Legume Lectins: Proteins with Diverse Applications
Abstract
:1. Introduction
2. Structure of Legume Lectins
3. Specificity of Legume Lectins
4. Antimicrobial Activity
4.1. Bacteria
4.2. Fungi and Yeasts
4.3. Virus
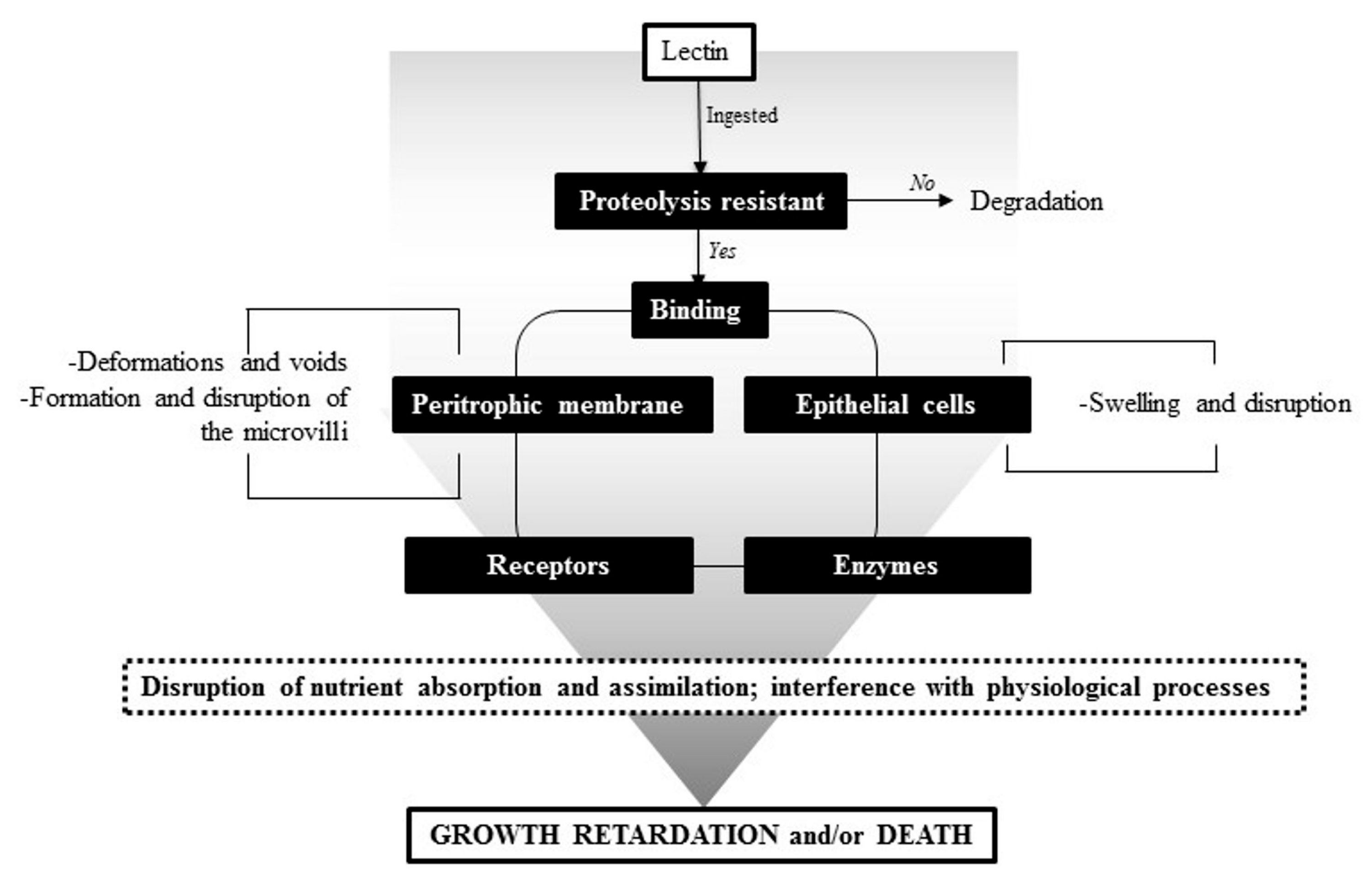
5. Insecticidal Activity
6. Antitumor Activity
7. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| CRD | Carbohydrate recognition domain |
| PM | Peritrophic membrane |
| PG | Peritrophic gel |
| PHA | Phaseolous vulgaris agglutinin |
| MAL-1 | Maackia amurensis lectin 1 |
| DCIS | Human breast ductal carcinoma in situ |
| GS IV | Griffonia simplicifolia IV lectin |
| MAH and MAL-1 | Maackia amurensis lectins |
| VLs | Acacia constricta isolectins |
| WGA | Wheat germ agglutinin |
| LCA | Lens culinaris agglutinin |
| PSA | Pisum sativum agglutinin |
| GS II | Griffonia simplicifolia II lectin |
| UEA | Ulex europeus agglutinin |
| ASAI and ASAII | Allium sativum L. bulbs |
| MuLL | Myracrodruon urundeuva leaf lectin |
| BmoLL | Bauhinia monandra leaf lectin |
| ASAL | Allium sativum Leaf Agglutinin |
| CEA | Colocasia esculenta tuber agglutinin |
| GS I | Griffonia simplicifolia lectin-I |
| VVA | Vicia vilosa agglutinin |
References
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53r–62r. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Masood, A.; Wani, I.H.; Rafiq, S. Lectins: Proteins with diverse applications. J. Appl. Pharm. Sci. 2013, 3, S93–S103. [Google Scholar]
- Loris, R.; Hamelryck, T.; Bouckaert, J.; Wyns, L. Legume lectin structure. BBA-Protein Struct. Mol. Enzymol. 1998, 1383, 9–36. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Legume lectins a large family of homologous proteins. FASEB J. 1990, 4, 3198–3208. [Google Scholar] [PubMed]
- Vázquez-Moreno, L.; Ortega-Nieblas, M.; Robles-Burgueño, M.R.; Ramos-Clamont, G. Purification of complex carbohydrate specific lectins from Olneya tesota seeds using tandem affinity chromatography. Int. J. Biochromatogr. 2000, 5, 83–90. [Google Scholar]
- Guzman-Partida, A.M.; Robles-Burgueno, M.R.; Ortega-Nieblas, M.; Vazquez-Moreno, I. Purification and characterization of complex carbohydrate specific isolectins from wild legume seeds: Acacia constricta is (vinorama) highly homologous to Phaseolus Vulgaris lectins. Biochimie 2004, 86, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Nasi, A.; Picariello, G.; Ferranti, P. Proteomic approaches to study structure, functions and toxicity of legume seeds lectins. Perspectives for the assessment of food quality and safety. J. Proteom. 2009, 72, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Lagarda-Diaz, I.; Guzman-Partida, A.M.; Urbano-Hernandez, G.; Ortega-Neblas, M.M.; Robles-Burgueno, M.R.; Winzerling, J.; Vazquez-Moreno, L. Insecticidal action of PF2 lectin from Olneya tesota (palo fierro) against Zabrotes subfasciatus larvae and midgut glycoconjugate binding. J. Agric. Food Chem. 2009, 57, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Surolia, A. Analyses of carbohydrate recognition by legume lectins: Size of the combining site loops and their primary specificity. J. Mol. Biol. 1997, 267, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Hamelryck, T.W.; Loris, R.; Bouckaert, J.; Doa-Thi, M.H.; Strecker, G.; Imberty, A.; Fernandez, E.; Wyns, L.; Etzer, M.E. Carbohydrate binding, quaternary structure and a novel hydrophobic binding site in two legume lectin oligomers from Dolichos biflorus. J. Mol. Biol. 1999, 288, 1037. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Chandra, N. Lectins. Curr. Opin. Struct. Biol. 1999, 9, 707–714. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. How proteins bind carbohydrates: Lessons from legume lectins. J. Agric. Food Chem. 2002, 50, 6586–6591. [Google Scholar] [CrossRef] [PubMed]
- Hamelryck, T.W.; Loris, R.; Bouckaert, J.; Wyns, L. Structural features of the legume lectins. Trends Glycosci. Glycotechnol. 1998, 10, 349–360. [Google Scholar] [CrossRef]
- Bouckaert, J.; Hamelryck, T.; Wyns, L.; Loris, R. Novel structures of plant lectins and their complexes with carbohydrates. Curr. Opin. Struct. Biol. 1999, 9, 572–577. [Google Scholar] [CrossRef]
- Buts, L.; Dao-Thi, M.H.; Loris, R.; Wyns, L.; Etzler, M.; Hamelryck, T. Weak protein–protein interactions in lectins: The crystal structure of a vegetative lectin from the legume Dolichos biflorus. J. Mol. Biol. 2001, 309, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Brinda, K.V.; Mitra, N.; Surolia, A.; Vishveshwara, S. Determinants of quaternary association in legume lectins. Protein Sci. 2004, 13, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Etzler, M.E.; Surolia, A.; Cummings, R.D. L-type lectins. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R., Esko, J., Freeze, H., Stanley, P., Bertozzi, C., Hart, G., Etzler, M., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; Chapter 28; pp. 403–414. [Google Scholar]
