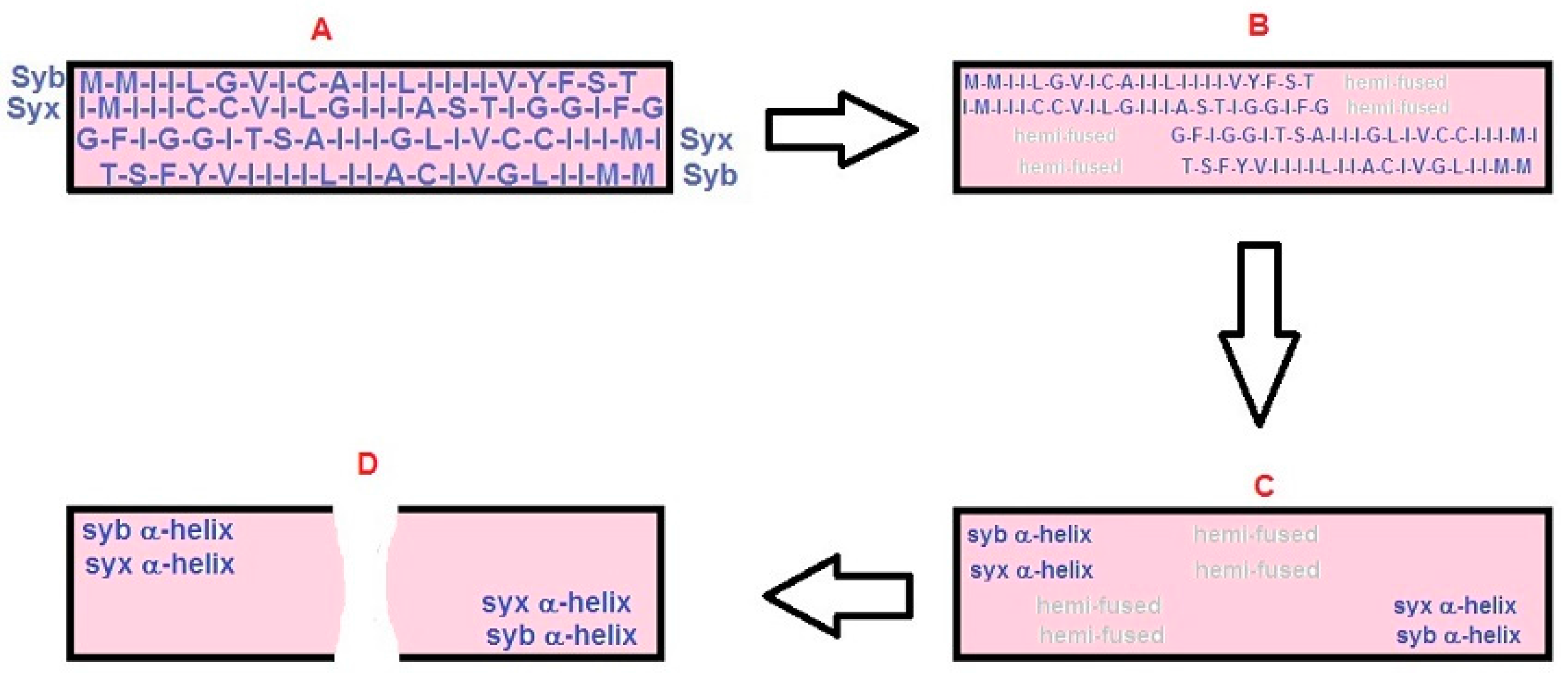
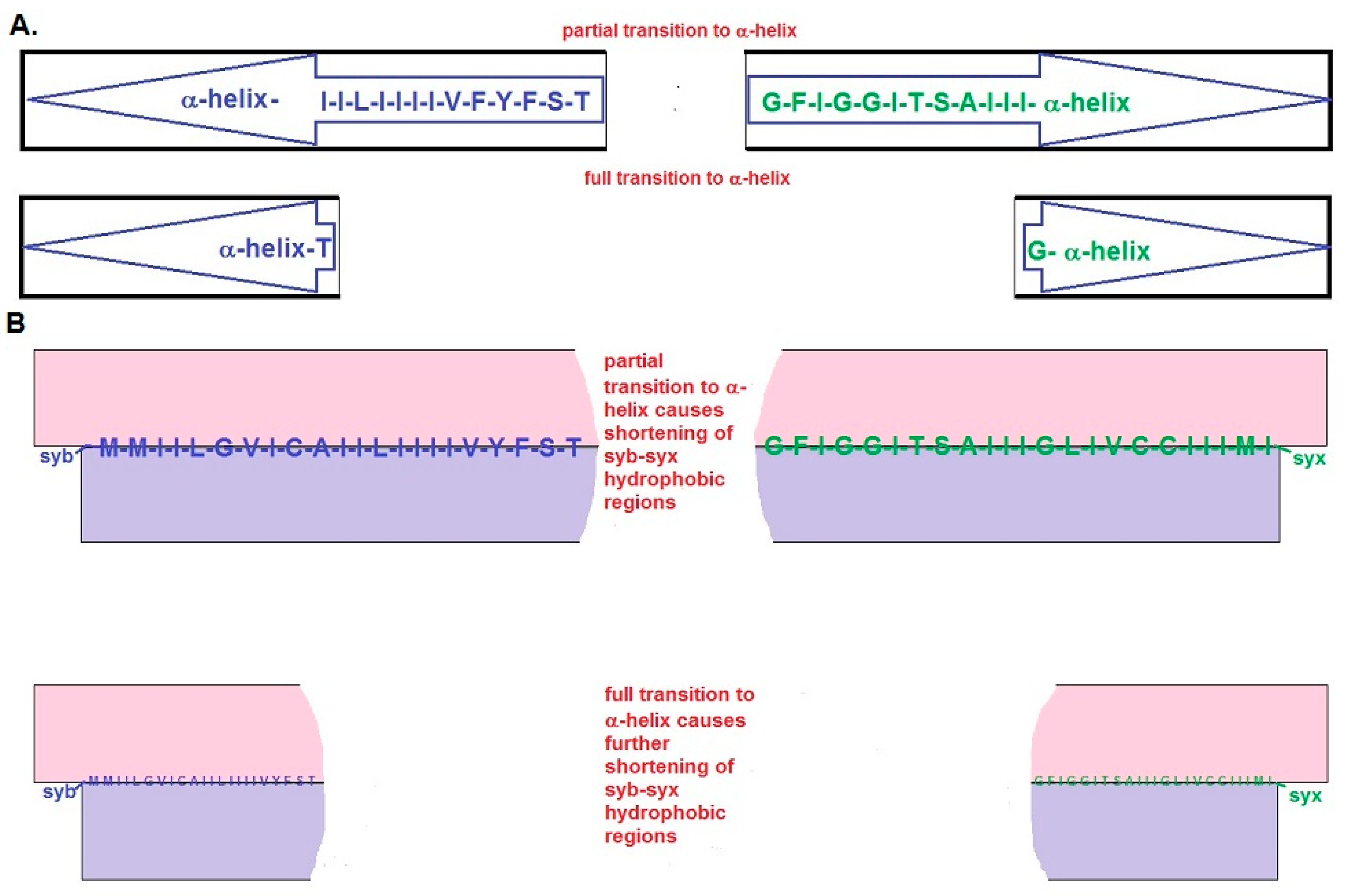
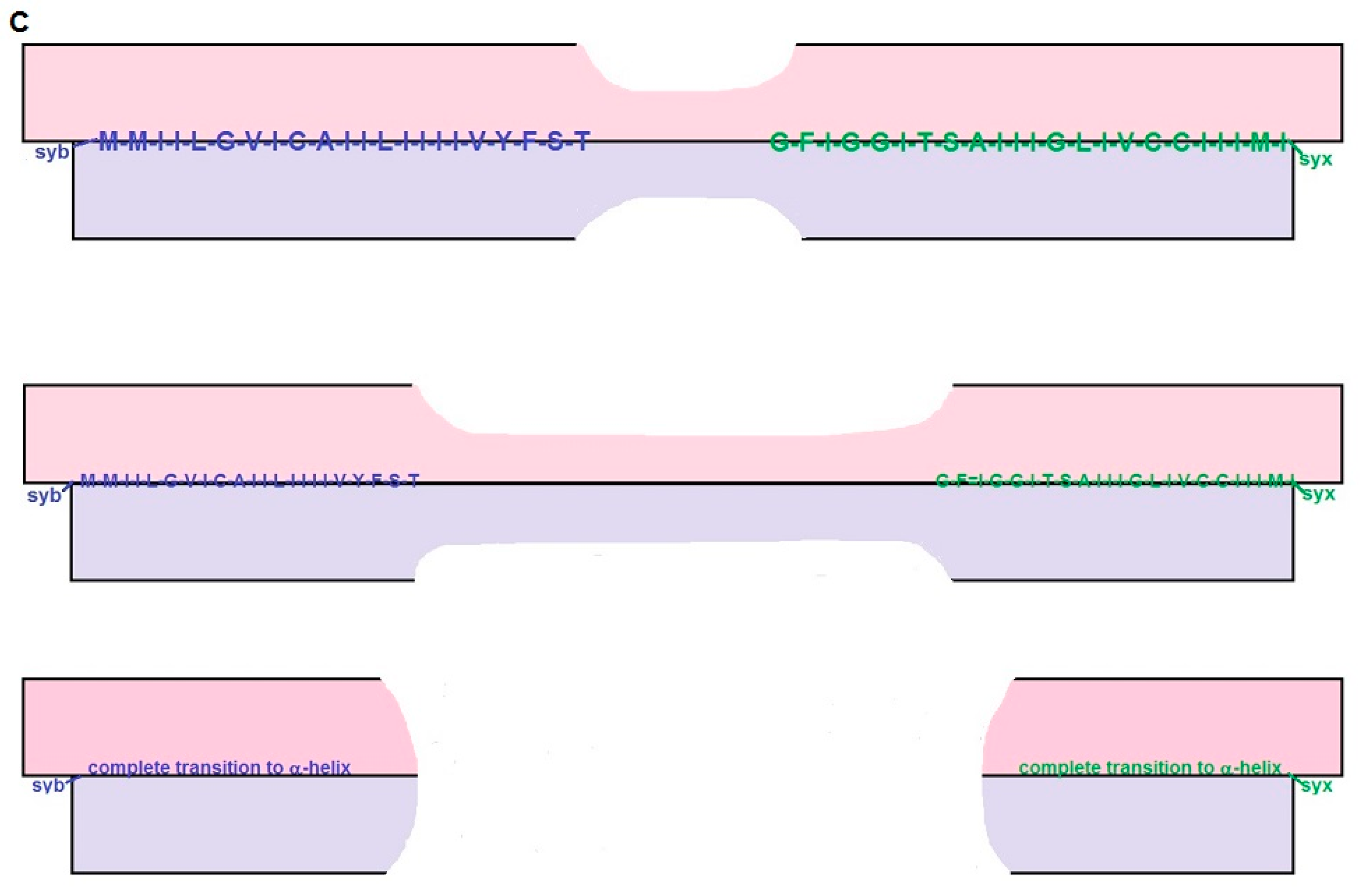
2.2. The “BAT” Model: Step-by-Step Description
2.3. The Short BAT Model: Step-by-Step Description
3. Discussion
Acknowledgments
Conflicts of Interest
Abbreviations
| Syb | Synaptobrevin |
| Syx | Syntaxin |
| SNAP-25 | Synaptosome-associated protein of 25 kDa |
| SNARE | Soluble, N-ethylmaleimide sensitive factor attachment protein receptor |
| BAT | β-to-α transition |
References
- Söllner, T.; Whiteheart, S.W.; Brunner, M.; Erdjument-Bromage, H.; Geromanos, S.; Tempst, P.; Rothman, J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993, 362, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Söllner, T.; Bennett, M.K.; Whiteheart, S.W.; Scheller, R.H.; Rothman, J.E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 1993, 75, 409–418. [Google Scholar] [CrossRef]
- Hanson, P.; Heuser, J.E.; Jahn, R. Neurotransmitter release—four years of SNARE complexes. Curr. Opin. Neurobiol. 1997, 7, 10–15. [Google Scholar] [CrossRef]
- Hanson, P.I.; Roth, R.; Morisaki, H.; Jahn, R.; Heuser, J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 1997, 90, 523–525. [Google Scholar] [CrossRef]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [PubMed]
- Meriney, S.D.; Umbach, J.A.; Gundersen, C.B. Fast, Ca2+-dependent exocytosis at nerve terminals: Shortcomings of SNARE-based models. Prog. Neurobiol. 2014, 121, 55–90. [Google Scholar] [CrossRef] [PubMed]
- Brunger, A.T.; Cipriano, D.J.; Diao, J. Towards reconstitution of membrane fusion mediated by SNAREs and other synaptic proteins. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Shin, Y.K. SNARE zippering. Biosci. Rep. 2016, 36, e00327. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Liang, B.; Kreutzberger, A.J.; Tamm, L.K. Planar supported membranes with mobile SNARE proteins and quantitative fluorescence microscopy assays to study synaptic vesicle fusion. