Changes of Cerebral and/or Peripheral Adenosine A1 Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats
Abstract
1. Introduction
2. Results
2.1. Body and Adrenal Gland Weights
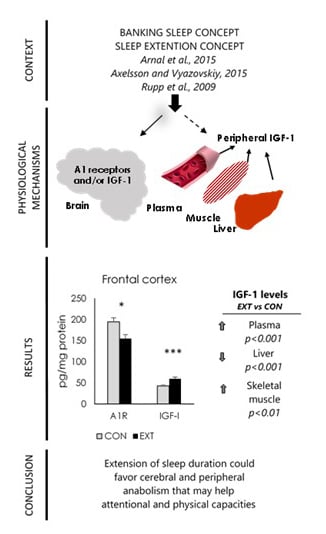
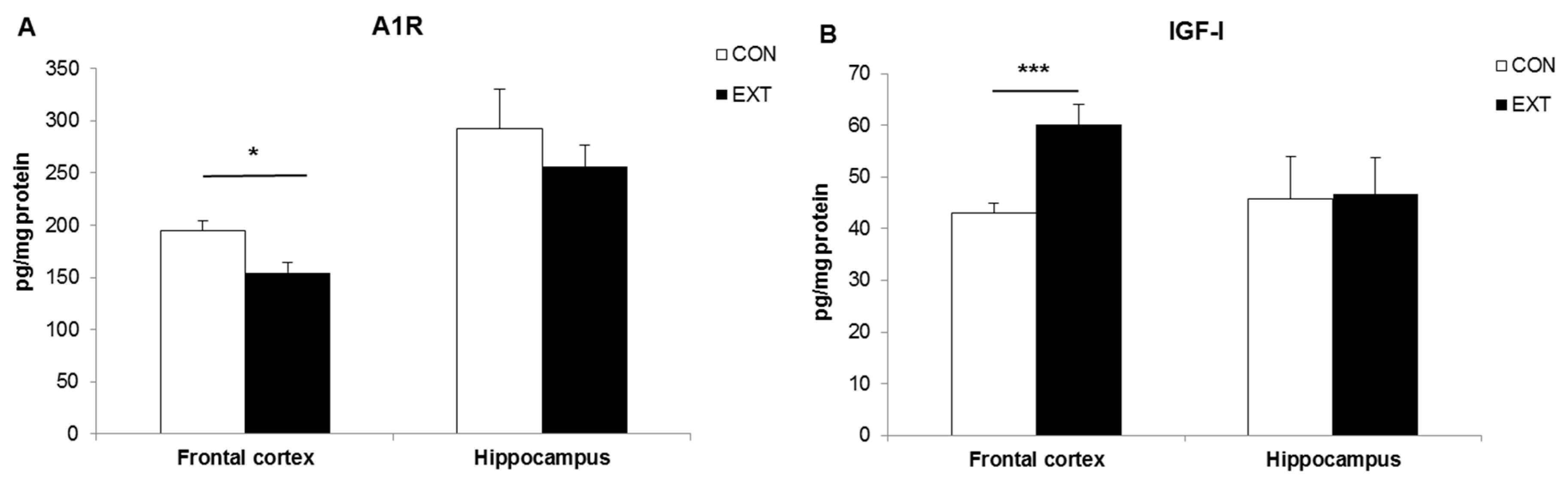
2.2. A1R and IGF-I Concentrations (Expressed in pg/mg Protein) in Brain Areas
2.3. IGF-I Concentrations (Expressed in pg/mg Protein) in Liver and Skeletal Muscle
2.4. Plasma IGF-I and Hormone Concentrations
2.5. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Blood and Tissue Processing
4.3. Assays of Hormones in Blood
4.4. Assays of IGF-1 and A1 Receptor (A1R) in Tissues
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eugene, A.R.; Masiak, J. The neuroprotective aspects of sleep. Medtube Sci. 2015, 3, 35–40. [Google Scholar] [PubMed]
- Goel, N.; Rao, H.; Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Rabat, A.; Gomez-Merino, D.; Roca-Paixao, L.; Bougard, C.; van Beers, P.; Dispersyn, G.; Guillard, M.; Bourrilhon, C.; Drogou, C.; Arnal, P.J.; et al. Differential kinetics in alteration and recovery of cognitive processes from a chronic sleep restriction in young healthy men. Front. Behav. Neurosci. 2016, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Rupp, T.L.; Wesensten, N.J.; Bliese, P.D.; Balkin, T.J. Banking sleep: Realization of benefits during subsequent sleep restriction and recovery. Sleep 2009, 32, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Arnal, P.J.; Sauvet, F.; Leger, D.; van Beers, P.; Bayon, V.; Bougard, C.; Rabat, A.; Millet, G.Y.; Chennaoui, M. Benefits of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and recovery. Sleep 2015, 38, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Vyazovskiy, V.V. Banking sleep and biological sleep need. Sleep 2015, 38, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Drogou, C.; Leger, D.; Sauvet, F.; Gomez-Merino, D. Leukocyte Expression of Type 1 and Type 2 Purinergic Receptors and Pro-Inflammatory Cytokines during Total Sleep Deprivation and/or Sleep Extension in Healthy Subjects. Front. Neurosci. 2017, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.C.; Landolt, H.P. Sleep homeostasis, metabolism, and adenosine. Curr. Sleep Med. Rep. 2015, 1, 27–37. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef] [PubMed]
- Yaar, R.; Jones, M.R.; Chen, J.F.; Ravid, K. Animal models for the study of adenosine receptor function. J. Cell. Physiol. 2005, 202, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Maire, M.; Schmidt, C.; Cajochen, C. Sleep–wake regulation and its impact on working memory performance: The role of adenosine. Biology 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.A.; Bolortuya, Y.; Chen, L.C.; McKenna, J.T.; McCarley, R.W.; Strecker, R.E. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep 2008, 31, 1393–1398. [Google Scholar] [PubMed]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J. Neurochem. 