- Ambrosi, M.; Cameron, N.R.; Davis, B.G. Lectins: Tools for the molecular understanding of the glycocode. Org. Biomol. Chem. 2005, 3, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Manoj, N.; Suguna, K. Signature of quaternary structure in the sequences of legume lectins. Protein Eng. 2001, 14, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, H.; Gabius, H.J. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconj. J. 2001, 18, 589–613. [Google Scholar] [CrossRef] [PubMed]
- Felsted, R.L.; Leavitt, R.D.; Bachur, N.R. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim. Biophys. Acta 1975, 405, 72–81. [Google Scholar] [CrossRef]
- Felsted, R.L.; Li, J.; Pokrywka, G.; Egorin, M.J.; Spiegel, J.; Dale, R.M. Comparison of Phaseolus vulgaris cultivars on the basis of isolectin differences. Int. J. Biochem. 1981, 13, 549–557. [Google Scholar] [CrossRef]
- Cummings, R.D.; Kornfeld, S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J. Biol. Chem. 1982, 257, 11230–11234. [Google Scholar] [PubMed]
- Fleischmann, G.; Mauder, I.; Illert, W.; Rudiger, H. A one-step procedure for isolation and resolution of the Phaseolus vulgaris isolectins by affinity-chromatography. Biol. Chem. Hoppe-Seyler 1985, 366, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Hamelryck, T.W.; DaoThi, M.H.; Poortmans, F.; Chrispeels, M.J.; Wyns, L.; Loris, R. The crystallographic structure of phytohemagglutinin-l. J. Biol. Chem. 1996, 271, 20479–20485. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Soga, K.; Morita-Matsumoto, K.; Hanashima, S.; Ikeda, A.; Yamamoto, K.; Yamaguchi, Y. Phytohemagglutinin from Phaseolus vulgaris (PHA-E) displays a novel glycan recognition mode using a common legume lectin fold. Glycobiology 2014, 24, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Sarkar, A.; Rivet, A.; Breton, C.; Imberty, A. Glyco3d: A portal for structural glycosciences. Glycoinformatics 2015, 1273, 241–258. [Google Scholar]
- Bouckaert, J.; Loris, R.; Poortmans, F.; Wyns, L. Crystallographic structure of metal-free concanavalin a at 2.5 angstrom resolution. Proteins 1995, 23, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Loris, R.; Casset, F.; Bouckaert, J.; Pletinckx, J.; Daothi, M.H.; Poortmans, F.; Imberty, A.; Perez, S.; Wyns, L. The monosaccharide binding-site of lentil lectin: An X-ray and molecular modeling study. Glycoconj. J. 1994, 11, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ruzheinikov, S.N.; Mikhailova, I.Y.; Tsygannik, I.N.; Pangborn, W.; Duax, W.; Pletnev, V.Z. The structure of the pea lectin-d-mannopyranose complex at a 2.1 angstrom resolution. Bioorganicheskaya Khimiya 1998, 24, 313–315. [Google Scholar]
- Babino, A.; Tello, D.; Rojas, A.; Bay, S.; Osinaga, E.; Alzari, P.M. The crystal structure of a plant lectin in complex with the Tn antigen. FEBS. Lett. 2003, 536, 106–110. [Google Scholar] [CrossRef]
- Imberty, A.; Gohier, A.; Jordan, E.; Goldstein, I.J.; Perez, S. Molecular modeling of native and mutated lima bean lectin: Dissection of lectin/blood group a trisaccharide interactions. Internet J. Chem. 1998, 1, 10. [Google Scholar]
- Felger, R.S.; Nabhan, G.P. Agroecosystem diversity: A model from the Sonoran Desert. In Social and Technological Management in the Dry Lands, American Association for the Advancement of Sciences (AAAS) Selected Symposium 10; Gonzalez, N.L., Ed.; Westview Press: Boulder, CO, USA, 1978; pp. 128–149. [Google Scholar]
- Sousa, M.; Delgado, A. Mexican leguminosae: Phytogeography, endemism, and origins. In Biological Diversity of Mexico: Origins and Distribution; Oxford University Press: New York, NY, USA, 1993; pp. 459–511. [Google Scholar]
- Sousa, S.M.; Medina, R.L.; Andrade, G.; Rico, M.L. Leguminosas. In Biodiversidad de Oaxaca; García, A., Diaz, M., Briones, M., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la Conservación de la Naturaleza, World Wildlife Fund: México, D.F., México, 2004; pp. 249–269. [Google Scholar]
- Ortega-Nieblas, M.M. Estudio de Algunas Semillas de Leguminosas del Desierto de Sonora. Factores Antinutricionales y Calidad de sus Proteínas y Aceites. Master’s Thesis, Centro de Investigación en Alimentación y Desarrollo, A.C., Sonora, México, 1993. [Google Scholar]
- Felipe-Ortega, X. Aislamiento y Caracterización de las Lectinas de Leguminosas Silvestres del Desierto de Sonora: Cercidium praecox (palo de brea) y Caesalpinia caladenia (palo dorado). Bachelor´s Thesis, Universidad de Sonora, Sonora, México, August 1996. [Google Scholar]
