Enhancement of Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and New Bone Formation in Rats by Obtusilactone A
Abstract
:1. Introduction
2. Results
2.1. Determination of the Bioactivity Effects of Purified Compounds of Cinnamomum kotoense on BMSCs
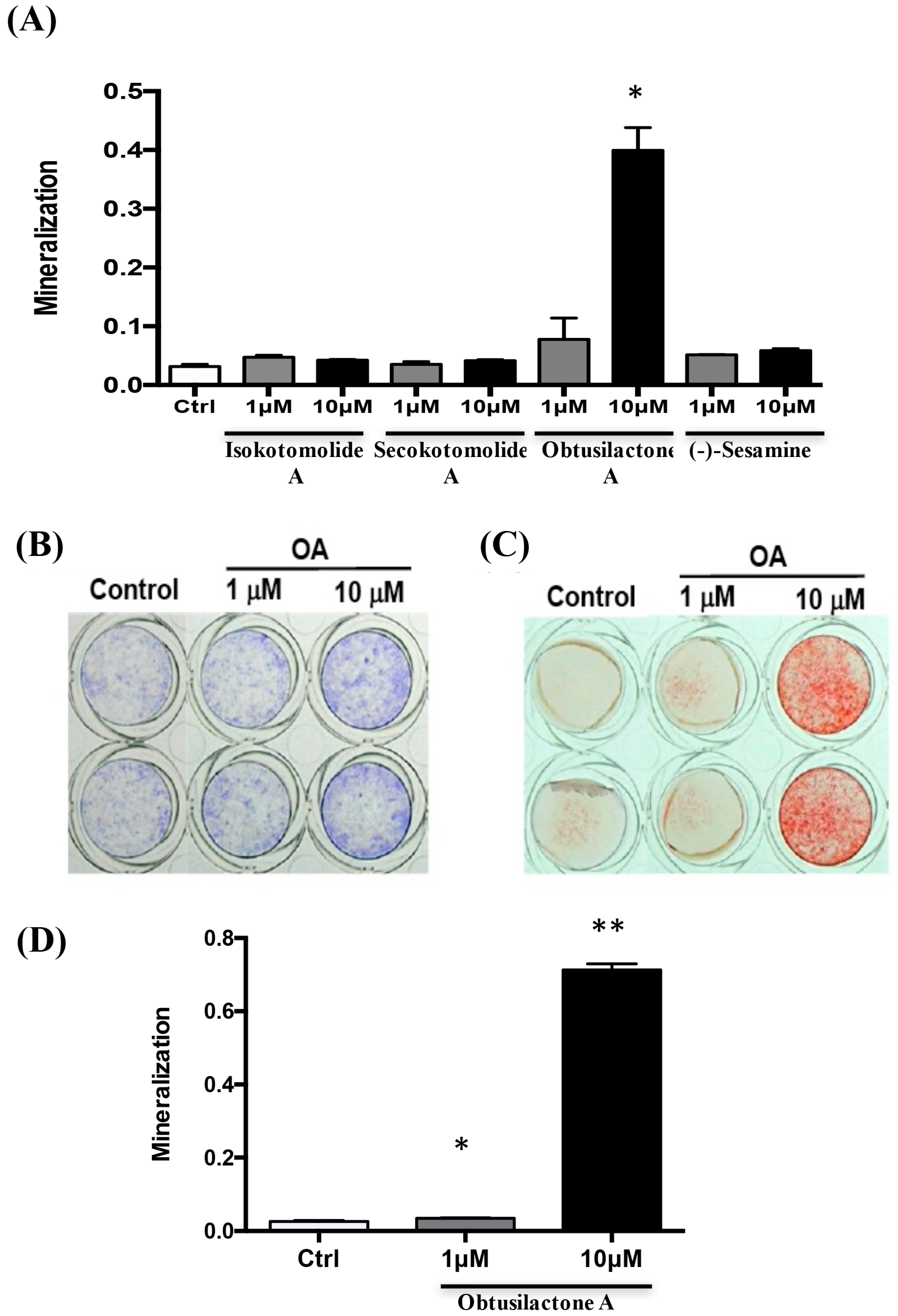
2.2. Osteoinductive Effect of Pure Cinnamomum kotoense Compounds on BMSCs Osteogenesis
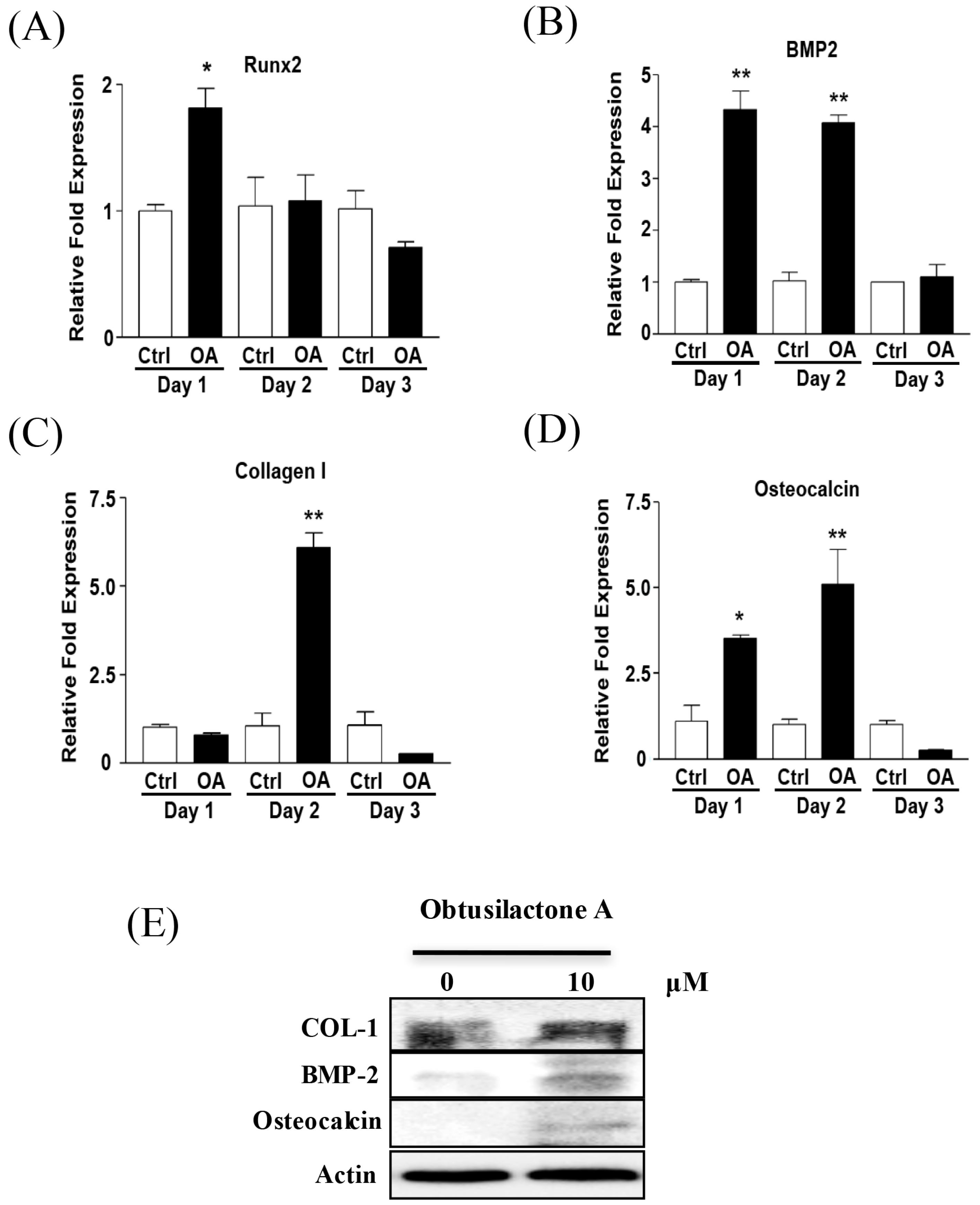
2.3. Enhancement of Osteogenic Gene Expression of BMSCs after OA Treatment
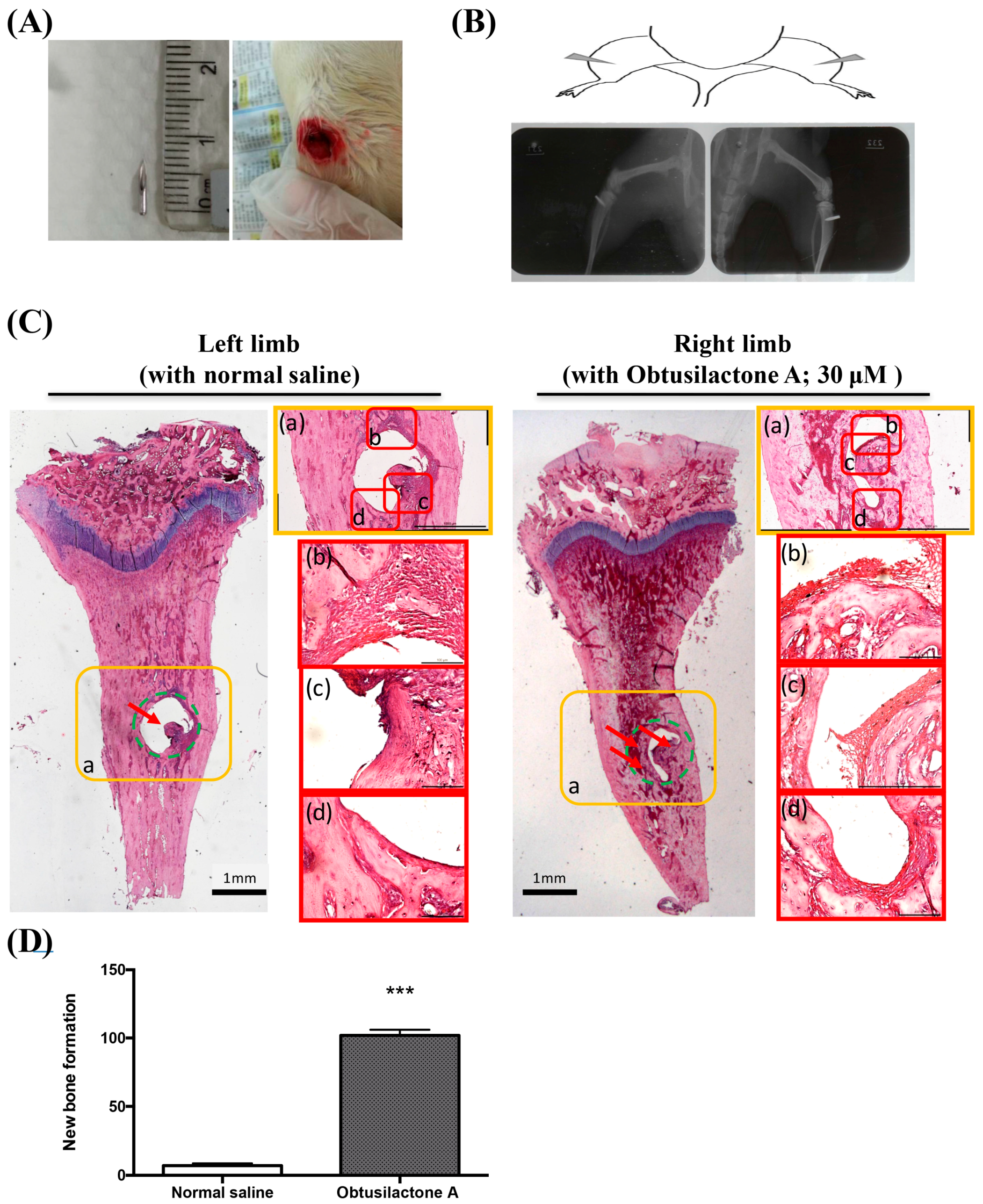
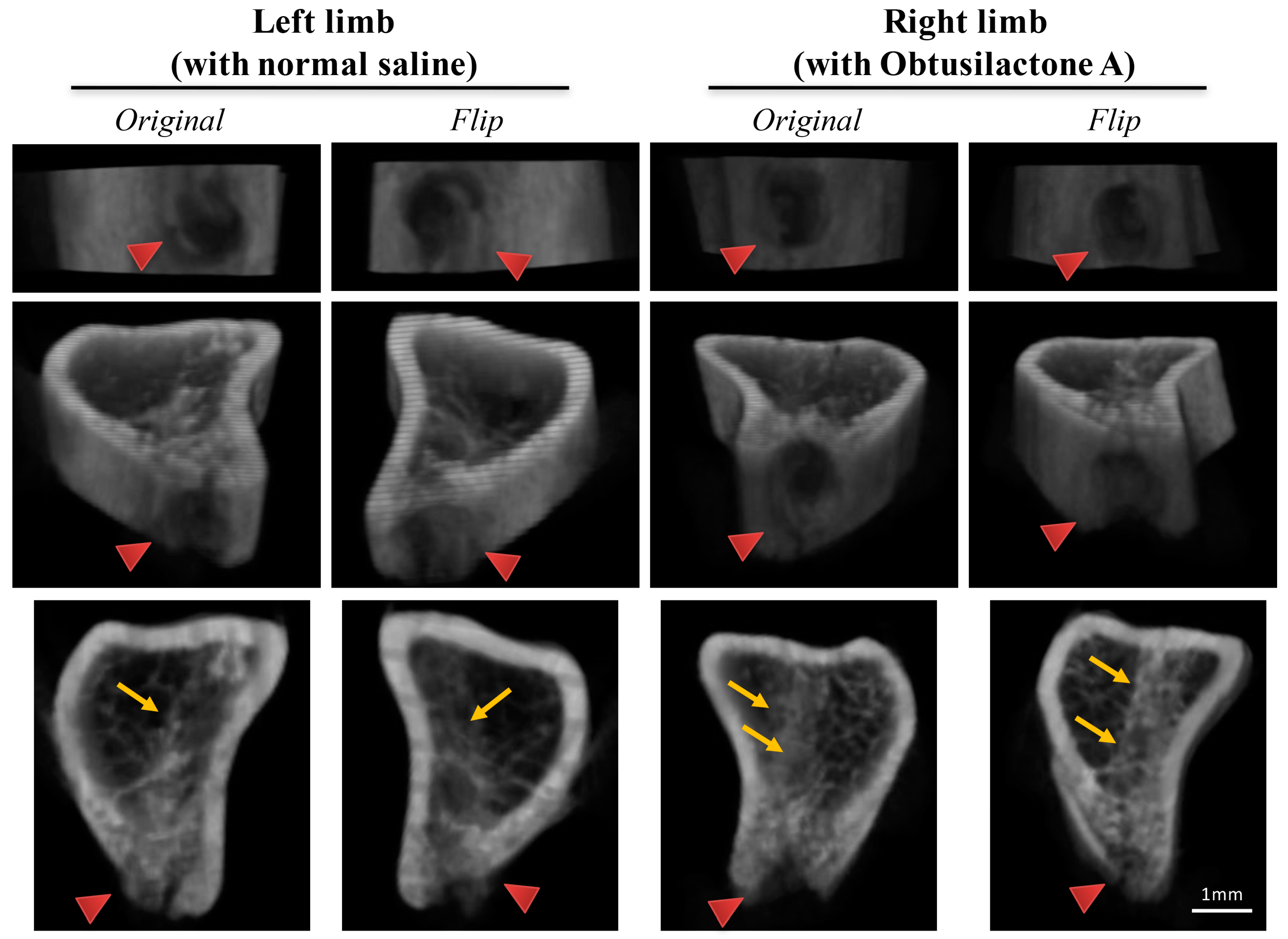
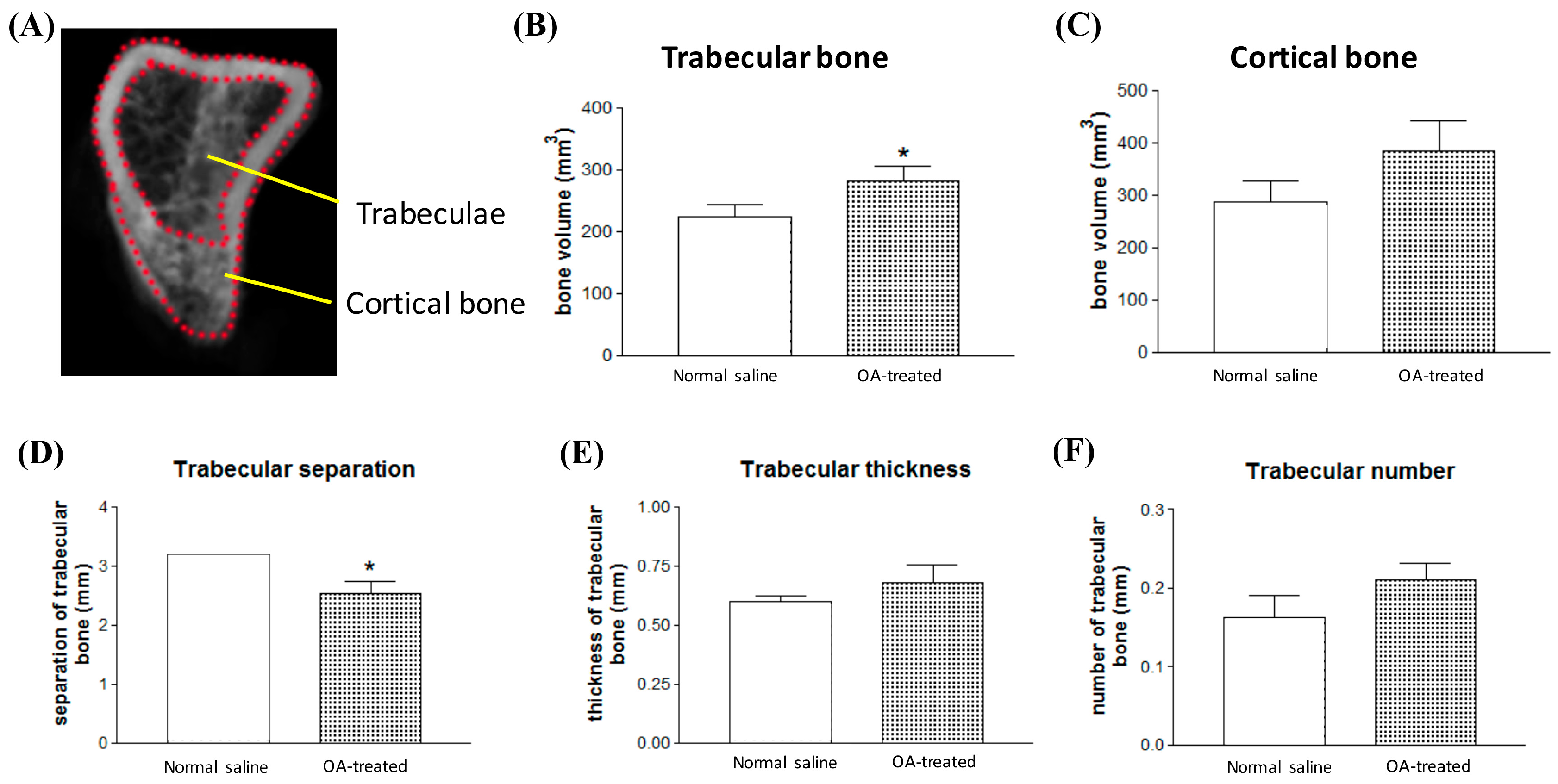
2.4. Local Administration of OA Enhanced New Bone Formation in the Tibia in Rats
3. Discussion
4. Materials and Methods
4.1. Bone Marrow-Derived Mesenchymal Stem Cell Preparation
4.2. Natural Pure Compound Treatment
4.3. MTS Cell Proliferation Assay
4.4. LDH Cytotoxicity Assay
4.5. Quantitative Real-Time PCR Analysis
4.6. Alkaline Phosphatase Activity Assay
4.7. Mineralization Assay by Alizarin Red S Staining
4.8. Needle Implantation in the Tibias of Rats
