Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities
Abstract
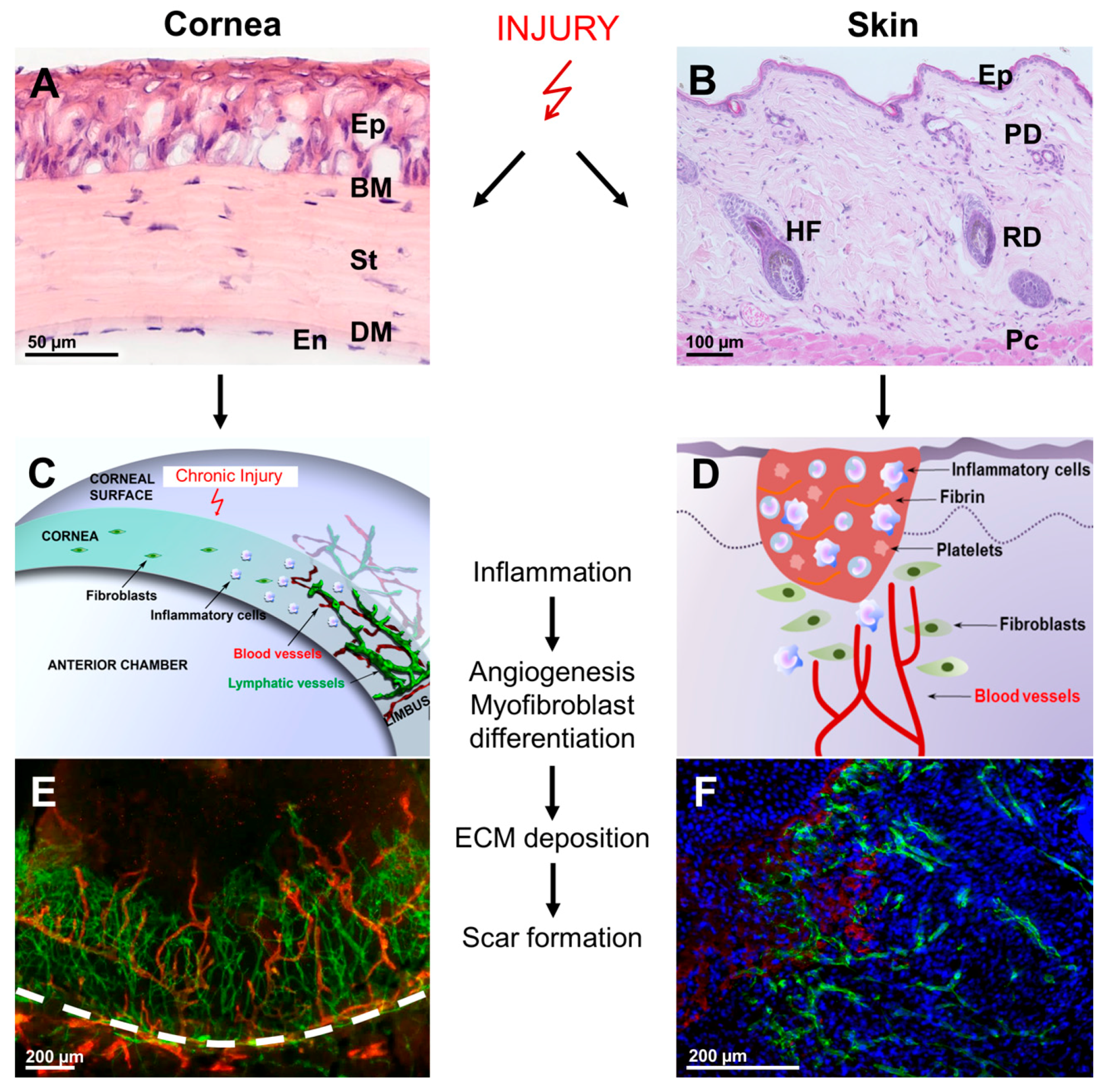
:1. Introduction
2. Corneal Anatomy
3. Distinct Regenerative Capacities of the Different Cellular Corneal Layers
4. The Corneal Angiogenic and Immune Privilege
5. Immune Cells in the Resting Cornea
6. Corneal Wound Healing
6.1. Epithelial Healing
6.2. Stromal Healing
6.3. Endothelial Damage Response
7. The Fibrotic Response in Corneal Repair
8. Cellular Events in Corneal Inflammation
9. Stem Cells in Corneal Regeneration
10. Concluding Remarks and Future Directions
Acknowledgments
Conflicts of Interest
References
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kang, Y.G.; Kim, H.J. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: Morphological changes during the repair process. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 537–545. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Gritz, D.C.; Lee, T.Y.; Kwitko, S.; McDonnell, P.J. Topical anti-inflammatory agents in an animal model of microbial keratitis. Arch. Ophthalmol. 1990, 108, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Tuli, S.S. Topical Corticosteroids in the Management of Bacterial Keratitis. Curr. Ophthalmol. Rep. 2013, 1, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Klenkler, B.; Sheardown, H.; Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 2007, 5, 228–239. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Kabosova, A.; Azar, D.T.; Bannikov, G.A.; Campbell, K.P.; Durbeej, M.; Ghohestani, R.F.; Jones, J.C.R.; Kenney, M.C.; Koch, M.; Ninomiya, Y.; et al. Compositional differences between infant and adult human corneal basement membranes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4989–4999. [Google Scholar] [CrossRef] [PubMed]
- Herwig, M.C.; Müller, A.M.; Holz, F.G.; Loeffler, K.U. Immunolocalization of different collagens in the cornea of human fetal eyes: A developmental approach. Curr. Eye Res. 2013, 38, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Michael, A.F.; Sun, T.T.; Kenney, M.C. Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Investig. 1995, 72, 461–473. [Google Scholar] [PubMed]
- Marshall, G.E.; Konstas, A.G.; Lee, W.R. Immunogold fine structural localization of extracellular matrix components in aged human cornea. II. Collagen types V and VI. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991, 229, 164–171. [Google Scholar] [CrossRef]
- Newsome, D.A.; Foidart, J.M.; Hassell, J.R.; Krachmer, J.H.; Rodrigues, M.M.; Katz, S.I. Detection of specific collagen types in normal and keratoconus corneas. Investig. Ophthalmol. Vis. Sci. 1981, 20, 738–750. [Google Scholar]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.F.; Galbavy, E.J. Connective tissue remodeling in corneal and scleral wounds. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1478–1484. [Google Scholar]
- Fini, M.E.; Stramer, B.M. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Radtke, F. Corneal epithelial stem cells and their niche at a glance. J. Cell Sci. 2017, 130, 1021–1025. [Google Scholar] [PubMed]
- Nowell, C.S.; Odermatt, P.D.; Azzolin, L.; Hohnel, S.; Wagner, E.F.; Fantner, G.E.; Lutolf, M.P.; Barrandon, Y.; Piccolo, S.; Radtke, F. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 2016, 18, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Cintron, C.; Covington, H.I.; Kublin, C.L. Morphologic analyses of proteoglycans in rabbit corneal scars. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1789–1798. [Google Scholar]
- Jumblatt, M.M.; Willer, S.S. Corneal endothelial repair. Regulation of prostaglandin E2 synthesis. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1294–1301. [Google Scholar]
- Albuquerque, R.J.C.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.C.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; Ambati, B.K. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Gabison, E.; Chang, J.-H.; Hernández-Quintela, E.; Javier, J.; Lu, P.C.S.; Ye, H.; Kure, T.; Kato, T.; Azar, D.T. Anti-angiogenic role of angiostatin during corneal wound healing. Exp. Eye Res. 2004, 78, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1117–1124. [Google Scholar] [CrossRef]