- López-Laredo, A.R. Caracterización de los oligosacáridos de las lectinas de Olneya tesota pf2 y su isoforma más abundante (IF2) y establecer la relación con la función de reconocimiento. Master´s Thesis, Centro de Investigación en Alimentación y Desarrollo, A.C., Sonora, México, May 2005. [Google Scholar]
- Félix-Favela, F. Isoformas de la lectina PF2, Características Moleculares y Reconocimiento de Leucocitos de Sangre Periférica de Adultos. Master´s Thesis, Centro de Investigación en Alimentación y Desarrollo, A.C., Sonora, México, April 2009. [Google Scholar]
- Quirós-Quintero, C.E. Purificación y Caracterización de la Lectina de Prosopis Juliflora y Evaluación del Desarrollo Larval de Zabrotes Subfasciatus en las Semillas. Bachelor´s Thesis, Universidad de la Sierra, Sonora, México, February 2010. [Google Scholar]
- Valadez-Vega, C.; Guzmán-Partida, A.M.; Soto-Cordova, F.J.; Álvarez-Manilla, G.; Morales-González, J.A.; Madrigal-Santillán, E.; Villagómez-Ibarra, J.R.; Zúñiga-Pérez, C.; Gutiérrez-Salinas, J.; Becerril-Flores, M.A. Purification, biochemical characterization, and bioactive properties of a lectin purified from the seeds of white tepary bean (Phaseolus acutifolius variety latifolius). Molecules 2011, 16, 2561–2582. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Partida, A.; Félix-Favela, F.; Mata-Haro, V.; Lopez-Laredo, A.; Urbano-Hernández, G.; Robles-Burgueño, M.; Candia-Plata, M.; Vázquez-Moreno, L. Identificación de la interacción de monocitos humanos con las lectinas de Olneya tesota (IF2) y Phaseolus vulgaris (PHA-L) por citometría de flujo. Biotecnia 2013, 15, 3–7. [Google Scholar] [CrossRef]
- Miller, J.B.; Noyes, C.; Heinrikson, R.; Kingdon, H.S.; Yachnin, S. Phytohemagglutinin mitogenic proteins. Structural evidence for a family of isomitogenic proteins. J. Exp. Med. 1973, 138, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, R.D.; Felsted, R.L.; Bachur, N.R. Biological and biochemical properties of Phaseolus vulgaris isolectins. J. Biol. Chem. 1977, 252, 2961–2966. [Google Scholar] [PubMed]
- Roberts, D.D.; Etzler, M.E.; Goldstein, I.J. Subunit heterogeneity in the lima bean lectin. J. Biol. Chem. 1982, 257, 9198–9204. [Google Scholar] [PubMed]
- Sparvoli, F.; Lanave, C.; Santucci, A.; Bollini, R.; Lioi, L. Lectin and lectin-related proteins in lima bean (Phaseolus lunatus L.) seeds: Biochemical and evolutionary studies. Plant Mol. Biol. 2001, 45, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Villanueva, A.; Caballero-Ortega, H.; Abdullaev-Jafarova, F.; Garfias, Y.; Jimenez-Martinez, M.D.C.; Bouquelet, S.; Martinez, G.; Mendoza-Hernandez, G.; Zenteno, E. Lectin from Phaseolus acutifolius var. Escumite: Chemical characterization, sugar specificity, and effect on human T-lymphocytes. J. Agric. Food Chem. 2007, 55, 5781–5787. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Moreno, L. Analysis of Olneya tesota lectin peptides with liquid chromatography in two dimensions and tandem mass spectrometry (LC–MS/MS). Manuscript in preparation. 2017. [Google Scholar]
- Hoffman, L.M.; Donaldson, D.D. Characterization of 2 Phaseolus-vugaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985, 4, 883–889. [Google Scholar] [PubMed]
- Lioi, L.; Sparvoli, F.; Galasso, I.; Lanave, C.; Bollini, R. Lectin-related resistance factors against bruchids evolved through a number of duplication events. Theor. Appl. Genet. 2003, 107, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Karnchanatat, A. Antimicrobial activity of lectins from antimicrobial activity of lectins from plants. In Antimicrobial Agents; Bobbarala, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 145–177. [Google Scholar]
- Grandhi, N.J.; Mamidi, A.S.; Surolia, A. Pattern recognition in legume lectins to extrapolate amino acid variability to sugar specificity. In Biochemical Roles of Eukaryotic Cell Surface Macromolecules; Chakrabarti, A., Surolia, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 199–215. [Google Scholar]
- Cruz, P.H.; Campos, E.P.; Martínez, L.M.; Ortiz, B.; Martínez, G. Las lectinas vegetales como modelo de estudio de las interacciones proteína-carbohidrato. Revista de Educación Bioquímica 2005, 24, 21–27. [Google Scholar]
- Sharon, N. Lectin-carbohydrate complexes of plants and animals: An atomic view. Trends Biochem. Sci. 1993, 18, 221–226. [Google Scholar] [CrossRef]