4.9. Hematoxylin and Eosin Staining and X-ray Radiological Photography of Rats’ Limbs
4.10. Micro-Computed Tomography and Bone Volume Evaluation
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, C.H.; Lo, W.L.; Liu, Y.C.; Chen, C.Y. Chemical and cytotoxic constituents from the leaves of Cinnamomum kotoense. J. Nat. Prod. 2006, 69, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Cheng, K.C.; Lin, C.J.; Hsu, S.W.; Fang, W.C.; Hsu, T.F.; Chiu, C.C.; Chang, H.W.; Hsu, C.H.; Lee, A.Y. Obtusilactone A and (-)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010, 101, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Hsueh, M.C.; Chang, H.C.; Lee, A.Y.; Wang, H.M.; Chen, C.Y. Antioxidants from the leaves of Cinnamomum kotoense. Nat. Prod. Commun. 2010, 5, 911–912. [Google Scholar] [PubMed]
- Potier, E.; Noailly, J.; Ito, K. Directing bone marrow-derived stromal cell function with mechanics. J. Biomech. 2010, 43, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Rosset, P.; Deschaseaux, F.; Layrolle, P. Cell therapy for bone repair. Orthop. Traumatol. Surg. Res. 2014, 100, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Both, S.K.; Yang, F.; Cui, F.Z.; Pan, J.; Meijer, G.J.; Jansen, J.A.; van den Beucken, J.J. Concise review: Cell-based strategies in bone tissue engineering and regenerative medicine. Stem Cells Transl. Med. 2014, 3, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Bianchi, G.; Derubeis, A.; Quarto, R. Cell therapy for bone disease: A review of current status. Stem Cells 2003, 21, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, G.; Sivalingam, N. Molecular mechanism of TGF-β signaling pathway in colon carcinogenesis and status of curcumin as chemopreventive strategy. Tumour Biol. 2014, 35, 7295–7305. [Google Scholar] [CrossRef] [PubMed]
- Choron, R.L.; Chang, S.; Khan, S.; Villalobos, M.A.; Zhang, P.; Carpenter, J.P.; Tulenko, T.N.; Liu, Y. Paclitaxel impairs adipose stem cell proliferation and differentiation. J. Surg. Res. 2015, 196, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Bosukonda, A.; Carlson, W.D. Harnessing the BMP signaling pathway to control the formation of cancer stem cells by effects on epithelial-to-mesenchymal transition. Biochem. Soc. Trans. 