- Lin, H.C.; Chang, J.H.; Jain, S.; Gabison, E.E.; Kure, T.; Kato, T.; Fukai, N.; Azar, D.T. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2517–2524. [Google Scholar]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Cao, R.; Svensson, K.; Bertilsson, G.; Asman, M.; Tanaka, H.; Cao, Y.; Berkenstam, A.; Poellinger, L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001, 414, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Küchle, M.; Naumann, G.O. Angiogenesis in corneal diseases: Histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea 1998, 17, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Schlötzer-Schrehardt, U.; Küchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2127–2135. [Google Scholar]
- Bock, F.; Maruyama, K.; Regenfuss, B.; Hos, D.; Steven, P.; Heindl, L.M.; Cursiefen, C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013, 34, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Immune privilege in the anterior chamber of the eye. Crit. Rev. Immunol. 2002, 22, 13–46. [Google Scholar] [CrossRef] [PubMed]
- Kezuka, T.; Streilein, J.W. Evidence for multiple CD95-CD95 ligand interactions in anteriorchamber-associated immune deviation induced by soluble protein antigen. Immunology 2000, 99, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, P.; Li, B.; Zhou, H.; Huang, X.; Zhu, L.; Ren, Y.; Kijlstra, A. CD4+PD-1+ T cells acting as regulatory cells during the induction of anterior chamber-associated immune deviation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W.; Masli, S.; Takeuchi, M.; Kezuka, T. The eye’s view of antigen presentation. Hum. Immunol. 2002, 63, 435–443. [Google Scholar] [CrossRef]
- Skelsey, M.E.; Mayhew, E.; Niederkorn, J.Y. CD25+, interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology 2003, 110, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K.-H.; Stein-Streilein, J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur. J. Immunol. 2002, 32, 848–857. [Google Scholar] [CrossRef]
- Lin, H.-H.; Faunce, D.E.; Stacey, M.; Terajewicz, A.; Nakamura, T.; Zhang-Hoover, J.; Kerley, M.; Mucenski, M.L.; Gordon, S.; Stein-Streilein, J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 2005, 201, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W.; Niederkorn, J.Y. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J. Exp. Med. 1981, 153, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 2007, 92, 50–57. [Google Scholar] [PubMed]
- Niederkorn, J.Y. High-risk corneal allografts and why they lose their immune privilege. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Cursiefen, C. Lymphatic vessels in the development of tissue and organ rejection. Adv. Anat. Embryol. Cell Biol. 2014, 214, 119–141. [Google Scholar] [PubMed]
- Niederkorn, J.Y. The immune privilege of corneal allografts. Transplantation 1999, 67, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Chinnery, H.R.; Humphries, T.; Clare, A.; Dixon, A.E.; Howes, K.; Moran, C.B.; Scott, D.; Zakrzewski, M.; Pearlman, E.; McMenamin, P.G. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology 2008, 125, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, Y.; Dong, D.; Xiao, C.; Lin, C.; Wang, H.; Song, F.; Fu, T.; Wang, Z.; Chen, J.; et al. CCR2− and CCR2+ corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Zhang, Q.; Liu, Y.; Dana, M.R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 639–646. [Google Scholar]
- Chen, W.; Hara, K.; Tian, Q.; Zhao, K.; Yoshitomi, T. Existence of small slow-cycling Langerhans cells in the limbal basal epithelium that express ABCG2. Exp. Eye Res. 2007, 84, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef]
- Nakamura, T.; Ishikawa, F.; Sonoda, K.-H.; Hisatomi, T.; Qiao, H.; Yamada, J.; Fukata, M.; Ishibashi, T.; Harada, M.; Kinoshita, S. Characterization and distribution of bone marrow-derived cells in mouse cornea. Investig. Ophthalmol. Vis. Sci. 2005, 46, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Ruitenberg, M.J.; Plant, G.W.; Pearlman, E.; Jung, S.; McMenamin, P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Koga, A.; Kikuta, J.; Yamada, K.; Kojima, S.; Shinomiya, K.; Ishii, M.; Kinoshita, S. Intravital imaging of the cellular dynamics of LysM-positive cells in a murine corneal suture model. Br. J. Ophthalmol. 2016, 100, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, T.; Song, F.; Xue, Y.; Xia, C.; Liu, P.; Wang, H.; Zhong, J.; Li, Q.; Chen, J.; et al. Mast cells participate in corneal development in mice. Sci. Rep. 2015, 5, 17569. [Google Scholar] [CrossRef] [PubMed]