- Imberty, A.; Casset, F.; Gegg, C.V.; Etzler, M.E.; Perez, S. Molecular modeling of the Dolichos-biflorus seed lectin and its specific interactions with carbohydrates: α-DN-Acetyl-Galactosamine, Forssman disaccharide and blood-group-a trisaccharide. Glycoconj. J. 1994, 11, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; Oomen, R.P. Analysis of sequence variation among legume lectins: A ring of hypervariable residues forms the perimeter of the carbohydrate-binding site. J. Mol. Biol. 1992, 228, 924–934. [Google Scholar] [CrossRef]
- Benevides, R.G.; Ganne, G.; Simoes Rda, C.; Schubert, V.; Niemietz, M.; Unverzagt, C.; Chazalet, V.; Breton, C.; Varrot, A.; Cavada, B.S.; et al. A lectin from Platypodium elegans with unusual specificity and affinity for asymmetric complex N-glycans. J. Biol. Chem. 2012, 287, 26352–26364. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Bose, P.P.; Ganguly, T.; Campana, P.T.; Ghosh, R.; Chatterjee, B.P. Comparison of the nature of interactions of two sialic acid specific lectins Saraca indica and Sambucus nigra with N-acetylneuraminic acid by spectroscopic techniques. J. Lumin. 2015, 160, 119–127. [Google Scholar] [CrossRef]
- Chrispeels, M.J.; Raikhel, N.V. Lectins, lectin genes, and their role in plant defense. Plant Cell 1991, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Van Damme, E. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, L.T.J.; Vandonselaar, M.; Prasad, L.; Quail, J.W.; Wilson, K.S.; Dauter, Z. Structures of the lectin-iv of Griffonia simplicifolia and its complex with the Lewis b human blood-group determinant at 2.0-angstrom resolution. J. Mol. Biol. 1993, 230, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Gautier, C.; Lescar, J.; Perez, S.; Wyns, L. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J. Biol. Chem. 2000, 275, 17541–17548. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, J.; Michaelsen, T.E.; Sletten, K. Properties of a lectin purified from the seeds of Cicer-arietinum. Hope-Seyler’s Z. Physiol. Chem. 1983, 364, 655–664. [Google Scholar] [CrossRef]
- Ray, S.; Chatterjee, B.P. Saracin a lectin from saraca-indica seed integument recognizes complex carbohydrates. Phytochem. 1995, 40, 643–649. [Google Scholar] [CrossRef]
- Irimura, T.; Tsuji, T.; Tagami, S.; Yamamoto, K.; Osawa, T. Structure of a complex-type sugar chain of human glycophorin A. Biochemistry 1981, 20, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, S.; Hammarstrom, M.L.; Sundblad, G.; Arnarp, J.; Lonngren, J. Mitogenic leukoagglutinin from Phaseolus-vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc. Natl. Acad. Sci. USA 1982, 79, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hitoi, A.; Kobata, A. Structural determinants of Phaseolus-vulgaris erythroagglutinating lectin for oligosaccharides. J. Biol. Chem. 1983, 258, 4753–4755. [Google Scholar]
- Kamemura, K.; Furuichi, Y.; Umekawa, H.; Takahashi, T. Purification and characterization of novel lectins from great northern bean, Phaseolus vulgaris L. BBA-Gen. Subj. 1993, 1158, 181–188. [Google Scholar] [CrossRef]
- Kaneda, Y.; Whittier, R.F.; Yamanaka, H.; Carredano, E.; Gotoh, M.; Sota, H.; Hasegawa, Y.; Shinohara, Y. The high specificities of Phaseolus vulgaris erythro- and leukoagglutinating lectins for bisecting GlcNac or beta 1–6-linked branch structures, respectively, are attributable to loop B. J. Biol. Chem. 2002, 277, 16928–16935. [Google Scholar] [CrossRef] [PubMed]
- Takeya, A.; Hosomi, O.; Nishijima, H.; Ohe, Y.; Sugahara, K.; Sagi, M.; Yamazaki, K.; Hayakawa, H.; Takeshita, H.; Sasaki, C.; et al. Presence of beta-linked GalNac residues on N-glycans of human thyroglobulin. Life Sci. 2007, 80, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Rinderle, S.J.; Goldstein, I.J.; Matta, K.L.; Ratcliffe, R.M. Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus-caudatus, that recognizes the T-antigen (or cryptic-T)-antigen. J. Biol. Chem. 1989, 264, 16123–16131. [Google Scholar] [PubMed]
- Ramos, M.V.; Sampaio, A.H.; Cavada, B.S.; Calvete, J.J.; Grangeiro, T.B.; Debray, H. Characterization of the sugar-binding specificity of the toxic lectins isolated from Abrus pulchellus seeds. Glycoconj. J. 2001, 18, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Braun, A.; Paschinger, K.; Wilson, I.B.H. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal. Biochem. 2009, 386, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.S.; Procopio, T.F.; Napoleao, T.H.; Coelho, L.C.; Paiva, P.M. Antimicrobial lectin from Schinus terebinthifolius leaf. J. Appl. Microbiol. 2013, 114, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012, 120, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.-H.; Jung, Y.P.; Cho, W.-K.; Kim, T.; Kim, A.; Im, M.; Ma, J.Y. Screening of aqueous extracts of medicinal herbs for antimicrobial activity against oral bacteria. Integr. Med. Res. 2013, 2, 18–24. [Google Scholar] [CrossRef]