2017, 45, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Shah, T.A.; Shaikh, N.N. Turning Bone Morphogenetic Protein 2 (BMP2) on and off in Mesenchymal Cells. J. Cell. Biochem. 2015, 116, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zhou, L.; Shi, X.; He, W.; Wang, H.; Wei, Q.; Chen, P.; Qi, L.; Tickner, J.; Lin, L.; et al. Bajijiasu Abrogates Osteoclast Differentiation via the Suppression of RANKL Signaling Pathways through NF-κB and NFAT. Int. J. Mol. Sci. 2017, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Deepak, V.; Kasonga, A.; Kruger, M.C.; Coetzee, M. Carvacrol Inhibits Osteoclastogenesis and Negatively Regulates the Survival of Mature Osteoclasts. Biol. Pharm. Bull. 2016, 39, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.; Pasco, J.A.; Kotowicz, M.A.; Nicholson, G.C.; Morrison, N.A. Alleles of RUNX2/CBFA1 gene are associated with differences in bone mineral density and risk of fracture. J. Bone Miner. Res. 2002, 17, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Doecke, J.D.; Day, C.J.; Stephens, A.S.; Carter, S.L.; van Daal, A.; Kotowicz, M.A.; Nicholson, G.C.; Morrison, N.A. Association of functionally different RUNX2 P2 promoter alleles with BMD. J. Bone Miner. Res. 2006, 21, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Osyczka, A.M.; Diefenderfer, D.L.; Bhargave, G.; Leboy, P.S. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs 2004, 176, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kaewsrichan, J.; Wongwitwichot, P.; Chandarajoti, K.; Chua, K.H.; Ruszymah, B.H. Sequential induction of marrow stromal cells by FGF2 and BMP2 improves their growth and differentiation potential in vivo. Arch. Oral Biol. 2011, 56, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Tasli, P.N.; Aydin, S.; Yalvac, M.E.; Sahin, F. Bmp 2 and bmp 7 induce odonto- and osteogenesis of human tooth germ stem cells. Appl. Biochem. Biotechnol. 