- Mott, K.R.; Osorio, Y.; Brown, D.J.; Morishige, N.; Wahlert, A.; Jester, J.V.; Ghiasi, H. The corneas of naive mice contain both CD4+ and CD8+ T cells. Mol. Vis. 2007, 13, 1802–1812. [Google Scholar] [PubMed]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Ahmadi, A.J.; Jakobiec, F.A. Corneal wound healing: Cytokines and extracellular matrix proteins. Int. Ophthalmol. Clin. 2002, 42, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-S.X.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D. Extracellular matrix and wound healing. Curr. Opin. Ophthalmol. 2001, 12, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C. Hemidesmosomes of normal and regenerating mouse corneal epithelium. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1982, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Giammaria, D.; Trabucco, P. Corneal wound healing after laser vision correction. Br. J. Ophthalmol. 2016, 100, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell. Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chikama, T.; Nishida, T. Up-regulation of integrin α 5 expression by combination of substance P and insulin-like growth factor-1 in rabbit corneal epithelial cells. Biochem. Biophys. Res. Commun. 1998, 246, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Mikulec, A.A.; Tanelian, D.L. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J. Ocul. Pharmacol. Ther. 1996, 12, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bonini, S.; Aloe, L.; Rama, P.; Bonini, S. Anti-inflammatory and healing properties of nerve growth factor in immune corneal ulcers with stromal melting. Arch. Ophthalmol. 2000, 118, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Rama, P.; Bonini, S.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998, 338, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.C.; Calonge, M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea 1992, 11, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Meduri, A.; Aragona, P.; Grenga, P.L.; Roszkowska, A.M. Effect of basic fibroblast growth factor on corneal epithelial healing after photorefractive keratectomy. J. Refract. Surg. 2012, 28, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Matsuda, R.; Morishige, N.; Yanai, R.; Chikama, T.-I.; Nishida, T.; Ishimitsu, T.; Kamiya, A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br. J. Ophthalmol. 2008, 92, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; He, Y.G.; Weng, J.; Li, Q.; McDowall, A.W.; Vital, M.; Chwang, E.L. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res. 1996, 62, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Liang, Q.; Kim, W.J.; Helena, M.C.; Baerveldt, F.; Wilson, S.E. Apoptosis in the cornea: Further characterization of Fas/Fas ligand system. Exp. Eye Res. 1997, 65, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Mohan, R.R.; Kim, W.J.; Wilson, S.E. Modulation of TNF-α-induced apoptosis in corneal fibroblasts by transcription factor NF-κB. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1327–1336. [Google Scholar]
- Ambrósio, R.; Kara-José, N.; Wilson, S.E. Early keratocyte apoptosis after epithelial scrape injury in the human cornea. Exp. Eye Res. 2009, 89, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrósio, R.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Petroll, W.M.; Kivanany, P.B.; Hagenasr, D.; Graham, E.K. Corneal fibroblast migration patterns during intrastromal wound healing correlate with ECM structure and alignment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7352–7361. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.L.; Ledet, T.; Ehlers, N. Keratocyte migration and peptide growth factors: The effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-β on human keratocyte migration in a collagen gel. Curr. Eye Res. 1997, 16, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Barry-Lane, P.A.; Petroll, W.M.; Olsen, D.R.; Cavanagh, H.D. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF β in the rabbit. Cornea 1997, 16, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Huang, J.; Barry-Lane, P.A.; Kao, W.W.; Petroll, W.M.; Cavanagh, H.D. Transforming growth factor(β)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1959–1967. [Google Scholar]