- Srivastava, P.; Upreti, D.K.; Dhole, T.N.; Srivastava, A.K.; Nayak, M.T. Antimicrobial property of extracts of Indian lichen against human pathogenic bacteria. Interdiscip. Perspect. Infect. Dis. 2013, 2013, 709348. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- De Brito Marques Ramos, D.; Gomes, F.S.; Napoleão, T.H.; Paiva, P.M.G.; da Silva, M.D.C.; Barroso Coelho, L.C.B. Antimicrobial activity of Cladonia verticillaris lichen preparations on bacteria and fungi of medical importance. Chin. J. Biol. 2014, 2014. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Cammue, B.P.A.; DeBolle, M.F.C.; Thevissen, K.; DeSamblanx, G.W.; Osborn, R.W. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997, 16, 297–323. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Ganz, T. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1999, 11, 23–27. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. The structural basis for carbohydrate recognition by lectins. Adv. Exp. Med. Biol. 2001, 491, 1–16. [Google Scholar] [PubMed]
- Charungchitrak, S.; Petsom, A.; Sangvanich, P.; Karnchanatat, A. Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem. 2011, 126, 1025–1032. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Fang, E.F.; Ng, T.B. Medicinal applications of plant lectins. In Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds; Fang, E., Tzi, B., Eds.; Springer International Publishing: Dordrecht, The Netherlands, 2013; pp. 55–74. [Google Scholar]
- Oliveira, M.D.L.; Andrade, C.A.S.; Santos-Magalhaes, N.S.; Coelho, L.C.B.B.; Teixeira, J.A.; Carneiro-Da-Cunha, M.G.; Correia, M.T.S. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol. 2008, 46, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Sammour, R.H.; El-Shanshoury, A. Antimicrobial activity of legume seed proteins. Bot. Bull. Acad. Sin. 1992, 31, 185–190. [Google Scholar]
- Ayouba, A.; Causse, H.; Vandamme, E.J.M.; Peumans, W.J.; Bourne, Y.; Cambillau, C.; Rouge, P. Interactions of plant-lectins with the components of the bacterial-cell wall peptidoglycan. Biochem. Syst. Ecol. 1994, 22, 153–159. [Google Scholar] [CrossRef]
- Santi-Gadelha, T.; Rocha, B.A.M.; Gadelha, C.A.A.; Silva, H.C.; Castellon, R.E.R.; Goncalves, F.J.T.; Toyama, D.O.; Toyama, M.H.; de Souza, A.J.F.; Beriam, L.O.S.; et al. Effects of a lectin-like protein isolated from Acacia Farnesiana seeds on phytopathogenic bacterial strains and root-knot nematode. Pestic. Biochem. Phys. 2012, 103, 15–22. [Google Scholar] [CrossRef]
- Islam, B.; Khan, A.U. Lectins: To combat infections. In Protein Purification; Ahmad, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 167–188. [Google Scholar]
- Bourne, Y.; Ayouba, A.; Rouge, P.; Cambillau, C. Interaction of a legume lectin with two components of the bacterial cell wall. A crystallographic study. J. Biol. Chem. 1994, 269, 9429–9435. [Google Scholar] [PubMed]
- Qadir, S.; Wani, I.H.; Rafiq, S.; Ganie, S.A.; Masood, A.; Hamid, R. Evaluation of antimicrobial activity of a lectin isolated and purified from Indigofera Heterantha. Adv. Biosci. Biotechnol. 2013, 4, 999. [Google Scholar] [CrossRef]
- Yan, Q.J.; Jiang, Z.Q.; Yang, S.Q.; Deng, W.; Han, L.J. A novel homodimeric lectin from Astragalus Mongholicus with antifungal activity. Arch. Biochem. Biophys. 2005, 442, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Ji, N.; Zhou, J.; Bian, H.J.; Li, C.Y.; Chen, F.; Bao, J.K. A novel sialic acid-specific lectin from Phaseolus coccineus seeds with potent antineoplastic and antifungal activities. Phytomedicine 2009, 16, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.C.; Pinto, L.D.; Teixeira, E.H.; Nascimento, K.S.; Cavada, B.S.; Silva, A.L.C. Bul: A novel lectin from Bauhinia Ungulata L. Seeds with fungistatic and antiproliferative activities. Process. Biochem. 2014, 49, 203–209. [Google Scholar] [CrossRef]
- Barkai-Golan, R.; Mirelman, D.; Sharon, N. Studies on growth inhibition by lectins of penicillia and aspergilli. Arch. Microbiol. 1978, 116, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Ng, T.B.; Tsang, P.W.K.; Wang, J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J. Protein Chem. 2001, 20, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.; Lopes, N.E.; Clark, A.T.; de Pontes Filho, N.T.; Beltrao, E.I.; Neves, R.P. Carbohydrate profiling of fungal cell wall surface glycoconjugates of Aspergillus species in brain and lung tissues using lectin histochemistry. Med. Mycol. 2012, 50, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.M.; Dan, X.L.; Chan, Y.S.; Pan, W.L.; Cheung, R.C.F. Lectins with anti-hiv activity: A review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef] [PubMed]