2011, 116, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Elmenhorst, D.; Weisshaupt, A.; Wedekind, F.; Kroll, T.; McCarley, R.W.; Strecker, R.E.; Bauer, A. Chronic sleep restriction induces long-lasting changes in adenosine and noradrenaline receptor density in the rat brain. J. Sleep Res. 2015, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Fernandez, A.M.; Lopez-Lopez, C.; Torres-Aleman, I. Emerging roles of insulin-like growth factor-I in the adult brain. Growth Horm. IGF Res. 2007, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Torres-Aleman, I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009, 89, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Szczesny, E.; Glombik, K.; Stachowicz, K.; Slusarczyk, J.; Nalepa, I.; Zelek-Molik, A.; Rafa-Zablocka, K.; Budziszewska, B.; Kubera, M.; et al. Prenatal stress affects insulin-like growth factor-1 (IGF-1) level and IGF-1 receptor phosphorylation in the brain of adult rats. Eur. Neuropsychopharmacol. 2014, 24, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.R.; Bondy, C.A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994, 135, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Nuñez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [PubMed]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.; Leroy, F.; Soya, H.; Nuñez, A.; et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Obál, F., Jr.; Krueger, J.M. GHRH and sleep. Sleep Med. Rev. 2004, 8, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Obál, F., Jr.; Fang, J.; Collins, B.J.; Krueger, J.M. Non-rapid eye movement sleep is suppressed in transgenic mice with a deficiency in the somatotropic system. Neurosci. Lett. 1996, 220, 97–100. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Zheng, J.; Chen, X.; Zou, J.; Shi, Y.; Liu, Z. The effect of IGF-1 on symptoms of sleep deprivation in a rat model of inflammatory heart disease and metabolic syndrome. Biochem. Biophys. Res. Commun. 2014, 446, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A.; Neuhaus, H.U. Daily pattern of sleep, motor activity and feeding in the rat: Effects of regular and gradually extended photoperiods. J. Comp. Physiol. 1978, 124, 1–14. [Google Scholar] [CrossRef]
- Franken, P.; Tobler, I.; Borbély, A.A. Varying photoperiod in the laboratory rat: Profound effect on 24-h sleep pattern but no effect on sleep homeostasis. Am. J. Physiol. 1995, 269, R691–R701. [Google Scholar] [PubMed]
- Dorey, R.P.; Arnal, F.; Sauvet, S.; Ciret, S.; Gallopin, T.; Lely, L.; Gomez-Merino, D.; Chennaoui, M. The interest of extended sleep on anxiety-like behaviour in rats, Abstracts of the 23rd Congress of the European Sleep Research Society, Bologna, Italy, 13–16 September 2016. J. Sleep Res. 2016, 25 (Suppl. S1), 376. [Google Scholar] [CrossRef]
- Ross, A.W.; Russell, L.; Helfer, G.; Thomson, L.M.; Dalby, M.J.; Morgan, P.J. Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet. PLoS ONE 2015, 10, e0119763. [Google Scholar] [CrossRef] [PubMed]