- Jester, J.V.; Huang, J.; Petroll, W.M.; Cavanagh, H.D. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp. Eye Res. 2002, 75, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chaurasia, S.S.; de Medeiros, F.W.; Agrawal, V.; Salomao, M.Q.; Singh, N.; Ambati, B.K.; Wilson, S.E. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009, 88, 960–965. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bazan, H.E.P. Epidermal growth factor synergism with TGF-β1 via PI-3 kinase activity in corneal keratocyte differentiation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Garana, R.M.; Petroll, W.M.; Chen, W.T.; Herman, I.M.; Barry, P.; Andrews, P.; Cavanagh, H.D.; Jester, J.V. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3271–3282. [Google Scholar]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of α-smooth muscle (α-SM) actin during corneal stromal wound healing. Investig. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [PubMed]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Wilson, S.E. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp. Eye Res. 2014, 129, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Mezquita, J.T.; Hutcheon, A.E.K.; Zieske, J.D. Role of thrombospondin-1 in repair of penetrating corneal wounds. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.L.; Chaurasia, S.S.; Cutler, A.; Asosingh, K.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Direkze, N.C.; Forbes, S.J.; Brittan, M.; Hunt, T.; Jeffery, R.; Preston, S.L.; Poulsom, R.; Hodivala-Dilke, K.; Alison, M.R.; Wright, N.A. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 2003, 21, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Agrawal, V.; Santhiago, M.R.; Wilson, S.E. Stromal fibroblast-bone marrow-derived cell interactions: Implications for myofibroblast development in the cornea. Exp. Eye Res. 2012, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roy, O.; Leclerc, V.B.; Bourget, J.-M.; Thériault, M.; Proulx, S. Understanding the process of corneal endothelial morphological change in vitro. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Kaimori, A.; Potter, J.; Kaimori, J.; Wang, C.; Mezey, E.; Koteish, A. Transforming growth factor-β1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J. Biol. Chem. 2007, 282, 22089–22101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Ko, M.K.; Kay, E.P. Endothelial mesenchymal transformation mediated by IL-1β-induced FGF-2 in corneal endothelial cells. Exp. Eye Res. 2012, 95, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Kay, E.P. NF-κB is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Kay, E.P.; Ueno, M.; Sakamoto, Y.; Nakamura, S.; Hamuro, J.; Kinoshita, S. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Sumioka, T.; Saika, S. Endothelial mesenchymal transition: A therapeutic target in retrocorneal membrane. Cornea 2010, 29, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Kinoshita, S.; Koizumi, N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea 2014, 33, S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Sumioka, T.; Ikeda, K.; Okada, Y.; Yamanaka, O.; Kitano, A.; Saika, S. Inhibitory effect of blocking TGF-β/Smad signal on injury-induced fibrosis of corneal endothelium. Mol. Vis. 2008, 14, 2272–2281. [Google Scholar] [PubMed]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.L.; Ledet, T.; Hager, H.; Josephsen, K.; Ehlers, N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp. Eye Res. 2000, 71, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.R.; Cintron, C.; Kublin, C.; Newsome, D.A. Proteoglycan changes during restoration of transparency in corneal scars. Arch. Biochem. Biophys. 1983, 222, 362–369. [Google Scholar] [CrossRef]
- Cintron, C.; Gregory, J.D.; Damle, S.P.; Kublin, C.L. Biochemical analyses of proteoglycans in rabbit corneal scars. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1975–1981. [Google Scholar]
- Stramer, B.M.; Zieske, J.D.; Jung, J.-C.; Austin, J.S.; Fini, M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: Implications for surgical outcomes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4237–4246. [Google Scholar] [CrossRef]
- Stramer, B.M.; Austin, J.S.; Roberts, A.B.; Fini, M.E. Selective reduction of fibrotic markers in repairing corneas of mice deficient in Smad3. J. Cell. Physiol. 2005, 203, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rumbaut, R.E.; Burns, A.R.; Smith, C.W. Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Monroy, D.; Ji, Z.; Yoshino, K.; Huang, A.; Pflugfelder, S.C. Transforming growth factor β-1 and β-2 in human tear fluid. Curr. Eye Res. 1996, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Vesaluoma, M.; Teppo, A.M.; Grönhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Mohan, R.R.; Mohan, R.R.; Wilson, S.E. Effect of PDGF, IL-1α, and BMP2/4 on corneal fibroblast chemotaxis: Expression of the platelet-derived growth factor system in the cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1364–1372. [Google Scholar]
- Kamiyama, K.; Iguchi, I.; Wang, X.; Imanishi, J. Effects of PDGF on the migration of rabbit corneal fibroblasts and epithelial cells. Cornea 1998, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J.D. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, 40548. [Google Scholar] [CrossRef] [PubMed]