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Fasshauer, D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Lindau, M. How could SNARE proteins open a fusion pore? Physiology 2014, 29, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Xu, J. The synaptic vesicle release machinery. Annu. Rev. Biophys. 2015, 44, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Wickner, W.; Rizo, J. A cascade of multiple proteins and lipids catalyzes membrane fusion. Mol. Biol. Cell 2017, 28, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Chiang, C.W.; Jackson, M.B. Fusion pores and their control of neurotransmitter and hormone release. J. Gen. Physiol. 2017, 149, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Kweon, D.H.; Kong, B.; Shin, Y.K. Hemifusion in synaptic vesicle cycle. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, C.B. The structure of the synaptic vesicle-plasma membrane interface constrains SNARE models of rapid, synchronous exocytosis at nerve terminals. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Szule, J.A.; Harlow, M.L.; Jung, J.H.; De-Miguel, F.F.; Marshall, R.M.; McMahan, U.J. Regulation of synaptic vesicle docking by different classes of macromolecules in active zone material. PLoS ONE 2012, 7, e33333. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.A.; Chen, X.; Reese, T.S. A network of three types of filaments organizes synaptic vesicles for storage, mobilization, and docking. J. Neurosci. 2016, 36, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, C.B.; Umbach, J.A. Synaptotagmins 1 and 2 as mediators of rapid exocytosis at nerve terminals: The dyad hypothesis. J. Theor. Biol. 2013, 332, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Trimble, W.S.; Cowan, D.M.; Scheller, R.H. VAMP-1: A synaptic vesicle-associated integral membrane protein. Proc. Natl. Acad. Sci. USA 1988, 85, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.E. The principle of membrane fusion in the cell (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12676–12694. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12696–12717. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Weber, G.; Wahl, M.C.; Jahn, R. Helical extension of the neuronal SNARE complex into the membrane. Nature 2009, 460, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Scheller, R. SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell. Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Veit, M.; Becher, A.; Ahnert-Hilger, G. Synaptobrevin 2 is palmitoylated in synaptic vesicles prepared from adult, but not from embryonic brain. Mol. Cell. Neurosci. 2000, 15, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Wan, J.; Arstikaitis, P.; Takahashi, H.; Huang, K.; Bailey, A.O.; Thompson, J.X.; Roth, A.F.; Drisdel, R.C.; Mastro, R.; et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 2008, 456, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C.; Baumert, M.; Perin, M.S.; Jahn, R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron 1989, 2, 1475–1481. [Google Scholar] [CrossRef]
- Bennett, M.K.; Calakos, N.; Scheller, R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992, 257, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.; Brunger, A.T. Conformation of the synaptobrevin transmembrane domain. Proc. Natl. Acad. Sci. USA 2006, 103, 8378–8383. [Google Scholar] [CrossRef] [PubMed]
- Langosch, D.; Crane, J.M.; Brosig, B.; Hellwig, A.; Tamm, L.K.; Reed, J. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J. Mol. Biol. 2001, 311, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ellena, J.F.; Liang, B.; Wiktor, M.; Stein, A.; Cafiso, D.S.; Jahn, R.; Tamm, L.K. Dynamic structure of lipid-bound synaptobrevin suggests a nucleation-propagation mechanism for trans-SNARE complex formation. Proc. Natl. Acad. Sci. USA 2009, 106, 20306–20311. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.E.; Arcario, M.J.; Schulten, K.; Tajkhorshid, E. A highly tilted membrane configuration for the prefusion state of synaptobrevin. Biophys. J. 2014, 107, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M.; Yarzagaray, A.; Makke, M.; Schindeldecker, B.; Schwarz, Y.; Shaaban, A.; Sharma, S.; Böckmann, R.A.; Lindau, M.; Mohrmann, R.; et al. v-SNARE transmembrane domains function as catalysts for vesicle fusion. eLife 2016, 5, e17571. [Google Scholar] [CrossRef] [PubMed]