- Havekes, R.; Vecsey, C.G.; Abel, T. The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cell Signal. 2012, 24, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Bjorness, T.E.; Kelly, C.L.; Gao, T.; Poffenberger, V.; Greene, R.W. Control and function of the homeostatic sleep response by adenosine A1 receptors. J. Neurosci. 2009, 29, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Ahlberg, S.; van der Ploeg, I.; Brené, S.; Lindefors, N.; Persson, H.; Fredholm, B.B. Effect of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1993, 347, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dworak, M.; McCarley, R.W.; Kim, T.; Kalinchuk, A.V.; Basheer, R. Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 2010, 30, 9007–9016. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Mohan, S.; Sjögren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; Jansson, J.O.; Svensson, J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Gluckman, P.D. IGF-1 derived small neuropeptides and analogues: A novel strategy for the development of pharmaceuticals for neurological conditions. Br. J. Pharmacol. 2009, 157, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Almengló, C.; Devesa, P.; Devesa, J.; Arce, V.M. GPE promotes the proliferation and migration of mouse embryonic neural stem cells and their progeny in Vitro. Int. J. Mol. Sci. 2017, 18, 1280. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Drogou, C.; Sauvet, F.; Gomez-Merino, D. Sleep extension increases IGF-I concentrations before and during sleep deprivation in healthy young men. Appl. Physiol. Nutr. Metab. 2016, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; Colecchia, E.F.; L’Hermite-Balériaux, M.; Nie, Z.; Copinschi, G.; van Cauter, E. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R874–R883. [Google Scholar] [PubMed]
- Grønli, J.; Dagestad, G.; Milde, A.M.; Murison, R.; Bramham, C.R. Post-transcriptional effects and interactions between chronic mild stress and acute sleep deprivation: Regulation of translation factor and cytoplasmic polyadenylation element-binding protein phosphorylation. Behav. Brain Res. 2012, 235, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zagaar, M.A.; Dao, A.T.; Alhaider, I.A.; Alkadhi, K.A. Prevention by Regular Exercise of Acute Sleep Deprivation-Induced Impairment of Late Phase LTP and Related Signaling Molecules in the Dentate Gyrus. Mol. Neurobiol. 2016, 53, 2900–2910. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.; Visser, H.; Daan, S. Effect of photoperiod on body mass, and daily energy intake and energy expenditure in young rats. Physiol. Behav. 1997, 62, 913–919. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Mershon, J.L.; Allen, D.L.; Steinmetz, R.W. Extrapituitary prolactin: Distribution, regulation, functions, and clinical aspects. Endocr. Rev. 1996, 17, 639–669. [Google Scholar] [PubMed]
- Ignacak, A.; Kasztelnik, M.; Sliwa, T.; Korbut, R.A.; Rajda, K.; Guzik, T.J. Prolactin—Not only lactotrophin. A “new” view of the “old” hormone. J. Physiol. Pharmacol. 2012, 63, 435–443. [Google Scholar] [PubMed]
- Zhang, S.Q.; Inoué, S.; Kimura, M. Sleep-promoting activity of prolactin-releasing peptide (PrRP) in the rat. Neuroreport 2001, 12, 3173–3176. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A.; Crowley, W.R. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E1060–E1070. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sawai, K.; Hashizume, T. Effects of photoperiod on secretory patterns of growth hormone in adult male goats. Anim. Sci. J. 2013, 84, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A.; Moul, D.E.; Barbato, G.; Giesen, H.A.; Seidel, J.A.; Barker, C.; Bender, C. Conservation of photoperiod-responsive mechanisms in humans. Am. J. Physiol. 1993, 265, R846–R857. [Google Scholar] [PubMed]