- Spurlin, J.W.; Lwigale, P.Y. Wounded embryonic corneas exhibit nonfibrotic regeneration and complete innervation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.-Y.; Yang, C.-C.; Chao, S.-C.; Wong, T.-W. Histopathological differential diagnosis of keloid and hypertrophic scar. Am. J. Dermatopathol. 2004, 26, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Baudrimont, M.; Boutboul, S.; Thomas, F.; Borderie, V.; Laroche, L. Corneal keloid: Clinical, ultrasonographic, and ultrastructural characteristics. J. Cataract Refract. Surg. 2004, 30, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, P.; Agarwal, D.R.; Fernandez, A.A.; Milman, T.; Glasgow, B.; Starr, C.E.; Aldave, A.J. Corneal keloid: Report of natural history and outcome of surgical management in two cases. Cornea 2013, 32, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Choi, H.J.; Kim, M.K.; Wee, W.R.; Oh, J.Y. Corneal keloid: Four case reports of clinicopathological features and surgical outcome. BMC Ophthalmol. 2016, 16, 198. [Google Scholar] [CrossRef] [PubMed]
- Holbach, L.M.; Font, R.L.; Shivitz, I.A.; Jones, D.B. Bilateral keloid-like myofibroblastic proliferations of the cornea in children. Ophthalmology 1990, 97, 1188–1193. [Google Scholar] [CrossRef]
- Mejía, L.F.; Acosta, C.; Santamaría, J.P. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea 2001, 20, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Wojno, T.H.; Grossniklaus, H.E. Giant corneal keloid: Case report and review of the literature. Cornea 2010, 29, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- McElvanney, A.M.; Adhikary, H.P. Corneal keloid: Aetiology and management in Lowe’s syndrome. Eye 1995, 9, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, S.; Eustis, H.S.; Bazan, H.E.; Leon, A.; He, J. Corneal keloid in Lowe syndrome. J. Pediatr. Ophthalmol. Strabismus 2005, 42, 308–310. [Google Scholar] [PubMed]
- Rao, S.K.; Fan, D.S.P.; Pang, C.P.; Li, W.W.Y.; Ng, J.S.K.; Good, W.V.; Lam, D.S.C. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein-Taybi syndrome. Cornea 2002, 21, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.D.; Greer, K.E. Rubinstein-Taybi syndrome with multiple flamboyant keloids. Cutis 1996, 57, 346–348. [Google Scholar] [PubMed]
- Van de Kar, A.L.; Houge, G.; Shaw, A.C.; de Jong, D.; van Belzen, M.J.; Peters, D.J.M.; Hennekam, R.C.M. Keloids in Rubinstein-Taybi syndrome: A clinical study. Br. J. Dermatol. 2014, 171, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Shilpashree, P.; Jaiswal, A.K.; Kharge, P.M. Keloids: An unwanted spontaneity in rubinstein-taybi syndrome. Indian J. Dermatol. 2015, 60, 214. [Google Scholar] [PubMed]
- Gipson, I.K.; Kiorpes, T.C. Epithelial sheet movement: Protein and glycoprotein synthesis. Dev. Biol. 1982, 92, 259–262. [Google Scholar] [CrossRef]
- Dana, M.R.; Streilein, J.W. Loss and restoration of immune privilege in eyes with corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2485–2494. [Google Scholar]
- Mahoney, J.M.; Waterbury, L.D. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr. Eye Res. 1985, 4, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Zieske, J.D.; Trinkaus-Randall, V.; Kyne, B.M.; Pal-Ghosh, S.; Tadvalkar, G.; Pajoohesh-Ganji, A. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014, 121, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Tadvalkar, G.; Jurjus, R.A.; Zieske, J.D.; Stepp, M.A. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 2008, 87, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Tellaetxe-Isusi, M.; Elner, V.; Strieter, R.M.; Lausch, R.N.; Oakes, J.E. Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Β-chemokine synthesis in corneal cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 987–996. [Google Scholar]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Stapleton, W.M.; Chaurasia, S.S.; Medeiros, F.W.; Mohan, R.R.; Sinha, S.; Wilson, S.E. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008, 86, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, C.; He, J.; Matsumoto, Y.; Kita, M.; Imanishi, J.; Kinoshita, S. Cytokine expression in the alkali-burned cornea. Curr. Eye Res. 1997, 16, 670–676. [Google Scholar] [CrossRef] [PubMed]