2014, 172, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Son, W.S.; Park, M.C.; Kim, C.M.; Cha, B.H.; Yoon, K.J.; Lee, S.H.; Park, S.G. ARS-interacting multi-functional protein 1 induces proliferation of human bone marrow-derived mesenchymal stem cells by accumulation of β-catenin via fibroblast growth factor receptor 2-mediated activation of Akt. Stem Cells Dev. 2013, 22, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Fu, Y.C.; Jian, S.C.; Wang, Y.H.; Liu, P.L.; Ho, M.L.; Wang, C.K. Synthesis and characterization of cationic polymeric nanoparticles as simvastatin carriers for enhancing the osteogenesis of bone marrow mesenchymal stem cells. J. Colloid Interface Sci. 2014, 432, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Wang, C.Z.; Chang, J.K.; Hsu, C.Y.; Ho, M.L. The susceptive alendronate-treatment timing and dosage for osteogenesis enhancement in human bone marrow-derived stem cells. PLoS ONE 2014, 9, e105705. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chen, C.-Y.; Chou, L.-Y.; Chen, C.-H.; Kang, L.; Wang, C.-Z. Enhancement of Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and New Bone Formation in Rats by Obtusilactone A. Int. J. Mol. Sci. 2017, 18, 2422. https://doi.org/10.3390/ijms18112422
Lin Y-H, Chen C-Y, Chou L-Y, Chen C-H, Kang L, Wang C-Z. Enhancement of Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and New Bone Formation in Rats by Obtusilactone A. International Journal of Molecular Sciences. 2017; 18(11):2422. https://doi.org/10.3390/ijms18112422
Chicago/Turabian StyleLin, Yi-Hsiung, Chung-Yi Chen, Liang-Yin Chou, Chung-Hwan Chen, Lin Kang, and Chau-Zen Wang. 2017. "Enhancement of Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and New Bone Formation in Rats by Obtusilactone A" International Journal of Molecular Sciences 18, no. 11: 2422. https://doi.org/10.3390/ijms18112422
APA StyleLin, Y.-H., Chen, C.-Y., Chou, L.-Y., Chen, C.-H., Kang, L., & Wang, C.-Z. (2017). Enhancement of Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and New Bone Formation in Rats by Obtusilactone A. International Journal of Molecular Sciences, 18(11), 2422. https://doi.org/10.3390/ijms18112422