- Ross, A.W.; Johnson, C.E.; Bell, L.M.; Reilly, L.; Duncan, J.S.; Barrett, P.; Heideman, P.D.; Morgan, P.J. Divergent regulation of hypothalamic neuropeptide Y and agouti-related protein by photoperiod in F344 rats with differential food intake and growth. J. Neuroendocrinol. 2009, 21, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Gomez Merino, D.; Lesage, J.; Drogou, C.; Guezennec, C.Y. Effects of moderate and intensive training on the hypothalamo-pituitary-adrenal axis in rats. Acta Physiol. Scand. 2002, 75, 113–121. [Google Scholar] [CrossRef] [PubMed]
| Tissue Contents | IGF-I | |
|---|---|---|
| CON | EXT | |
| Liver (pg/mg protein) | 674 ± 47 | 377 ± 10 *** |
| Skeletal muscle (pg/mg protein) | 84 ± 7 | 102 ± 5 ** |
| Hormones | CON | EXT |
|---|---|---|
| Corticosterone (ng/mL) | 6.26 ± 1.26 | 6.32 ± 0.72 |
| Prolactin (ng/mL) | 22.60 ± 4.66 | 41.18 ± 6.36 * |
| Testosterone (ng/mL) | 6.79 ± 1.01 | 7.52 ± 0.97 |
| Insulin (µU/mL) | 34.3 ± 6.1 | 49.9 ± 6.4 |
| IGF-I (pg/mL) | 1109 ± 38 | 1354 ± 39 *** |
| Variables | A1R Cortex | IGF-I Cortex | IGF-I Liver | IGF-I Muscle | IGF-I Plasma | Prolactin Plasma |
|---|---|---|---|---|---|---|
| A1R Cortex | - | −0.635 * | 0.344 | −0.430 * | −0.461 * | −0.323 |
| IGF-I Cortex | −0.635 * | - | −0.415 * | 0.347 | 0.522 * | 0.240 |
| IGF Liver | 0.344 | −0.415 * | - | 0.058 | −0.406 * | −0.427 * |
| IGF-I Muscle | −0.430 * | 0.347 | 0.058 | - | 0.546 * | 0.070 |
| IGF-I Plasma | −0.461 * | 0.522 * | −0.406 * | 0.546 * | - | 0.263 |
| Prolactin Plasma | −0.323 | 0.240 | −0.427 * | 0.070 | 0.263 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chennaoui, M.; Arnal, P.J.; Dorey, R.; Sauvet, F.; Ciret, S.; Gallopin, T.; Leger, D.; Drogou, C.; Gomez-Merino, D. Changes of Cerebral and/or Peripheral Adenosine A1 Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats. Int. J. Mol. Sci. 2017, 18, 2439. https://doi.org/10.3390/ijms18112439
Chennaoui M, Arnal PJ, Dorey R, Sauvet F, Ciret S, Gallopin T, Leger D, Drogou C, Gomez-Merino D. Changes of Cerebral and/or Peripheral Adenosine A1 Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats. International Journal of Molecular Sciences. 2017; 18(11):2439. https://doi.org/10.3390/ijms18112439
Chicago/Turabian StyleChennaoui, Mounir, Pierrick J. Arnal, Rodolphe Dorey, Fabien Sauvet, Sylvain Ciret, Thierry Gallopin, Damien Leger, Catherine Drogou, and Danielle Gomez-Merino. 2017. "Changes of Cerebral and/or Peripheral Adenosine A1 Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats" International Journal of Molecular Sciences 18, no. 11: 2439. https://doi.org/10.3390/ijms18112439
APA StyleChennaoui, M., Arnal, P. J., Dorey, R., Sauvet, F., Ciret, S., Gallopin, T., Leger, D., Drogou, C., & Gomez-Merino, D. (2017). Changes of Cerebral and/or Peripheral Adenosine A1 Receptor and IGF-I Concentrations under Extended Sleep Duration in Rats. International Journal of Molecular Sciences, 18(11), 2439. https://doi.org/10.3390/ijms18112439