- B́rien, T.P.O.; Li, Q.; Ashraf, M.F.; Matteson, D.M.; Stark, W.J.; Chan, C.C. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch. Ophthalmol. 1998, 116, 1470–1474. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Smith, C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: Distinct adhesion molecule requirements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, G.; Bellner, L.; Halilovic, A.; Volti, G.L.; Drago, F.; Dunn, M.W.; Schwartzman, M.L. The role of neutrophils in corneal wound healing in HO-2 null mice. PLoS ONE 2011, 6, e21180. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Han, L.; Rumbaut, R.E.; Smith, C.W. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am. J. Pathol. 2011, 178, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fukuoka, S.; Karagianni, N.; Guaiquil, V.H.; Rosenblatt, M.I. Vascular endothelial growth factor promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea. FASEB J. 2013, 27, 2756–2767. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; DÁmore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Watari, K.; Nakao, S.; Fotovati, A.; Basaki, Y.; Hosoi, F.; Bereczky, B.; Higuchi, R.; Miyamoto, T.; Kuwano, M.; Ono, M. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-κB activation. Biochem. Biophys. Res. Commun. 2008, 377, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; van Rooijen, N.; Takenaka, H.; DÁmore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Barnett, F.H.; Rosenfeld, M.; Wood, M.; Kiosses, W.B.; Usui, Y.; Marchetti, V.; Aguilar, E.; Friedlander, M. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 2016, 6, 35569. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, B.; Jiang, H.; Wang, Y.; Qu, M.; Duan, H.; Zhou, Q.; Shi, W. Macrophage depletion impairs corneal wound healing after autologous transplantation in mice. PLoS ONE 2013, 8, e61799. [Google Scholar] [CrossRef] [PubMed]
- Bellner, L.; Marrazzo, G.; van Rooijen, N.; Dunn, M.W.; Abraham, N.G.; Schwartzman, M.L. Heme oxygenase-2 deletion impairs macrophage function: Implication in wound healing. FASEB J. 2015, 29, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Hamrah, P.; Miyamoto, K.; Miyazaki, D.; Dekaris, I.; Dawson, T.; Lu, B.; Gerard, C.; Dana, M.R. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, H.; Dong, N.; Sanae, T.; Liu, Z.; Yoshitomi, T. Cauterization of central cornea induces recruitment of major histocompatibility complex class II+ Langerhans cells from limbal basal epithelium. Cornea 2010, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Hassan, N.; Mehta, P.; Burns, A.R.; Tang, X.; Smith, C.W. NK cells are necessary for recovery of corneal CD11c+ dendritic cells after epithelial abrasion injury. J. Leukoc. Biol. 2013, 94, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yin, J.; Yoon, G.S.; Mi, Q.-S.; Yu, F.-S.X. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am. J. Pathol. 2011, 179, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Smith, C.W.; Zhang, W.; Burns, A.R.; Li, Z. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am. J. Pathol. 2012, 181, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Rumbaut, R.E.; Smith, C.W. Gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am. J. Pathol. 2007, 171, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Byeseda, S.E.; Burns, A.R.; Dieffenbaugher, S.; Rumbaut, R.E.; Smith, C.W.; Li, Z. ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am. J. Pathol. 2009, 175, 571–579. [Google Scholar] [CrossRef] [PubMed]
- B́rien, R.L.O.; Taylor, M.A.; Hartley, J.; Nuhsbaum, T.; Dugan, S.; Lahmers, K.; Aydintug, M.K.; Wands, J.M.; Roark, C.L.; Born, W.K. Protective role of gammadelta T cells in spontaneous ocular inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Spandau, U.H.M.; Toksoy, A.; Verhaart, S.; Gillitzer, R.; Kruse, F.E. High expression of chemokines Gro-α (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch. Ophthalmol. 2003, 121, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shen, L.; Chong, E.-M.; Hamrah, P.; Zhang, Q.; Chen, L.; Dana, M.R. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol. Vis. 2007, 13, 626–634. [Google Scholar] [PubMed]
- Oshima, T.; Sonoda, K.-H.; Tsutsumi-Miyahara, C.; Qiao, H.; Hisatomi, T.; Nakao, S.; Hamano, S.; Egashira, K.; Charo, I.F.; Ishibashi, T. Analysis of corneal inflammation induced by cauterisation in CCR2 and MCP-1 knockout mice. Br. J. Ophthalmol. 2006, 90, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, N.; Yamagami, S.; Yokoo, S.; Amano, S.; Murakami, A. Involvement of C-C chemokine ligand 2-CCR2 interaction in monocyte-lineage cell recruitment of normal human corneal stroma. J. Immunol. 2007, 178, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Brogelli, L.; Ziche, M.; Augustin, H.G. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int. J. Cancer 1999, 82, 765–770. [Google Scholar] [CrossRef]