- Ingale, A.G.; Hivrale, A.U. Plant as a plenteous reserve of lectin. Plant Signal Behav. 2013, 8, e26595. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, B.; Martins, M.B.; Karamanska, R.; Russell, D.A.; Field, R.A. Bacterial detection using carbohydrate-functionalised cds quantum dots: A model study exploiting E. coli recognition of mannosides. Tetrahedron Lett. 2009, 50, 886–889. [Google Scholar] [CrossRef]
- Charan, R.D.; Munro, M.H.G.; O’Keefe, B.R.; Sowder, R.C.; McKee, T.C.; Currens, M.J.; Pannell, L.K.; Boyd, M.R. Isolation and characterization of Myrianthus holstii lectin, a potent HIV-1 inhibitory protein from the plant Myrianthus Holstii. J. Nat. Prod. 2000, 63, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kobayashi, S.; Yoshida, O.; Ishii, S.; Abe, Y.; Yamamoto, N. Effects of succinylated concanavalin a on infectivity and syncytial formation of human-immunodeficiency-virus. Med. Microbiol. Immun. 1990, 179, 225–235. [Google Scholar] [CrossRef]
- Hansen, J.E.; Nielsen, C.M.; Nielsen, C.; Heegaard, P.; Mathiesen, L.R.; Nielsen, J.O. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. Aids 1989, 3, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.L.; Liu, W.L.; Ng, T.B. Development and applications of lectins as biological tools in biomedical research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B.; Cheung, R.C.; Ye, X.J.; Wang, H.X.; Lam, S.K.; Lin, P.; Chan, Y.S.; Fang, E.F.; Ngai, P.H.; et al. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl. Microbiol. Biotechnol. 2010, 87, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Lis, H.; Sharon, N. Lectins in higher plants. In The Biochemistry of Plants; Marcus, A., Ed.; Academic Press Inc.: New York, NY, USA, 1981; Volume 6, pp. 371–447. [Google Scholar]
- Ciopraga, J.; Gozia, O.; Tudor, R.; Brezuica, L.; Doyle, R.J. Fusarium sp growth inhibition by wheat germ agglutinin. BBA-Gen. Subj. 1999, 1428, 424–432. [Google Scholar] [CrossRef]
- Van Parijs, J.; Broekaert, W.F.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 183, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Boleti, A.P.D.; Freire, M.D.M.; Coelho, M.B.; da Silva, W.; Baldasso, P.A.; Gomes, V.M.; Marangoni, S.; Novello, J.C.; Macedo, M.L.R. Insecticidal and antifungal activity of a protein from Pouteria torta seeds with lectin-like properties. J. Agric. Food Chem. 2007, 55, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.R.; Lam, K.; Qasba, P.K. Three dimensional structure of the soybean agglutinin-Gal/GalNac complexes by homology modeling. J. Biomol. Struct. Dyn. 1998, 15, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, N.E.; Wlodawer, A. Structural studies of algal lectins with anti-hiv activity. Acta Biochim. Pol. 2006, 53, 617–626. [Google Scholar] [PubMed]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007, 75, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An antiviral lectin with outstanding therapeutic potential. Viruses 2016, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Fei Fang, E.; Ho Wong, J.; Lin, P.; Bun Ng, T. Biochemical and functional properties of a lectin purified from korean large black soybeans a cultivar of glycine max. Protein Pept. Lett. 2010, 17, 690–698. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; das Graças Machado Freire, M.; da Silva, M.B.R.; Coelho, L.C.B.B. Insecticidal action of Bauhinia monandra leaf lectin (bmoll) against Anagasta kuehniella (lepidoptera: Pyralidae), Zabrotes subfasciatus and Callosobruchus maculatus (coleoptera: Bruchidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Melander, M.; Åhman, I.; Kamnert, I.; Strömdahl, A.-C. Pea lectin expressed transgenically in oilseed rape reduces growth rate of pollen beetle larvae. Transgenic Res. 2003, 12, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Michiels, K.; Van Damme, E.J.; Smagghe, G. Plant–insect interactions: What can we learn from plant lectins? Arch. Insect Biochem. Physiol. 2010, 73, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Dandagi, P.; Mastiholimath, V.; Patil, M.; Gupta, M. Biodegradable microparticulate system of captopril. Int. J. Pharm. 2006, 307, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Walski, T.; Van Damme, E.J.; Smagghe, G. Penetration through the peritrophic matrix is a key to lectin toxicity against Tribolium castaneum. J. Insect Physiol. 