- Ngatchou, A.N.; Kisler, K.; Fang, Q.; Walter, A.M.; Zhao, Y.; Bruns, D.; Sørensen, J.B.; Lindau, M. Role of the synaptobrevin C terminus in fusion pore formation. Proc. Natl. Acad. Sci. USA 2010, 107, 18463–18468. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Szule, J.A.; Marshall, R.M.; McMahan, U.J. Variable priming of a docked synaptic vesicle. Proc. Natl. Acad. Sci. USA 2016, 113, E1098–E1107. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Kreutzberger, A.J.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zampighi, G.A.; Zampighi, L.M.; Fain, N.; Lanzavecchia, S.; Simon, S.A.; Wright, E.M. Conical electron tomography of a chemical synapse: Vesicles docked to the active zone are hemi-fused. Biophys. J. 2006, 91, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, B.L.; Regehr, W.G. Timing of neurotransmission at fast synapses in the mammalian brain. Nature 1996, 384, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zorman, S.; Gundersen, G.; Xi, Z.; Ma, L.; Sirinakis, G.; Rothman, J.E.; Zhang, Y. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science 2012, 337, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Kim, K.; Hyeon, C.; Cho, Y.H.; Shin, Y.K.; Yoon, T.Y. Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism. Nat. Commun. 2013, 4, 1705. [Google Scholar] [CrossRef] [PubMed]
- Zorman, S.; Rebane, A.A.; Ma, L.; Yang, G.; Molski, M.A.; Coleman, J.; Pincet, F.; Rothman, J.E.; Zhang, Y. Common intermediates and kinetics, but different energetics, in the assembly of SNARE proteins. eLife 2014, 3, e03348. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, J.; Hofrichter, J.; Eaton, W.A. The protein folding “speed limit”. Curr. Opin. Struct. Biol. 2004, 14, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Mohrmann, R.; Dhara, M.; Bruns, D. Complexins: Small but capable. Cell. Mol. Life Sci. 2015, 72, 4221–4235. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Busnadiego, R.; Zuber, B.; Maurer, U.E.; Cyrklaff, M.; Baumeister, W.; Lucic, V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J. Cell Biol. 2010, 188, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.J.; Vites, O.; Stein, A.; Heintzmann, R.; Jahn, R.; Fasshauer, D. Determinants of synaptobrevin regulation in membranes. Mol. Biol. Cell 2007, 18, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.L.; Dong, M.; Earles, C.A.; Chapman, E.R. The transmembrane domain of syntaxin 1A is critical for cytoplasmic domain protein-protein interactions. J. Biol. Chem. 2001, 276, 15458–15465. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; McMahon, H.T. Mechanisms of membrane fusion: Disparate players and common principles. Nat. Rev. Mol. Cell. Biol. 2008, 9, 543–556. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundersen, C.B. A Membrane-Fusion Model That Exploits a β-to-α Transition in the Hydrophobic Domains of Syntaxin 1A and Synaptobrevin 2. Int. J. Mol. Sci. 2017, 18, 1582. https://doi.org/10.3390/ijms18071582
Gundersen CB. A Membrane-Fusion Model That Exploits a β-to-α Transition in the Hydrophobic Domains of Syntaxin 1A and Synaptobrevin 2. International Journal of Molecular Sciences. 2017; 18(7):1582. https://doi.org/10.3390/ijms18071582
Chicago/Turabian StyleGundersen, Cameron B. 2017. "A Membrane-Fusion Model That Exploits a β-to-α Transition in the Hydrophobic Domains of Syntaxin 1A and Synaptobrevin 2" International Journal of Molecular Sciences 18, no. 7: 1582. https://doi.org/10.3390/ijms18071582
APA StyleGundersen, C. B. (2017). A Membrane-Fusion Model That Exploits a β-to-α Transition in the Hydrophobic Domains of Syntaxin 1A and Synaptobrevin 2. International Journal of Molecular Sciences, 18(7), 1582. https://doi.org/10.3390/ijms18071582