- Goyal, S.; Chauhan, S.K.; Zhang, Q.; Dana, R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch. Ophthalmol. 2009, 127, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Shimizu, A.; Masuda, Y.; Nagasaka, S.; Fukuda, Y.; Takahashi, H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013, 19, 2135–2150. [Google Scholar] [PubMed]
- Dua, H.S.; Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef]
- Branch, M.J.; Hashmani, K.; Dhillon, P.; Jones, D.R.E.; Dua, H.S.; Hopkinson, A. Mesenchymal stem cells in the human corneal limbal stroma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5109–5116. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, M.L.; Du, Y.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005, 19, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- O’callaghan, A.R.; Daniels, J.T. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells 2011, 29, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; van Acker, S.I.; van Gerwen, V.; Ní Dhubhghaill, S.; Koppen, C.; Tassignon, M.-J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent stem cells in human corneal stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Sundarraj, N.; Funderburgh, M.L.; Harvey, S.A.; Birk, D.E.; Funderburgh, J.L. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Karamichos, D.; Funderburgh, M.L.; Hutcheon, A.E.K.; Zieske, J.D.; Du, Y.; Wu, J.; Funderburgh, J.L. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS ONE 2014, 9, e86260. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rnjak-Kovacina, J.; Du, Y.; Funderburgh, M.L.; Kaplan, D.L.; Funderburgh, J.L. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 2014, 35, 3744–3755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, H.; Li, X.; Yuan, X.; Zhang, L.; Zhao, S. Utilization of human limbal mesenchymal cells as feeder layers for human limbal stem cells cultured on amniotic membrane. J. Tissue Eng. Regen. Med. 2010, 4, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-T.; Chen, S.-Y.; Li, G.-G.; Tseng, S.C.G. Isolation and expansion of human limbal stromal niche cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hertsenberg, A.J.; Funderburgh, M.L.; Burrow, M.K.; Mann, M.M.; Du, Y.; Lathrop, K.L.; Syed-Picard, F.N.; Adams, S.M.; Birk, D.E.; et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014, 6, 266ra172. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.-Y.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Krishna, P.V.; Stratis, A.K.; Gopinathan, U. The value of corneal transplantation in reducing blindness. Eye 2005, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; de Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Harkin, D.G.; Foyn, L.; Bray, L.J.; Sutherland, A.J.; Li, F.J.; Cronin, B.G. Concise reviews: Can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells 2015, 33, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Takeda, K.; Inatomi, T.; Sotozono, C.; Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J. Ophthalmol. 2011, 95, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, H.; Kawasaki, S.; Yamasaki, K.; Ang, L.P.K.; Koizumi, N.; Nakamura, T.; Yokoi, N.; Komuro, A.; Inatomi, T.; Kinoshita, S. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3820–3827. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yokoo, S.; Mimura, T.; Usui, T.; Miyata, K.; Araie, M.; Yamagami, S.; Amano, S. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol. Vis. 2007, 13, 1138–1143. [Google Scholar] [PubMed]
- Ricardo, J.R.S.; Cristovam, P.C.; Filho, P.A.N.; Farias, C.C.; de Araujo, A.L.; Loureiro, R.R.; Covre, J.L.; de Barros, J.N.; Barreiro, T.P.; dos Santos, M.S.; et al. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 2013, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Saichanma, S.; Bunyaratvej, A.; Sila-Asna, M. In vitro transdifferentiation of corneal epithelial-like cells from human skin-derived precursor cells. Int. J. Ophthalmol. 2012, 5, 158–163. [Google Scholar] [PubMed]
- Meyer-Blazejewska, E.A.; Call, M.K.; Yamanaka, O.; Liu, H.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Kao, W.W. From hair to cornea: Toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011, 29, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Moldovan, N.I.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol. Vis. 2008, 14, 1064–1070. [Google Scholar] [PubMed]