2014, 70, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Gupta, S.; Hess, D.; Das, K.P.; Das, S. Binding of insecticidal lectin Colocasia esculenta tuber agglutinin (cea) to midgut receptors of Bemisia tabaci and Lipaphis erysimi provides clues to its insecticidal potential. Proteomics 2014, 14, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Marty-Detraves, C.; Poincloux, R.; Baricault, L.; Fournier, D.; Paquereau, L. Fungal lectin, xcl, is internalized via clathrin-dependent endocytosis and facilitates uptake of other molecules. Eur. J. Cell Biol. 2003, 82, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Van Damme, E.J.; De Vos, W.H.; Smagghe, G. Mechanism of entomotoxicity of the plant lectin from Hippeastrum hybrid (amaryllis) in spodoptera Littoralis larvae. J. Insect Physiol. 2012, 58, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Salzman, R.A. Functional mechanics of the plant defensive Griffonia simplicifolia lectin II: Resistance to proteolysis is independent of glycoconjugate binding in the insect gut. J. Econ. Entomol. 2001, 94, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Fitches, E.; Wiles, D.; Douglas, A.E.; Hinchliffe, G.; Audsley, N.; Gatehouse, J.A. The insecticidal activity of recombinant garlic lectins towards aphids. Insect Biochem. Mol. Biol. 2008, 38, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Foissac, X.; Carss, A.; Gatehouse, A.M.; Gatehouse, J.A. Ferritin acts as the most abundant binding protein for snowdrop lectin in the midgut of rice brown planthoppers (Nilaparvata lugens). Insect Biochem. Mol. Biol. 2000, 30, 297–305. [Google Scholar] [CrossRef]
- Sadeghi, A.; Smagghe, G.; Proost, P.; Van Damme, E.J. Ferritin acts as a target site for the snowdrop lectin (gna) in the midgut of the cotton leafworm spodoptera littoralis. Insect Sci. 2008, 15, 513–519. [Google Scholar] [CrossRef]
- Napoleão, T.H.; Pontual, E.V.; de Albuquerque Lima, T.; de Lima Santos, N.D.; Sá, R.A.; Coelho, L.C.B.B.; do Amaral Ferraz Navarro, D.M.; Paiva, P.M.G. Effect of myracrodruon urundeuva leaf lectin on survival and digestive enzymes of Aedes aegypti larvae. Parasitol. Res. 2012, 110, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Sprawka, I.; Goławska, S.; Goławski, A.; Chrzanowski, G.; Czerniewicz, P.; Sytykiewicz, H. Entomotoxic action of jackbean lectin (con a) in bird cherry-oat aphid through the effect on insect enzymes. J. Plant Interact. 2014, 9, 425–433. [Google Scholar] [CrossRef]
- Bala, A.; Roy, A.; Behura, N.; Hess, D.; Das, S. Insight to the mode of action of allium sativum leaf agglutinin (asal) expressing in t3 rice lines on brown planthopper. Am. J. Plant Sci. 2013, 4, 400–407. [Google Scholar] [CrossRef]
- Lagarda-Diaz, I.; Guzman-Partida, A.M.; Huerta-Ocampo, J.A.; Winzerling, J.; Vazquez-Moreno, L. Identification of membrane proteins of the midgut of Zabrotes subfasciatus larvae associated with the insecticidal mechanism of PF2 lectin. J. Asia-Pacif. Entomol. 2016, 19, 677–682. [Google Scholar] [CrossRef]
- Lagarda-Diaz, I.; Robles-Burgeño, M.R.; Guzman-Partida, A.M.; Geiser, D.; Winzerling, J.; Vazquez-Moreno, L. Binding of PF2 lectin from Olneya tesota to gut proteins of Zabrotes subfasciatus larvae associated with the insecticidal mechanism. J. Agric. Food Chem. 2012, 60, 2398–2402. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, P.K. Receptors of garlic (Allium sativum) lectins and their role in insecticidal action. Protein J. 2012, 31, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Luo, Z.; Wu, J.; Qiu, L.; Luo, M.; Ke, Q.; Dong, X. Enhanced expression of α2, 3-linked sialic acids promotes gastric cancer cell metastasis and correlates with poor prognosis. Int. J. Oncol. 2017, 50, 1201–1210. [Google Scholar] [PubMed]
- Litynska, A.; Przybyuulo, M.; Pochec, E.; Hoja-uuLukowicz, D.; Ciouulczyk, D.; Laidler, P.; Gil, D. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Res. 2001, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Korourian, S.; Siegel, E.; Kieber-Emmons, T.; Monzavi-Karbassi, B. Expression analysis of carbohydrate antigens in ductal carcinoma in situ of the breast by lectin histochemistry. BMC Cancer 2008, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Blomme, B.; Van Steenkiste, C.; Callewaert, N.; Van Vlierberghe, H. Alteration of protein glycosylation in liver diseases. J. Hepatol. 2009, 50, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Zhou, C.C.; Yao, S.; Yu, J.Y.; Liu, B.; Bao, J.K. Plant lectins: Targeting programmed cell death pathways as antitumor agents. Int. J. Biochem. Cell Biol. 2011, 43, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Stauder, H.; Kreuser, E.-D. Mistletoe extracts standardised in terms of mistletoe lectins (ml i) in oncology: Current state of clinical research. Oncol. Res. Treat. 2002, 25, 374–380. [Google Scholar] [CrossRef]