- Syed-Picard, F.N.; Du, Y.; Lathrop, K.L.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L. Dental pulp stem cells: A new cellular resource for corneal stromal regeneration. Stem Cells Transl. Med. 2015, 4, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.W.; McGhee, C.N.J.; Cowan, C.A.; Davidson, A.J.; Holm, T.M.; Sherwin, T. Derivation of Corneal Keratocyte-Like Cells from Human Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0165464. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Chen, M.; Sun, X.; Ge, J. Differentiation of mouse induced pluripotent stem cells into corneal epithelial-like cells. Cell Biol. Int. 2013, 37, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Saghizadeh, M.; Ornelas, L.; Winkler, M.A.; Narwani, K.; Sahabian, A.; Funari, V.A.; Tang, J.; Spurka, L.; Punj, V.; et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl. Med. 2014, 3, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Wahlin, K.; Adams, S.M.; Birk, D.E.; Zack, D.J.; Chakravarti, S. Cornea organoids from human induced pluripotent stem cells. Sci. Rep. 2017, 7, 41286. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Liao, Y.-W.; Liu, D.-M.; Lin, H.-L.; Chen, S.-J.; Chen, H.-L.; Peng, C.-H.; Liang, C.-M.; Mou, C.-Y.; Chiou, S.-H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.P.; Lizier, N.F.; Maranduba, C.M.C.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.P.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; da Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.M.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar] [PubMed]
- Katikireddy, K.R.; Dana, R.; Jurkunas, U.V. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 2014, 32, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Roh, D.S.; Funderburgh, M.L.; Mann, M.M.; Marra, K.G.; Rubin, J.P.; Li, X.; Funderburgh, J.L. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol. Vis. 2010, 16, 1680–2689. [Google Scholar]
- Liu, H.; Zhang, J.; Liu, C.-Y.; Hayashi, Y.; Kao, W.W.-Y. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J. Cell. Mol. Med. 2012, 16, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, B.; Yüksel, N.; Çelik, O.S.; Subaşı, C.; Duruksu, G.; Unal, Z.S.; Yıldız, D.K.; Karaöz, E. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp. Eye Res. 2016, 151, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, Z.-R.; Su, W.-R.; Li, Y.-P.; Lin, M.-L.; Zhang, W.-X.; Liu, Y.; Wan, Q.; Liang, D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS ONE 2012, 7, e30842. [Google Scholar] [CrossRef] [PubMed]
- Almaliotis, D.; Koliakos, G.; Papakonstantinou, E.; Komnenou, A.; Thomas, A.; Petrakis, S.; Nakos, I.; Gounari, E.; Karampatakis, V. Mesenchymal stem cells improve healing of the cornea after alkali injury. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Zeppieri, M.; Salvetat, M.L.; Beltrami, A.P.; Cesselli, D.; Bergamin, N.; Russo, R.; Cavaliere, F.; Varano, G.P.; Alcalde, I.; Merayo, J.; et al. Human adipose-derived stem cells for the treatment of chemically burned rat cornea: Preliminary results. Curr. Eye Res. 2013, 38, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-F.; Lai, Y.-C.; Tai, C.-F.; Tsai, J.-L.; Hsu, H.-C.; Hsu, R.-F.; Lu, S.-N.; Feng, N.-H.; Chai, C.-Y.; Lee, C.-H. Effects of cultured human adipose-derived stem cells transplantation on rabbit cornea regeneration after alkaline chemical burn. Kaohsiung J. Med. Sci. 2013, 29, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Shimomura, N.; Usui, T.; Noda, Y.; Kaji, Y.; Yamgami, S.; Amano, S.; Miyata, K.; Araie, M. Magnetic attraction of iron-endocytosed corneal endothelial cells to Descemet’s membrane. Exp. Eye Res. 2003, 76, 745–751. [Google Scholar] [CrossRef]
- Patel, S.V.; Bachman, L.A.; Hann, C.R.; Bahler, C.K.; Fautsch, M.P. Human corneal endothelial cell transplantation in a human ex vivo model. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. https://doi.org/10.3390/ijms18061257
Bukowiecki A, Hos D, Cursiefen C, Eming SA. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. International Journal of Molecular Sciences. 2017; 18(6):1257. https://doi.org/10.3390/ijms18061257
Chicago/Turabian StyleBukowiecki, Anne, Deniz Hos, Claus Cursiefen, and Sabine A. Eming. 2017. "Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities" International Journal of Molecular Sciences 18, no. 6: 1257. https://doi.org/10.3390/ijms18061257
APA StyleBukowiecki, A., Hos, D., Cursiefen, C., & Eming, S. A. (2017). Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. International Journal of Molecular Sciences, 18(6), 1257. https://doi.org/10.3390/ijms18061257