- Jiang, Q.L.; Zhang, S.; Tian, M.; Zhang, S.Y.; Xie, T.; Chen, D.Y.; Chen, Y.J.; He, J.; Liu, J.; Ouyang, L. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015, 48, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hajela, K.; Kojima, M.; Ambrus, G.; Wong, K.H.; Moffatt, B.E.; Ferluga, J.; Hajela, S.; Gál, P.; Sim, R.B. The biological functions of MBL-associated serine proteases (MASPs). Immunobiology 2002, 205, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Dey, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rao, M.; Tweardy, D.; Prakash, M.; Galili, U.; Gorelik, E. Lectin-induced apoptosis of tumour cells. Glycobiology 1993, 3, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Lei, H.Y. Autophagy induction in t cell-independent acute hepatitis induced by concanavalin a in scid/nod mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 817–826. [Google Scholar] [CrossRef] [PubMed]

| Lectin | Carbohydrate/Glycoprotein Receptor | Type of Microorganism | Mechanism of Action | Reference |
|---|---|---|---|---|
| From Leguminosae, tribe: Vicieae Diocleae Phaseoleae Erithrinea Glycineae Sophoreae Galegeae Genisteae Loteae Acacieae | Components of the cell Wall: Muramic acid, N-acetylmuramic acid, N-acetylglucosamine, Muramyl-dipeptides, glucosaminyl-muyamyl-dipeptide, lipopolysacharides | Bacteria | Form a channel or pore on cell membrane and the cell dies as a result of the out flowing of cellular contents. Bacterial aggregation and inhibition bacterial cell division | [79,83,87,89,90,91] |
| Astragalus mongholicus agglutinin P. vulgaris agglutinin P. coccineus agglutinin Soy bean agglutinin Peanut agglutinin | Components of fungal cell wall: Chitin, sialic acid | Fungi | Binding to hyphae, swollen hyphal, vacuolization of the cell content, and enhanced susceptibility to cell wall lysis of the hyphal induced by osmotic shock, producing more susceptibility to other stress conditions. This condition produces poor absorption of nutrients, interference spore germination and rupture of the cell wall. | [79,83,91,92,93,94,95,96,97,98] |
| Concanavalin A Psophocarpus tetragonolubus agglutinin Lens culinaris agglutinin Vicia faba agglutinin Pisum sativum agglutinin Erythroagglutinin | Components of viral envelope: Glycoproteins Gp120/Gp41, sialic acid | Virus | Bind to the glycosylated envelope protein and block cellular entry (interfere with replication cycle) | [98,99,100,101,102,103,104,105] |
| Lectin | Receptor | Insect | Reference |
|---|---|---|---|
| Allium sativum L. bulbs (ASAI and ASAII) | Aminopeptidase N Sucrase | Acyrthosiphon pisum | [126] |
| Galantus nivalis lectin | Ferritin | Nilaparvata lunges Spodoptera littoralis | [127] [128] |
| Myracrodruon urundeuva leaf lectin (MuLL) | Trypsin α-amylase | Aedes aegypti | [129] |
| Bauhinia monandra leaf lectin (BmoLL) | α-amylase | Callosobruchus maculatus | [117] |
| Concanavalin A | β-glucosidases cathepsin L | Rhopalosiphum padi L. | [130] |
| Allium sativum Leaf Agglutinin (ASAL) | (Nicotinamide adenine dinucleotide reduced) quinone oxidoreductase | Brown planthopper | [131] |
| Colocasia esculenta tuber agglutinin (CEA) | Vacuolar ATP synthase ATP synthase Heat shock protein 70 clathrin heavy chain | Lipaphis erysimi | [122] |
| Sarcoplasmic endoplasmic reticulum typ Ca2+ATPase | Bemisia tabaci | [122] | |
| PF2 lectin | α-amylase V-type proton ATPase Arginine kinase Prohibitin Polyubiquitin Actin ATP-dependent RNA helicase ATP synthase subunit alpha Mitochondrial-processing peptidase α-tubulin Odorant receptor Cytochrome c oxidase | Zabrotes subfasciatus | [132] [133] |
| Allium sativum lectin | Aminopeptidase Cadherin-N Polycalin Alkaline phosphatase Cytochrome P450 | Helicoverpa Armigera | [134] |
| Alanyl Aminopeptidase N Sucrase | Acyrthosiphon Pisum | [134] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagarda-Diaz, I.; Guzman-Partida, A.M.; Vazquez-Moreno, L. Legume Lectins: Proteins with Diverse Applications. Int. J. Mol. Sci. 2017, 18, 1242. https://doi.org/10.3390/ijms18061242
Lagarda-Diaz I, Guzman-Partida AM, Vazquez-Moreno L. Legume Lectins: Proteins with Diverse Applications. International Journal of Molecular Sciences. 2017; 18(6):1242. https://doi.org/10.3390/ijms18061242
Chicago/Turabian StyleLagarda-Diaz, Irlanda, Ana Maria Guzman-Partida, and Luz Vazquez-Moreno. 2017. "Legume Lectins: Proteins with Diverse Applications" International Journal of Molecular Sciences 18, no. 6: 1242. https://doi.org/10.3390/ijms18061242
APA StyleLagarda-Diaz, I., Guzman-Partida, A. M., & Vazquez-Moreno, L. (2017). Legume Lectins: Proteins with Diverse Applications. International Journal of Molecular Sciences, 18(6), 1242. https://doi.org/10.3390/ijms18061242